Abstract
The complement system is a key component regulation influences susceptibility to age-related macular degeneration, meningitis, and kidney disease. Variation includes genomic rearrangements within the complement factor H-related (CFHR) locus. Elucidating the mechanism underlying these associations has been hindered by the lack of understanding of the biological role of CFHR proteins. Here we present unique structural data demonstrating that three of the CFHR proteins contain a shared dimerization motif and that this hitherto unrecognized structural property enables formation of both homodimers and heterodimers. Dimerization confers avidity for tissue-bound complement fragments and enables these proteins to efficiently compete with the physiological complement inhibitor, complement factor H (CFH), for ligand binding. Our data demonstrate that these CFHR proteins function as competitive antagonists of CFH to modulate complement activation in vivo and explain why variation in the CFHRs predisposes to disease.
Keywords: deregulation, structure, innate immunity
The complement system is a key component of innate immunity and host defense. Regulation of complement activation is of major importance to enable activation on pathogens while preventing activation on healthy host tissue. Complement factor H (CFH) is an abundant plasma protein, the major function of which is to down-regulate C3 activation through the alternative pathway and C3b amplification loops. Complete CFH deficiency is associated with severe secondary C3 deficiency because of uncontrolled consumption through these pathways (1). CFH mutations increase susceptibility to the renal diseases, atypical hemolytic uraemic syndrome and dense deposit disease (1), and polymorphic variation of CFH has been strongly associated with important human diseases, including age-related macular degeneration and meningococcal sepsis (2). It is now evident that variation in the complement factor H-related (CFHR) genes is also important in disease susceptibility and a role for the CFHR proteins in pathology has been unequivocally demonstrated by diseases associated with both mutations and polymorphisms in the CFHR genes (2)
The five CFHR proteins (CFHR1 to -5), together with CFH, comprise a family of structurally related proteins. CFH is a well-characterized negative regulator of complement C3 activation but the biological roles of the CFHR proteins are poorly understood. The frequent finding among healthy individuals of an allele lacking both CFHR3 and CFHR1 genes (∆CFHR3-1) (3) and, less commonly, an allele lacking both CFHR1 and CFHR4 (4), demonstrated that these proteins were biologically nonessential. However, genetic variation across the CFHR locus influences susceptibility to disease: the ∆CFHR3-1 deletion copy number variation polymorphism confers protection against IgA nephropathy (5) and age-related macular degeneration (6), and susceptibility to systemic lupus erythematosus (7). Two rare copy number variation within the CFHR locus are associated with familial C3 glomerulopathy. Among individuals with Cypriot ancestry, the disease segregated with an internal duplication affecting the CFHR5 gene (8), and in an Irish family the disease was associated with a heterozygous hybrid CFHR3-1 gene that was present on an allele that contained intact copies of both the CFHR3 and CFHR1 genes (9). Here we demonstrate that CFHR1, CFHR2, and CFHR5 contain a shared dimerization motif that resides within their common two amino-terminal domains. This motif enables the formation of three homodimers (CFHR1-CFHR1, CFHR2-CFHR2, and CFHR5-CFHR5) and three heterodimers (CFHR1-CFHR2, CFHR1-CFHR5, and CFHR2-CFHR5). In the presence of the ∆CFHR3-1 deletion polymorphism, the absence of CFHR1 reduces the potential combinations to two homodimers (CFHR2-CFHR2 and CFHR5-CFHR5) and a single heterodimer, CFHR2-CFHR5. We show that dimerization significantly enhances the avidity of these proteins for ligand in vivo and that this property enables these proteins to out-compete CFH at physiologically relevant concentrations. This dimerization-driven avidity enables these proteins to function as deregulators of complement by acting as competitive antagonists of CFH. Our data demonstrate that qualitative and quantitative variation within the CFHR family provides an unprecedented means by which complement activation is modulated in vivo.
Results
CFHR1, CFHR2, and CFHR5 Contain a Unique Dimerization Motif.
