Abstract
Objective
To investigate the relationship between HIV-1 drug resistance and adherence and the accumulation rate of resistance mutations in 1191 HIV-infected, antiretroviral-naive adults initiating highly active antiretroviral therapy in British Columbia, Canada.
Methods
Plasma samples with plasma viral load >1000 copies per milliliter collected within 30 months of follow-up were genotyped for drug resistance. Adherence was estimated using prescription refills and plasma drug levels. The primary outcome measure was time to detection of drug resistance. Cox proportional hazard regression was used to calculate hazard ratios (HRs) associated with baseline variables.
Results
The accumulation rates of multiple primary and secondary mutations were similar in patients initiating highly active antiretroviral therapy with protease inhibitor versus nonnucleoside reverse transcriptase inhibitor (NNRTI). Rates decreased approximately 50% per additional mutation. At 80%–90% adherence based on refills, there was greater risk of detecting lamivudine (3TC) [HR 3.0, 95% confidence interval (CI): 1.9 to 4.7; P < 0.0001] and NNRTI mutations (HR 6.0, 95% CI: 3.3 to 10.9; P < 0.0001) compared with the ≥95% refill reference group. In a multivariate model, individuals with <95% refills and consistently detectable plasma drug levels were at increased risk for 3TC (HR 4.5, 95% CI: 2.6 to 7.9; P = 0.0001) and NNRTI resistance (HR 7.0, 95% CI: 3.4 to 14.5; P = 0.0001) compared with the reference group of ≥95% refills with consistently detectable drug levels. Adherence–resistance relationships were much weaker for protease inhibitors and nucleoside reverse transcriptase inhibitors as there was little variance in HRs among the different adherence strata compared with 3TC and NNRTIs.
Conclusion
The relationships between resistance, adherence, and mutation accumulation differ between HIV drug classes.
Keywords: adherence, HAART, HIV-1 drug resistance, mutations
INTRODUCTION
The introduction of highly active antiretroviral therapy (HAART) has revolutionized clinical care for HIV-1–infected individuals. The benefits of HAART in minimizing morbidity and mortality are well established.1–3 However, the emergence of drug resistance mutations limits the efficacy of these treatments. 4 Suboptimal adherence to HAART has been associated with the emergence of drug resistance mutations.5, 6 Studies characterizing the relationship between nonadherence and the development of resistance reveal that patients in this cohort with good but not perfect adherence (80%–90%) were at the greatest risk of developing resistant HIV variants.7 Computer simulations have modeled the adherence–resistance relationship in both drug-naive and experienced patient populations.8 More recently, small studies including both populations have reported that the relationship between resistance and adherence may be specific with respect to each drug class.9, 10
To further investigate this issue, we characterized the relationship between adherence and resistance for the individual drug classes and the progressive accumulation of primary and secondary mutations in a large population of antiretroviral-naive adults initiating HAART in British Columbia, Canada, between August 1996 and September 1999.
METHODS
British Columbia Centre for Excellence in HIV/AIDS Drug Treatment Program
In the province of British Columbia (BC), Canada, antiretrovirals are distributed free of charge to HIV-1–infected individuals through a centralized HIV drug treatment program (DTP). Antiretrovirals are prescribed according to specific guidelines set by the BC Therapeutic Guidelines Committee in concordance with international guidelines.11 A complete prospective profile of therapy use is maintained in the DTP database for all individuals enrolled in the program. Routine clinical monitoring of patients occurs at approximately 3-month intervals, when plasma viral load (pVL) testing and CD4 cell counts are carried out. Enrolled DTP patients provided written informed consent to participate in a sociodemographic survey for research purposes. Ethical approval for this study was obtained from the ethics board of Providence Health Care–University of British Columbia.
The HAART Observational Medical Evaluation and Research Cohort
The HAART Observational Medical Evaluation and Research (HOMER) cohort of the BC Centre for Excellence in HIV/AIDS DTP has been described in detail elsewhere.11, 12 In brief, all HIV-1–positive, antiretroviral-naive adults who initiated HAART in BC between August 1, 1996, and September 30, 1999, were eligible for inclusion in the study (N = 1312); however, our analysis was restricted to individuals who had pretherapy CD4 cell count and HIV-1 RNA pVL data (n = 1191) collected within 180 days of therapy initiation. Subjects were followed up for 30 months or until March 31, 2002, depending on whichever came first (ie, if subject followup time was less than 30 months before this date). Participants were prescribed HAART regimens consisting of 2 nucleoside reverse transcriptase inhibitors (NRTIs) and either a protease inhibitor (PI) or a nonnucleoside reverse transcriptase inhibitor (NNRTI). Further details of the population studied and the resistance analyses are available in Harrigan et al.7
Plasma Viral Load
The pVL was measured at baseline, after 1 month and approximately quarterly thereafter using the Roche Amplicor Monitor assay (Roche Molecular Systems, Pleasanton, CA) as part of routine individual patient monitoring. The level of detection was either <400 or <50 copies per milliliter for pVL monitoring. These values correspond to the level of detection for the standard assay or ultrasensitive assay, respectively. Plasma samples were frozen indefinitely for future use.