Comparing the amino acid conservation between CFHR1, CFHR2, and CFHR5 and CFH demonstrated that the CFHR proteins do not possess the residues implicated in the complement regulatory activity of CFH (Fig. 1A, cyan) but that these CFHRs shared a unique pair of highly conserved N-terminal domains (>85% sequence identity) (Fig. 1A). Duplication of these conserved domains has previously been linked to C3 glomerulonephritis (8, 9) but the mechanism underlying the association was not understood. We therefore determined the crystal structure of the first two short consensus repeat (SCR) domains of CFHR1 (CFHR112), which revealed that these domains assemble as a tight head-to-tail dimer with residues Tyr34, Ser36, and Tyr39 playing key roles in stabilizing the assembly (Fig. 1 B–D and Table S1). The recombinant CFHR112 fragment was also homogenously dimeric in solution (Fig. 2A) and the only conditions under which the chains could be separated was by SDS/PAGE (Fig. 2A). When we analyzed recombinant CFHR5 by multiangle laser light scattering (MALS) it too is seen to be dimeric, with no evidence of monomeric forms (Fig. 2A). The complete absence of monomeric CFHR1 and CFHR5 when the proteins are expressed recombinantly and the fact that partial unfolding of the proteins is required to separate the dimers both support the idea that the dimeric forms are the native state. The dimer interface is highly conserved among CFHR1, CFHR2, and CFHR5 (Fig. 1 C and D). CFHR1 and CFHR2 are identical in the interface and it is highly conserved between CFHR1 and CFHR5; the three major dimer contacts are identical, as are 11 of 15 of the minor contacts. Only one minor contact position has a nonconservative substitution that, inspection of the dimer structure suggests, will not disturb the interface. This high level of interface conservation implies that, in cells where more than one of these proteins are being synthesized simultaneously, CFHR1, CFHR2, and CFHR5 could assemble as hetero- as well as homodimers (Fig. S1). We therefore looked for the presence of hetero- and homodimers in vivo.
Fig. 1.
CFHR1, CFHR2, and CFHR5 contain an identical unique dimerization motif. (A) Alignment of the SCR domains of CFHR1, CFHR2, and CFHR5 with CFH. These proteins are comprised of subunits termed SCR domains and domains have been aligned according to the CFH domain with which they share the highest amino acid similarity, percentage identity indicated. Red boxing denotes domains for which unique X-ray structures are presented in this article. The complement regulatory domains of CFH reside within the first four amino-terminal domains (cyan). None of the CFHR proteins contain domains similar to these. CFH surface-recognition domains, which contain C3b/C3d and glycosaminoglycan binding sites reside within the carboxyl-terminal two domains (CFH19–20) and all three CFHR proteins contain highly similar domains. Mapping of the conserved residues onto the existing structure of CFH19–20 suggests that glycosaminoglycan but not C3b/C3d binding is altered or lost within CFHR23–4 (Figs. S1 and S2). The first two amino-terminal domains of CFHR1, CFHR2, and CFHR5 are highly conserved and have previously been described as CFH67-like, although the level of identity is less than 40%. (B) X-ray crystal structure of CFHR11–2. The two copies of CFHR112 that form the head-to-tail dimer are shown as gray cartoons with a semitransparent surface. Residues Tyr34, Ser36 and Tyr39 that are critical in stabilizing the dimer are shown in a ball-and-stick representation (Figure drawn using program PyMol, www.pymol.org). (C) Sequence alignment of CFHR11–2, CFHR21–2, and CFHR51–2 with CFH6–7. The dimerization interface is conserved between these CFHR proteins but not in CFH [interface residues determined using PISA; residues Tyr34, Ser36, and Tyr39 indicated by an asterisk (*); other interface residues by a dot (•)]. Red boxed residues, nonconservative; green boxed residues, conservative variation; and yellow boxed residues, residues unique to CFH67. (D) Mapping sequence variation onto the molecular surface of one copy of CFHR112. This analysis confirmed that the dimerization interface is conserved among CFHR112, CFHR212, and CFHR512 but not in CFH67 (positions of Tyr34, Ser36, and Tyr-39 indicated with an asterisk).
Fig. 2.
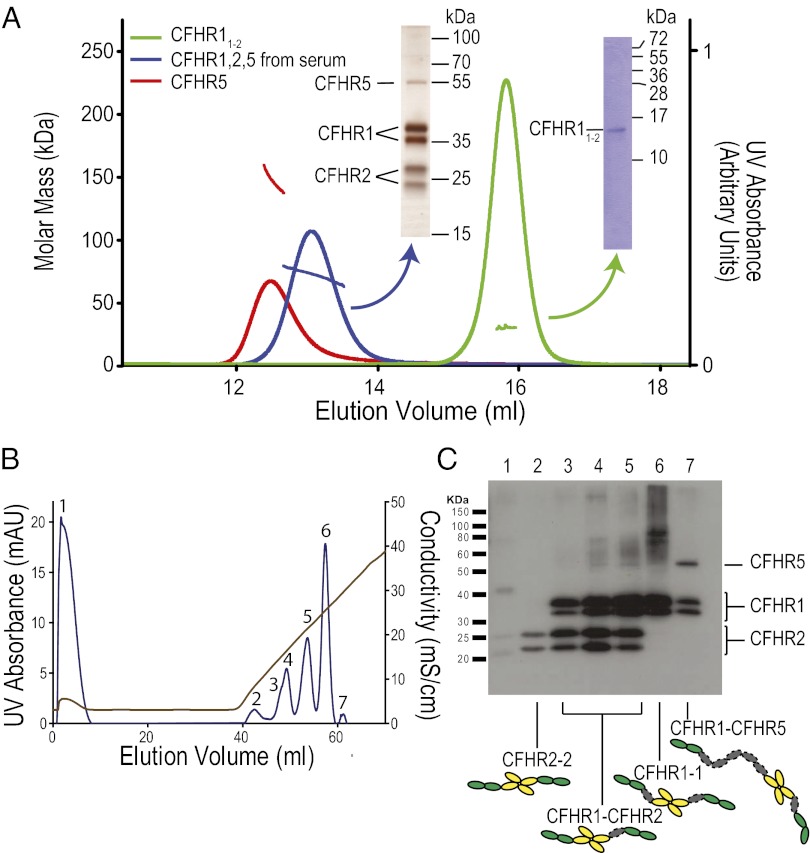
CFHR1, CFHR2, and CFHR5 are dimeric in serum. (A) MALS of a serum fraction containing CFHR1, CFHR2, and CFHR5, recombinant CFHR5 and recombinant CFHR11–2. MALS analysis of the serum fraction (green trace and mass profile) demonstrates that this mixture contains a mass range between 65 and 80 kDa. MALS using recombinant CFHR5 and CFHR11–2 (red and blue traces and mass profiles, respectively) demonstrates that both form dimers in solution. (B) Purification of homo- and heterodimers formed between CFHR1, CFHR2, and CFHR5. CFHR1, CFHR2, and CFHR5 were copurified from serum and the different species separated according to their affinity for heparin, eluting with a salt gradient. (C) Western blot analysis of each peak from the heparin elution in B using anti-CFHR1/2/5. Because our analyses above demonstrate that CFHR1/2/5 circulate in dimeric forms, we interpret peaks containing more than one CFHR coeluting as containing heterodimeric species.
CFHR1, CFHR2, and CFHR5 Exist as Dimeric Species in Vivo.
If, as our data predicted, these proteins exist entirely as dimeric species, the smallest mass would be 60 kDa, representing a CFHR2 homodimer. Next in size would be a CFHR1 homodimer (80 kDa), and CFHR1-CFHR2 heterodimer (70 kDa). The largest species would contain CFHR5 (CFHR2-CFHR5 = 95 kDa, CFHR1-CFHR5 = 105 kDa, CFHR5-CFHR5 = 130 kDa). In contrast, the mass range for monomeric forms would be 30–65 kDa (Mr for CFHR1, CFHR2, and CFHR5 is 40, 30, and 65 kDa, respectively).
We copurified CFHR1, CFHR2, and CFHR5 from serum using a monoclonal antibody (MBC125; anti-CFHR1/2/5) that recognizes a shared epitope within the first two SCR domains of these proteins. When this purified preparation was analyzed in solution by MALS (Fig. 2A), the observed mass range was 65–80 kDa. This range excluded monomeric forms of CFHR2 and CFHR1 (30 and 40 kDa, respectively). Because recombinant CFHR5 is entirely dimeric (Fig. 2A), we find it unlikely that the 65-kDa species are monomeric CFHR5. Therefore, the 65–80 kDa observed mass range supports our in vitro and structural data, suggesting that the native state of these proteins is dimeric. The upper mass range of 80 kDa was below that predicted for any of the CFHR5-containing dimers (all ≥95 kDa), suggesting (i) that the detected species in this assay were CFHR1 homodimers and CFHR1-CFHR2 heterodimers, and (ii) that CFHR5-containing species were either absent in vivo or below the assay detection limit.