Drug Resistance Genotyping
HIV drug resistance genotyping was attempted on all plasma samples with pVL ≥1000 copies per milliliter collected during the 30-month follow-up period after HAART initiation (n = 2805). Samples with pVL <1000 copies per milliliter were not systematically genotyped because genotyping does not yield consistent results for samples with low pVL; these samples were assumed to have no drug resistance mutations. HIV-1 RNA was extracted from plasma using the Qiagen viral RNA kit with a BioRobot 9600/9604, or manually using guanidinium-based buffer, followed by isopropanol and ethanol washes. HIV-1 protease (PR) and reverse transcriptase (RT) genes were amplified using nested reverse transcriptase–polymerase chain reaction and sequenced in both the 5# and 3# directions. Results were reported as amino acid changes in the HIV-1 PR and RT regions with respect to the wild-type reference sequence (HIV-1 HXB2).
PR and RT Mutation Frequencies and Interpretation of Genotypic Data
Mutations associated with drug resistance were defined according to a modification of the International AIDS Society–USA table [15 primary and 27 secondary PR mutations (8 and 15 codons, respectively) and 31 primary and 90 secondary RT mutations (22 and 44 codons, respectively)].7, 13 Additionally, HIV-1 isolates were assigned to 1 of 4 drug resistance categories based on these primary mutations:
Lamivudine (3TC) resistance (184I/V) which also confers resistance to emtricitabine;
Any other NRTI resistance (41L, 62V, 65R, 67N, 69D or insertion, 70R, 74V, 75I, 151M, 210W, 215F/Y, or 219E/Q);
Any NNRTI resistance (100I, 103N, 106A/M, 108I, 181C/I, 188C/H/L, 190A/S, 225H, 230L, or 236L);
Any PI resistance (30N, 46I/L, 48V, 50L/V, 54V/L/M, 82A/F/S/T, 84V, or 90M).
The 3TC resistance was classified separately from other NRTI resistance due to the common appearance of the M184V/I mutation and the lack of NRTI cross-resistance conferred by this mutation. Secondary mutations considered were as follows:
Mutations in the PR protein (10C/F/I/V/R, 20I/M/R/T/V, 24I, 32I, 33F/I/M/V, 47V/A, 54A/S/T, 58E, 62V, 71I/L/T/V, 73A/C/S/T, 88D/S);
Mutations in the RT protein (43E/N/Q, 44A/D, 67del/E/G/N, 69A/D/del/ins/N/S, 98G/S, 101I/E/H/P/Q/R, 115F, 179D/E/I, 190E/Q, 215C/D/S, 228H/R).
Estimates of Adherence
Prescription Refill Data
The proportion of time that patients spent taking HAART during their first year of therapy, calculated by dividing the number of months of prescriptions dispensed by the number of months of follow-up, was employed as an adherence estimate.14, 15 Subjects were stratified into 7 categories based on these estimates of adherence: 0%<20%, 20%<40%, 40%<60%, 60%<80%, 80%<90%, 90%<95%, and ≥95% of prescriptions refilled.
Untimed Plasma Drug Concentrations
Plasma concentrations of prescribed PIs and NNRTIs were determined for the first 2 plasma samples collected for pVL testing within the first year of follow-up by use of a sensitive, validated, simultaneous assay using reverse-phase high-pressure liquid chromatography coupled with tandem mass spectrometry.16 A breakdown of the different drug regimens is described under “Study Population” in the Results section. Plasma drug concentrations were classified as “untimed” because the time of dosing relative to sampling was unknown. Plasma drug concentrations were categorized as “abnormally low” if they were lower than the steady-state trough concentration minus 1 SD (Ctrough − 1 SD) reported in the product monographs,16 a concentration unlikely to be observed in most randomly sampled patients. Any measurements of plasma drug concentrations above the specified limits in Alexander et al16 were classified as “normal.” In addition to considering prescription refill records alone, adherence was stratified into 4 categories based on a combination of prescription refill data and drug level measurements. Adherence categories are as follows: (1) <95% adherence and 0 or 1 normal drug levels, (2) <95% adherence and 2 normal drug levels, (3) ≥95% adherence and 0 or 1 normal drug levels, and (4) ≥95% adherence and 2 normal drug levels.
Statistical Analysis
The primary outcome measure was time to detection of primary mutations in each drug resistance category, defined as the time from the date of HAART initiation to the date of collection of the first plasma sample containing at least 1 drug resistance mutation. Event-free subjects were censored on their last pVL date.