To explore this theory further, we separated these proteins using heparin affinity chromatography and an anti-CFHR1/2/5–purified serum fraction (Fig. 2B and Fig. S2). We detected sequential fractions containing CFHR2 alone, both CFHR2 and CFHR1, CFHR1 alone, and both CFHR1 and CFHR5. In light of our earlier experiments, these fractions will all contain dimeric proteins. Therefore, the fractions that contain only a single species indicate the presence of homodimers. These fractions were homodimers of CFHR2 (Fig. 2C, lane 2, interacting very weakly with the column and eluting at low ionic strength – conductivity <10 mS) and CFHR1 (Fig. 2C, lane 6, eluting at higher ionic strength conductivity >20 mS). The presence of CFHR2 containing fractions coeluting from the column at intermediate ionic strengths (10–20 mS) with CFHR1 (Fig. 2C, lanes 3, 4, and 5) is consistent with the presence of CFHR1-CFHR2 heterodimers. It is not clear why these CFHR2-CFHR1 fractions elute as two peaks (Fig. 2C, note lanes 3 and 4 represent the same peak). The apparent differences in the relative intensity of the bands arising from the differently glycosylated forms of each protein suggests subtle differences between the heparin-binding capacities of dimers made up of different glycoforms. The presence of CFHR1-CFHR5 heterodimers is also suggested by their coelution in a fraction at ∼30 mS but it is not possible to rule out that this reflects elution of CFHR5 homodimers with some CFHR1 homodimer from the preceding peak. However, using sequential immunoprecipitation steps, we were able to demonstrate CFHR1-CFHR2 and CFHR1-CFHR5 heterodimers (Fig. S3 A and B). Taken together, these data support our structural and sequence-based hypotheses that the dimerization interface does not encode sufficient specificity to limit assembly to homodimers. It is notable that in these experiments we did not definitively detect either CFHR5 homodimers or the CFHR5-CFHR2 heterodimer. We hypothesized that the abundance of CFHR1 resulted in a dominance of CFHR1-containing species. Consistent with this hypothesis, when we performed an ELISA to detect CFHR5-CFHR2 heterodimer we were only able to detect a signal using sera partially or totally deficient in CFHR1 (serum from individuals heterozygous and homozygous for the ∆CFHR3-1 deletion polymorphism respectively) (Fig. S3C). This finding demonstrated that the relative abundance of CFHR1, CFHR2, and CFHR5 influences the composition of the dimeric species in vivo.
Dimerization Enhances the Interaction of CFHR5 with Renal-Bound Mouse Complement C3 in Vivo.
We next explored the functional consequences of dimerization. We predicted that dimerization would enhance ligand interaction through avidity. To test this theory we generated monomeric and dimeric CFHR5 proteins. Monomeric CFHR5 (CFHR5dimer mutant) was generated in vitro by mutating the three key amino acids within the dimerization motif to the corresponding amino acids within CFH (Tyr34Ser, Ser36Tyr, Tyr39Glu) (Fig. 3 A and B). CFHR5dimer mutant was demonstrated to be monomeric using MALS (Fig. 3C). Next we examined the interaction of monomeric and dimeric CFHR5 with tissue-bound complement in a mouse model. Gene-targeted CFH-deficient mice have florid deposition of activated mouse C3 along the glomerular basement membrane (GBM) within the kidney (10). Human CFHR5 was able to interact with the GBM-bound C3 in a specific and dose-dependent manner (Fig. S4). Using this model the interaction of intravenously administered monomeric CFHR5 with GBM-bound mouse C3 was significantly reduced compared with that of the dimeric protein (median glomerular staining = 227 and 95 arbitrary fluorescence units, for wild-type and dimer mutant, respectively, P < 0.05, unpaired t test) (Fig. 3D). This finding indicated that dimerization of CFHR5 enhanced its ability to interact with mouse C3 in vivo.
Fig. 3.
Dimerization enhances the interaction of CFHR5 with complement C3 in vivo. (A) Generation of a CFHR5 protein lacking critical amino acids within the dimerization motif. Monomeric CFHR5 (CFHR5dimer mutant) was generated by mutating the three stabilizing amino acids (Tyr34Ser, Ser36Tyr, Tyr39Glu) within the dimerization motif to the corresponding amino acids within CFH. (B) Analysis of recombinant CFHR5 and CFHR5dimer mutant using SDS/PAGE. Both the wild-type and dimer mutants were purified to single homogenous species as visualized by denaturing electrophoresis. (C) Analysis of recombinant CFHR5 and CFHR5dimer mutant using size-exclusion chromatography. Size-exclusion chromatography was performed on a Superdex200 10/30 column (GE Healthcare) equilibrated in 50 mM Tris•HCl, pH 7.5, 150 mM NaCl at 0.4 mL/min. The column was followed in-line by an Optilab-Rex refractive index monitor (Wyatt Technologies). The CFHR5 dimer elutes early from the column (blue trace) but the monomeric CFHR5dimer mutant protein elutes at a larger column volume (red trace). (D) Interaction of CFHR5 and CFHR5dimer mutant with renal-bound mouse C3 in vivo. When recombinant CFHR5dimer mutant was injected at identical concentration to that of CFHR5, CFHR5dimer mutant binding to glomerular C3 was significantly reduced compared with that of wild-type CFHR5. Original magnification, ×40.
Dimerization Enhances the Ability of CFHR1 and CFHR5 to Compete with CFH for C3b Binding in Vitro.
We next speculated that dimerization of CFHR1, CFHR2, and CFHR5 would enable these proteins to efficiently compete with CFH for interaction with C3 in vivo. Because CFH, CFHR1, and CFHR5 contain the same carboxyl-terminal C3b/C3d binding site (Fig. 1A and Fig. S5), we developed an ELISA to determine if CFHR1 and CFHR5 influence the interaction of CFH with C3b. This process demonstrated that the CFH–C3b interaction was inhibited in a dose-dependent manner at physiologically relevant concentrations by native dimers of CFHR5 (dose range 0.005–0.6 μM) and CFHR1 (dose range 0.014–1.8 μM) (Fig. 4A). Monomeric constructs of CFHR1 and CFHR2 that lack the dimerization domains (denoted CFHR1345 and CFHR234, respectively) could also inhibit CFH binding but at higher concentrations (Fig. 4A).
Fig. 4.
CFHR1 and CHFR5 deregulate complement activation by competitively inhibiting of the interaxtion of CFH with C3b. (A) CFH binding to C3b is inhibited by either recombinant CFHR5 or serum-derived CFHR1. ELISA wells were coated with C3b and 0.07 μM CFH was incubated with increasing amounts of either CFHR1 (0.014–1.8 μM) or CFHR5 (0.005–0.6 μM). Both proteins reduced the CFH–C3b interaction in a dose-dependent manner. Similar results were obtained when recombinant CFHR1345 (0.14–18 μM) and CFHR234 (0.13–16 μM) were used. (B) CFH-dependent alternative pathway hemolytic assay. Using a CFH dose that reduced lysis of guinea pig erythrocytes to 50%, the addition of increasing concentrations of CFHR135, CFHR234, serum-derived CFHR1, and recombinant CFHR5 resulted in a dose-dependent increase in lysis. Full-length, dimeric, CFHR1, and CFHR5 were orders-of-magnitude more potent with respect to the monomeric CFHR1 and CFH2 fragments lacking the dimerization motif. (C) Enhanced de-regulation by plasma-derived preparations containing CFHR1, CFHR2, and CFHR5 from individuals with familial C3 glomerulopathy. Using the hemolytic assay described in B, serum-derived preparations from patients with either a CFHR5 mutation (CFHR51212–9) or a CFHR3-1 hybrid protein associated with C3 glomerulopathy showed significantly greater hemolysis than controls.
CFHR1 and CFHR5 Deregulate Complement Activation by Acting as Competitive Antagonists of CFH.
To determine the physiological relevance of the competition between CFHR1/CFHR5 and CFH for C3b binding we, like others, have studied the ability of CFHR1 and CFHR5 to regulate C3. Using surface plasmon resonance, in which the sensor surface was coated with either amine- or thioester-coupled C3b (monomeric or “clustered” C3b, respectively) (Fig. S6), or thioester-coupled iC3b and C3dg (Fig. S7), CFHR5 bound to C3b, iC3b, and C3dg, but there was no evidence of fluid-phase factor I cofactor activity (Fig. S8). CFHR1 has previously been reported to inhibit the C5 not C3 convertase by binding to C5/C5b6 (11) but we were unable to detect any significant interaction with C5 (Fig. S9). Moreover, we were unable to detect any evidence of complement regulatory activity when CFHR1 was investigated in alternative pathway hemolysis assays (Fig. S10).