Cox proportional hazard regression was used to calculate univariate and multivariate risk ratios17 associated with the following baseline variables: pVL (per log10 increment), CD4 cell count (per 100 cells/mL decrement), AIDS diagnosis (yes versus no), age (per 10-year increment), sex (male versus female), calendar year of initial HAART, adherence (prescription refill percentage and untimed drug concentration data), initial use of NNRTIs (yes versus no), and history of injection drug use (self-reported and/or physician reported, yes versus no). All tests for significance were 2 sided, with P < 0.05 indicating statistical significance. Subjects with missing baseline values were censored on their HAART initiation date. We also monitored the time to the sequential accumulation of additional resistance mutations.
RESULTS
Study Population
Between August 1, 1996, and September 30, 1999, 1312 antiretroviral-naive individuals initiated HAART in the HOMER cohort.11, 12 Of these individuals, 121 (9.2%) were excluded due to unavailable pretherapy CD4 and pVL data; therefore, the study cohort was comprised of the remaining 1191 subjects of which 1004 (84.3%) were males. At HAART initiation, the median age of subjects was 37 years [interquartile range (IQR) 32–44 years], the median CD4 cell count was 280 cells per microliter (IQR 130–420 cells/mL), and the median pVL was 120,000 copies per milliliter (IQR 42,000–310,000 copies/mL). Participants received a total of 26 different initial triple therapy combinations; over half (885; 74.3%) of these subjects initiated HAART with a PI. Indinavir (672; 75.9%) was the predominant PI used, followed by nelfinavir (105; 11.9%), saquinavir (75; 8.5%), and full-dose ritonavir (33; 3.7%). (All patients eligible for this study were on unboosted PIs.) The remaining study subjects (306; 25.7%) initiated HAART with an NNRTI. Among these individuals, nevirapine was prescribed most often (288; 94.1%), whereas efavirenz and delavirdine were less common (8; 2.6% and 10; 3.3%, respectively). Of the 1191 participants, 842 (70.7%) initiated HAART after July 1997, when HAART became widely recommended for individuals initiating drug therapy in BC. Additional details can be found in Harrigan et al.7
Accumulation of Resistance Mutations Stratified by Prescription Refills
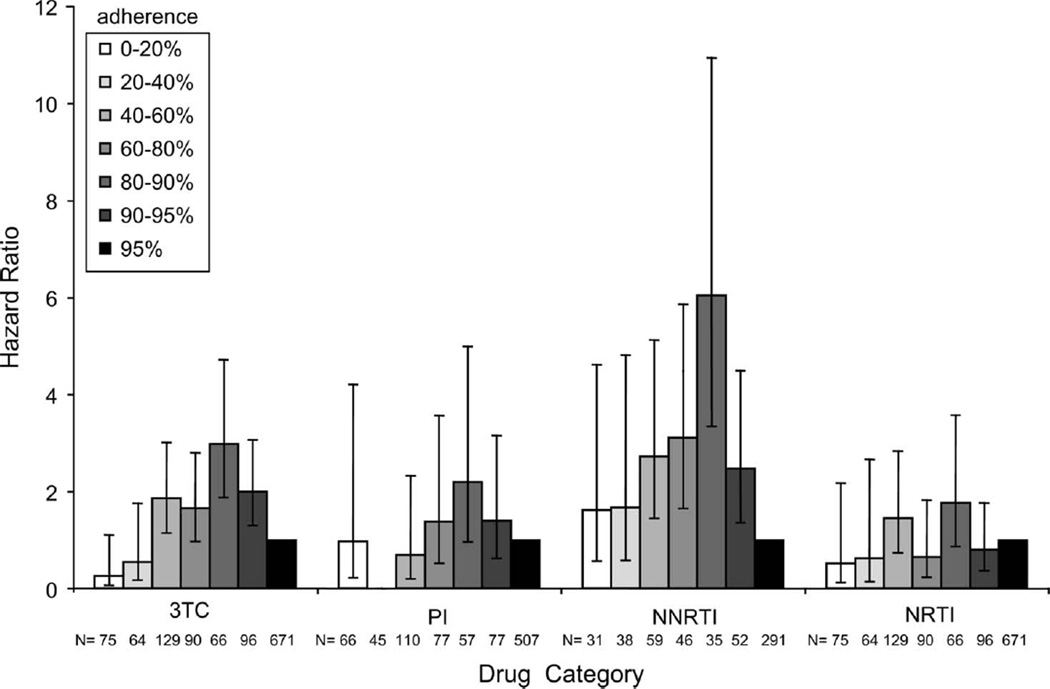
In a multivariate model adjusting for antiretroviral exposure as a time-dependent variable, we observed a strong association between adherence and the detection of 3TC or NNRTI mutations, but not for PI or NRTI resistance mutations. At relatively high levels of adherence (80%–90% prescription refills), there was a greater risk of developing resistance mutations than at any other level of adherence in any drug class (Fig. 1). Even more notably, at 80%–90% refills, there was a 3-fold increase in risk of detection of 3TC resistance mutations [95% confidence interval (CI): 1.9 to 4.7; P < 0.0001] and a 6-fold increase for NNRTI resistance (95% CI: 3.3 to 10.9; P < 0.0001), when compared with the ≥95% refill level (Fig. 1).
FIGURE 1.
HRs for the risk of detection of 3TC, PI, NNRTI, and NRTI resistance mutations in multivariable models adjusted for baseline demographics, pVL, and CD4 count. Antiretroviral exposure was adjusted as a time-dependent variable. Stratified adherence levels of (1) 0%<20%, (2) 20% <40%, (3) 40%<60%, (4) 60%<80%, (5) 80%<90%, and (6) 90%<95% were compared with the (7) ≥95% refill level (HR 1.0). Results are reported as HRs (bars) with 95% CIs (lines).
Accumulation of Resistance Mutations Stratified by a Combined Prescription Refill and Plasma Drug Level Adherence Model
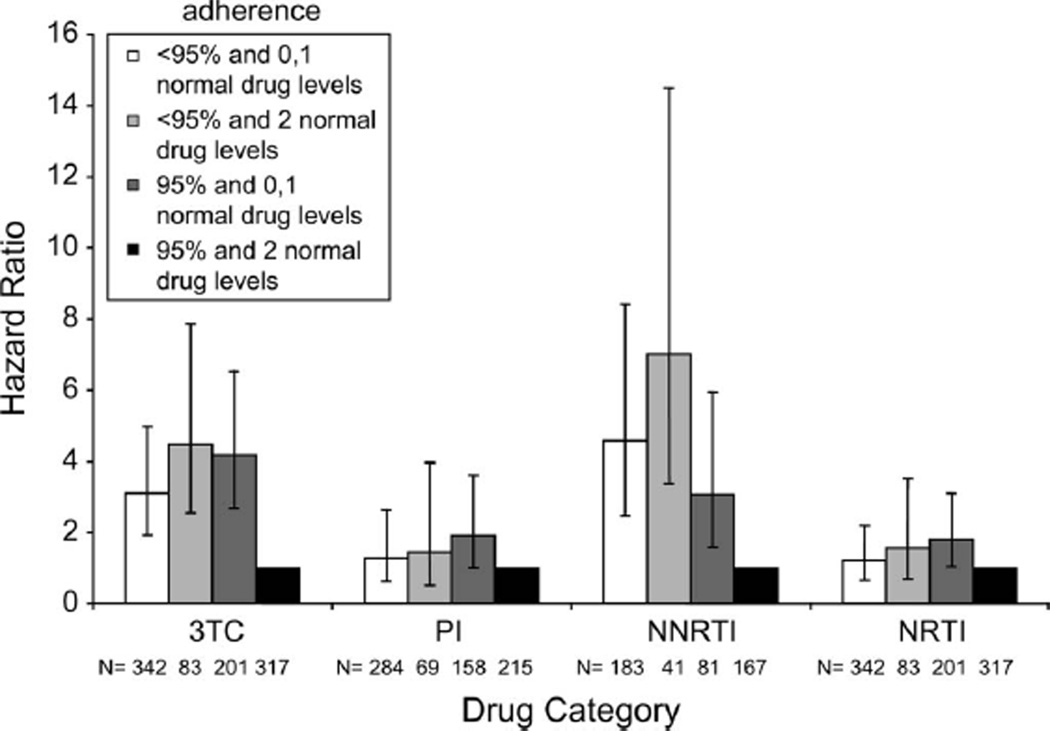
When we combined pharmacy refill data with drug level data, we found that the majority of NNRTI and 3TC mutations were occurring in the lower 2 adherence categories, 0%–20% and 20%–40%. Increased multivariate hazard ratios (HRs) of 4.5-fold for 3TC resistance (95% CI: 2.6 to 7.9; P = 0.0001) and 7-fold for NNRTI resistance (95% CI: 3.4 to 14.5; P = 0.0001) were observed in individuals with <95% prescription refills and consistent drug levels compared with the high-adherence reference group of ≥95% prescription refills with consistent drug levels (Fig. 2). In contrast, there was no significant association between adherence and resistance for either PI (HR 1.6, 95% CI: 0.7 to 3.5; P = 0.3) or the other NRTIs (HR 1.4, 95% CI: 0.5 to 4.0; P = 0.5).
FIGURE 2.
HRs for the risk of detection of 3TC, PI, NNRTI, and NRTI resistance mutations in multivariable models adjusted for baseline demographics, pVL, and CD4 count. Stratified adherence levels of (1) <95% with 0 or 1 normal drug levels, (2) <95% with 2 normal drug levels, and (3) ≥95% with 0 or 1 normal drug levels were compared with the (4) ≥95% with 2 normal drug levels (HR 1.0). Results are reported as HRs (bars) with 95% CIs (lines).
High levels of prescription refills (≥95%) with inconsistent drug levels were also significantly associated with an increased risk of detection of resistance-associated mutations for all the 4 drug categories (Fig. 2).
Accumulation of Resistance Mutations in Individuals Initiating HAART With PI Versus NNRTI
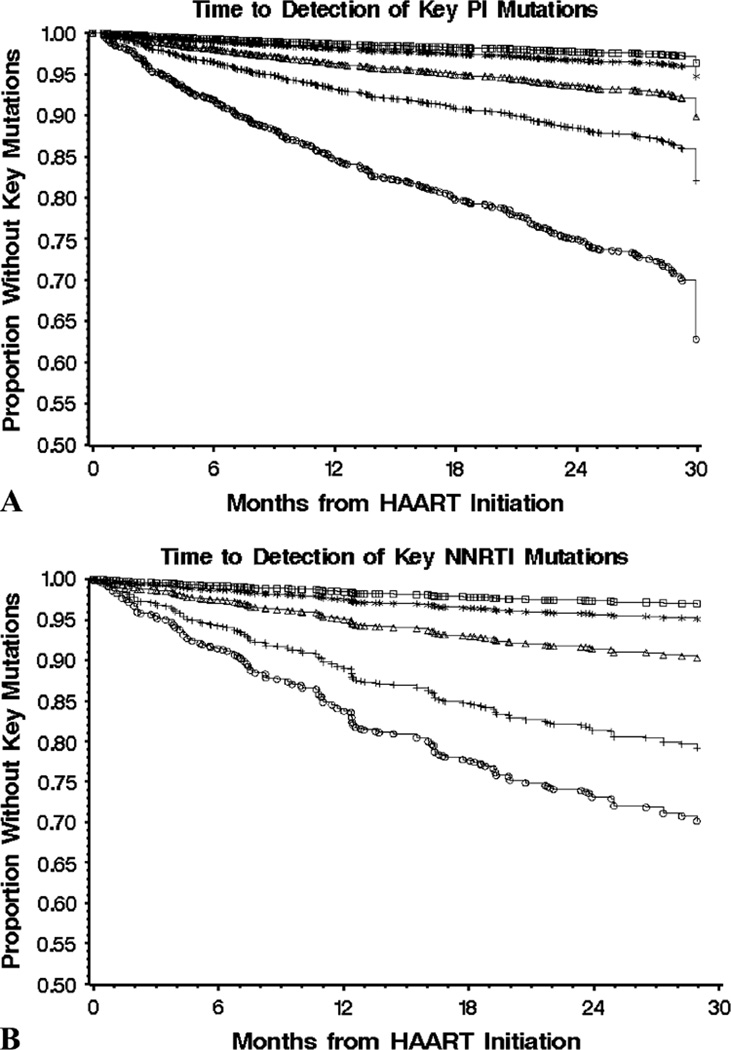
The rate of accumulating multiple primary mutations decreased approximately 50% per additional mutation (Fig. 3). For example, 218 patients had developed 1 resistance-associated mutation in HIV-1, 102 had developed 2, 57 had developed 3, 29 had developed 4, and 20 had developed 5 primary mutations after 30 months of initiating PI-based therapy (Fig. 3A). Similarly, 1, 2, 3, 4, or 5 primary mutations were selected in 80, 56, 26, 13, and 8 individuals, respectively, after 30 months on NNRTI-based triple therapy (Fig. 3B). The rate of accumulation of key resistance mutations was similar among individuals initiating HAART with PIs versus NNRTIs.
FIGURE 3.
The accumulation rate of multiple primary resistance mutations in antiretroviral-naive individuals initiating HAART with PIs (n = 885) (A) versus NNRTI (n = 306) (B). Results are reported as the time to accumulation of at least 1 (circles), 2 (plus signs), 3 (triangles), 4 (stars), and 5 (squares) primary mutations. A modified IAS-USA table defined 15 primary PR mutations and 31 primary RT mutations. IAS, International AIDS Society.
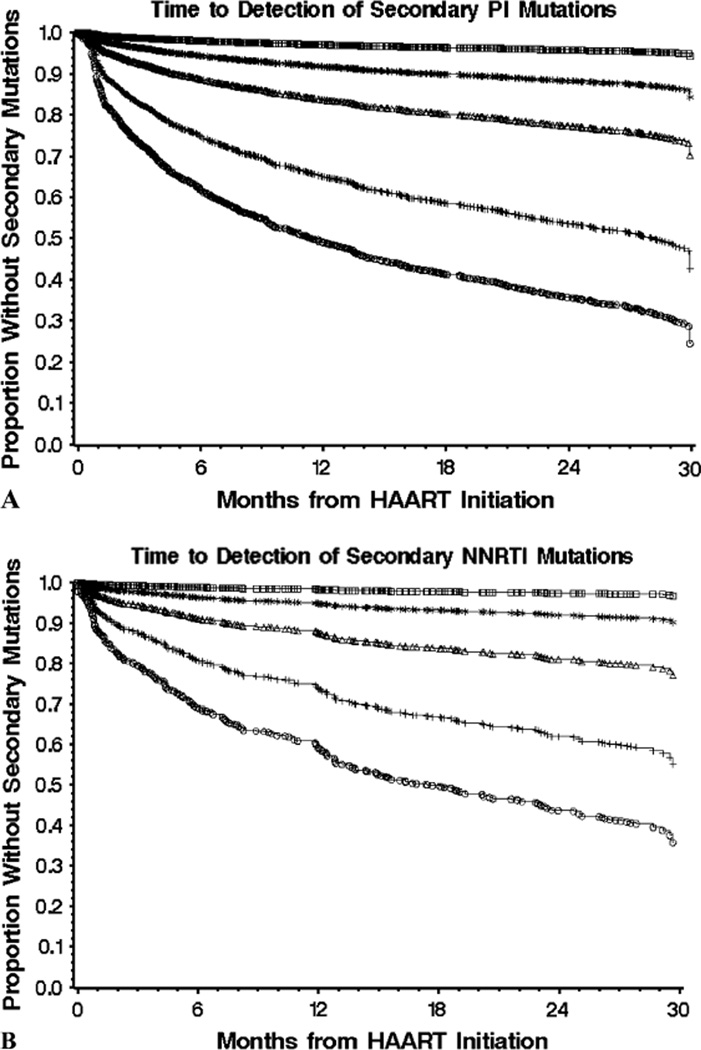
A total of 2, 3, 4, or ≥5 secondary mutations were observed in 406, 200, 103, and 37 individuals who initiated therapy on PIs (Fig. 4A) and in 117, 58, 25, and 8 individuals who started on NNRTIs (Fig. 4B), respectively, at 30 months after therapy initiation. Thus, similar to primary resistance mutations, the rate of accumulation of secondary mutations for individuals starting on either PIs or NNRTIs was also reduced by approximately 50% for each additional mutation.
FIGURE 4.
The accumulation rate of secondary resistance mutations in antiretroviral-naive individuals initiating HAART with PI (n = 885) (A) versus NNRTI (n = 306) (B). Results are reported as the time to accumulation of at least 1(circles), 2 (plus signs), 3 (triangles), 4 (stars), and 5 (squares) secondary mutations. A modified IAS-USA table defined 27 secondary PR mutations and 90 secondary RT mutations. IAS, International AIDS Society.
DISCUSSION
This population-based study of antiretroviral-naive individuals initiating HAART demonstrated the near linear relationship between time on therapy and the number of subjects with detectable resistance mutations. Previously, we describe the time to accumulation of resistance mutations for different drug classes.7 Here we show that the accumulation of drug resistance mutations for each of these drug classes is unique with respect to adherence. For each additional primary mutation, the accumulation rate of multiple drug resistance mutations decreased by approximately 50% in the population. These results were similar between PI- and NNRTI-based triple therapies. The accumulation rates of secondary resistance mutations, however, cannot be directly compared between PIs and NNRTIs because of the large difference in the number of established RT (90) versus PR (27) mutations.13 Nonetheless, primary and secondary mutations accumulated over time, and the rate of developing additional mutations was reduced by approximately 50% in each case.
The relationship between adherence and resistance in our study population was characterized according to drug class. In a multivariate model stratified by prescription refills, there was a strong association between adherence and the accumulation of resistance mutations for 3TC and NNRTIs only. As previously demonstrated,7 high but incomplete levels of adherence (80%–90% prescription refills) conferred the greatest risk of developing drug resistance mutations. These results are consistent with previous observations that state that suboptimal adherence contributes to the common development of resistance to 3TC and NNRTIs.18–22 These observations further emphasize the need for adequate adherence to prevent the loss of activity of these “fragile” drugs and the accumulation of additional mutations.
It should be noted that pharmacy refill adherence represents the maximum possible adherence level in a patient, and actual adherence is likely lower. Other modes of measuring adherence, such as self-reports and pill counts, may also overestimate adherence,23–25 whereas electronic medication monitoring may underestimate levels of adherence if multiple doses of prescription are removed from the bottle at the same time.25 A study by Grossberg et al23 showed that only pharmacy refill adherence was statistically significantly associated with viral load change when compared with self-reports. We assume that our results would not be greatly impacted by the addition of methods, which estimate adherence such as self-report, and restrict to pharmacy refill data because maintaining a comprehensive database of self-reports would be very difficult. Such data would be useful to clinicians and should be considered for future implementation. As there is no gold standard of adherence to date, combining measures of adherence likely provides more accurate and reliable results.25
In our study, combining prescription refill and plasma drug level data improved the model for adherence estimates. Patients may not adhere 100% to their prescription after refill, thus plasma drug levels would be further indication of a patient’s adherence level. Liechty et al26 have shown that a fairly linear relationship exists between plasma drug levels and adherence, providing support for use of this adherence measure at the cohort level. Our combined adherence results showed a strong association between adherence and the frequency of resistance mutations for 3TC and NNRTIs, with multivariate HRs of 7-fold and 4.5-fold for NNRTI and 3TC. In addition, we show that individuals with ≥95% prescription refills, but inconsistent plasma drug levels, were 4-fold and 3-fold more likely to have detectable 3 TC and NNRTI resistance mutations, respectively. This finding is consistent with a report suggesting that NNRTI may require relatively little drug pressure to select for resistant virus due to the relative small impact resistance mutations have on NNRTI replicative capacity in the presence of drug.10
For individuals with <95% adherence compared with the ≥95% group, there were no significant associations between adherence and resistance to PIs (HR 1.6) or other NRTIs (HR 1.4) (Fig. 2); these results are consistent with other studies.10, 27 However, contrasting studies show that higher levels of adherence lead to greater emergence of drug resistance, as more drug exposure under suboptimal levels of viral suppression select for drug-resistant virus.28, 29 Single-PI therapy may not be potent enough to fully suppress the virus at 100% adherence, thus stronger regimens would ideally shift the maximal selection for drug-resistant virus to a lower level of adherence.29 In our population study, it should be noted that all the patients were on unboosted PIs. Other reports have shown that resistance to unboosted PIs occurs at higher levels of adherence than NNRTI resistance, based on the observation that PI resistance mutations have a greater impact on replicative capacity relative to the degree of resistance conferred.10 As such, higher levels of drug exposure are needed to select for unboosted PI resistance. Our combined measure of pharmacy refill and drug level data was not able to confirm such a relationship. Our study was conducted on a drug-naive population, whereas others were conducted on treatment-experienced patients.10, 28, 29 This difference may account for the absence of a relationship between adherence and resistance for PIs as our study cohort did not have prior drug exposure.
There are several limitations to our study. Our study is retrospective and observational in nature. Samples with pVL <1000 copies per milliliter were not genotyped and were assumed to not harbor drug resistance mutations. Baseline drug resistance genotypes were not consistently available, and therefore all subjects were assumed to have wild-type virus at therapy initiation. This assumption was based on data from a representative subset of the HOMER cohort,7 but the presence of baseline drug resistance mutations in this population cannot be completely eliminated. Furthermore, plasma samples were only available at 3-month intervals. Because our analysis was restricted to genotypic drug resistance testing, which typically detects predominant circulating species, our study may lack information regarding minority HIV viral strains. Information from Figures 3 and 4 may represent an underestimation of the true degree of clinically relevant resistance as it does not reflect the cumulative resistance over time. Finally, no patients were on ritonavir-boosted PIs, so our data are not relevant for these regimens.
In this study, we found that multiple primary and secondary resistance mutations accumulated in individuals starting both PI-based and NNRTI-based HAART, where the accumulation rate decreased by approximately 50% for each additional mutation. In addition, our results showed that the relationship between resistance and nonadherence differed between drug classes. Most of the NNRTI and 3TC mutations occurred in the lower categories of adherence, suggesting that these medications have different adherence–resistance relationships than that of PIs and NRTIs. We believe that further definition of regimen-specific adherence–resistance relationships will be important for determining which regimens are best suited to individual patterns of adherence behavior.
REFERENCES
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Hogg RS, Yip B, Kully C, et al. Improved survival among HIV-infected patients after initiation of triple-drug antiretroviral regimens. CMAJ. 1999;160:659–665. [PMC free article] [PubMed] [Google Scholar]
- 3.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch MS, Brun-Vezinet F, D’Aquila RT, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: recommendations of an International AIDS Society-USA Panel. JAMA. 2000;283:2417–2426. doi: 10.1001/jama.283.18.2417. [DOI] [PubMed] [Google Scholar]
- 5.Wood E, Montaner JS, Yip B, et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ. 2003;169:656–661. [PMC free article] [PubMed] [Google Scholar]
- 6.Deeks SG. Determinants of virological response to antiretroviral therapy: implications for long-term strategies. Clin Infect Dis. 2000;30(Suppl 2):S177–S184. doi: 10.1086/313855. [DOI] [PubMed] [Google Scholar]
- 7.Harrigan PR, Hogg RS, Dong WW, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191:339–347. doi: 10.1086/427192. [DOI] [PubMed] [Google Scholar]
- 8.Braithwaite RS, Shecter S, Roberts MS, et al. Explaining variability in the relationship between antiretroviral adherence and HIV mutation accumulation. J Antimicrob Chemother. 2006;58:1036–1043. doi: 10.1093/jac/dkl386. [DOI] [PubMed] [Google Scholar]
- 9.Bangsberg DR, Moss AR, Deeks SG. Paradoxes of adherence and drug resistance to HIV-antiretroviral therapy. J Antimicrob Chemother. 2004;53:696–699. doi: 10.1093/jac/dkh162. [DOI] [PubMed] [Google Scholar]
- 10.Bangsberg DR, Acosta EP, Gupta R, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20:223–231. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- 11.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–2577. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 12.Wood E, Hogg RS, Yip B, et al. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350×10(9) cells/L. Ann Intern Med. 2003;139:810–816. doi: 10.7326/0003-4819-139-10-200311180-00008. [DOI] [PubMed] [Google Scholar]
- 13.Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: 2005. Top HIV Med. 2005;13:51–57. [PubMed] [Google Scholar]
- 14.Hogg RS, Heath K, Bangsberg D, et al. Intermittent use of triple combination therapy is predictive of mortality at baseline and after one year of follow-up. AIDS. 2002;16:1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 15.Low-Beer S, Yip B, O’Shaughnessy MV, et al. Adherence to triple therapy and viral load response. J Acquir Immune Defic Syndr. 2000;23:360–361. doi: 10.1097/00126334-200004010-00016. [DOI] [PubMed] [Google Scholar]
- 16.Alexander CS, Asselin JJ, Ting LS, et al. Antiretroviral concentrations in untimed plasma samples predict therapy outcome in a population with advanced disease. J Infect Dis. 2003;188:541–548. doi: 10.1086/376835. [DOI] [PubMed] [Google Scholar]
- 17.Cox DR. Regression models and life tables (with discussion) J R Stat Ser Soc B. 1972;34:187–202. [Google Scholar]
- 18.Descamps D, Flandre P, Calvez V, et al. Mechanisms of virologic failure in previously untreated HIV-infected patients from a trial of inductionmaintenance therapy. Trilege (Agence Nationale de Recherches sur le SIDA 072) Study Team. JAMA. 2000;283:205–211. doi: 10.1001/jama.283.2.205. [DOI] [PubMed] [Google Scholar]
- 19.Havlir DV, Hellmann NS, Petropoulos CJ, et al. Drug susceptibility in HIV infection after viral rebound in patients receiving indinavir-containing regimens. JAMA. 2000;283:229–234. doi: 10.1001/jama.283.2.229. [DOI] [PubMed] [Google Scholar]
- 20.Maguire M, Gartland M, Moore S, et al. Absence of zidovudine resistance in antiretroviral-naïve patients following zidovudine/lamivudine/protease inhibitor combination therapy: virological evaluation of the AVANTI 2 AVANTI 3 studies. AIDS. 2000;14:1195–1201. doi: 10.1097/00002030-200006160-00017. [DOI] [PubMed] [Google Scholar]
- 21.Gallego O, Ruiz L, Vallejo A, et al. Changes in the rate of genotypic resistance to antiretroviral drugs in Spain. AIDS. 2000;15:1894–1896. doi: 10.1097/00002030-200109280-00025. [DOI] [PubMed] [Google Scholar]
- 22.Walmsley S, Bernstein B, King M, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346:2039–2046. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 23.Grossberg R, Zhang Y, Gross R. A time-to-prescription-refill measure of antiretroviral adherence predicted changes in viral load in HIV. J Clin Epidemiol. 2004;57:1107–1110. doi: 10.1016/j.jclinepi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;134:968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 25.Bangsberg DR, Hecht FM, Charlebois ED, et al. Comparing objective measures of adherence to HIV antiretroviral therapy: electronic monitors and unannounced pill counts. AIDS Behav. 2001;5:275–281. [Google Scholar]
- 26.Liechty CA, Alexander CS, Harrigan PR, et al. Are untimed antiretroviral drug levels useful predictors of adherence behavior? AIDS. 2004;18:127–129. doi: 10.1097/00002030-200401020-00017. [DOI] [PubMed] [Google Scholar]
- 27.King MS, Brun SC, Kempf DJ. Relationship between adherence and the development of resistance in antiretroviral-naïve, HIV-1-infected patients receiving lopinavir/ritonavir or nelfinavir. J Infect Dis. 2005;191:2046–2052. doi: 10.1086/430387. [DOI] [PubMed] [Google Scholar]
- 28.Bangsberg DR, Charlebois ED, Grant RM, et al. High levels of adherence do not prevent accumulation of HIV drug resistance mutations. AIDS. 2003;17:1925–1932. doi: 10.1097/00002030-200309050-00011. [DOI] [PubMed] [Google Scholar]
- 29.Bangsberg DR, Porco TC, Kagay C, et al. Modeling the HIV protease inhibitor adherence-resistance curve by use of empirically derived estimates. J Infect Dis. 2004;190:162–165. doi: 10.1086/420790. [DOI] [PubMed] [Google Scholar]