These data indicated that CFHR1 and CFHR5 have no intrinsic C3 or C5 regulatory activity at physiological concentrations. We therefore hypothesized that these proteins, through their ability to compete with CFH for binding to C3b, actually prevent CFH-mediated complement regulation. To test this theory we used a complement-dependent hemolytic assay comprising unopsonized guinea pig erythrocytes (a complement activating surface) incubated with 20% (vol/vol) normal human sera. The addition of 100 nM CFH resulted in 50% inhibition of cell lysis and therefore enabled us to determine if exogenous CFHR proteins increased or decreased hemolysis. Using these conditions, in which the total CFH concentration in the assay was ∼0.5 μM (100 nM added to assay in addition to 20% normal human sera), we added increasing concentrations of concentrations of CFHR1345, CFHR234, serum-derived CFHR1, and recombinant CFHR5 (Fig. 4B). Strikingly, these preparations increased rather than decreased hemolysis in a dose-dependent fashion. Importantly, the IC50 was significantly lower for the dimeric CFHR1 (0.7 μM) and CFHR5 (0.15 μM) compared with the monomeric CFHR1 (3.6 μM) and CFHR2 (4.7 μM) fragments. These data demonstrated that CFHR1 and CFHR5 can interfere with the C3b inhibitory actions of CFH by acting as competitive antagonists and that this interference is enhanced by dimerization. We refer to this process as complement deregulation because it emphasizes the point that these proteins have no ability to influence complement regulation in the absence of CFH.
Deregulation by CFHR5 Mutation Associated with Familial C3 Glomerulopathy.
In patients with familial complement-mediated kidney disease, termed C3 glomerulopathy, there is a heterozygous CFHR5 mutation in which the initial two N-terminal domains are duplicated (8). Our data reveal that this results in duplication of the dimerization motif (denoted CFHR51212–9). When we generated recombinant CFHR51212–9 it was clear that the purified preparation readily “aggregated” and was associated with atypical C3 binding kinetics using surface plasmon resonance (Fig. S11). When we elucidated the dimerization domain we reinterpreted this aggregation as a direct consequence of duplicated dimerization domains (enabling multimeric interaction) rather than an in vitro artifact. A further consequence of our structural data was that examination of the isolated recombinant CFHR51212–9 was irrelevant pathophysiologically because it was likely that CFHR51212–9 interacted with CFHR1, CFHR2, and the wild-type CFHR5 (derived from the unaffected allele) in vivo. Consequently, we tested whether deregulation is influenced in these patients by comparing plasma preparations containing all CFHR1, CFHR2, and CFHR5 species from affected individuals and healthy controls without the CFHR3-1 deletion polymorphism (Fig. 4C). These preparations resulted in significantly greater hemolysis compared with controls. The same enhanced hemolysis was demonstrable using preparations containing the hybrid CFHR3-1 protein (9).
Discussion
Our data provide evidence that CFHR1, CFHR2, and CFHR5 at physiologically relevant concentrations interfere with the complement inhibitory activities of CFH. This process, which we term deregulation, is influenced by the ability of these proteins to form dimers (Fig. 5). This structural property confers avidity enabling these dimeric molecules to compete with CFH for ligand because of the fact that the C-terminal C3b/C3d recognition sites are essentially conserved between the CFHR proteins and CFH. The shared dimerization domain between CFHR1, CFHR2, and CFHR5 enabled the formation of both homo- and heterodimers. The dimerization motif we have characterized is not present within CFHR3 and CFHR4 but gel electrophoresis reported in earlier work suggests that CFHR4, at least, may also exist as a dimer (12). Whether CFHR3 and CFHR4 form dimers and behave as competitive antagonists of CFH is the subject of ongoing study.
Fig. 5.
Modulation of complement in vivo by CFHR1, CFHR2, and CFHR5. These proteins compete with CFH for interaction with C3b (18–20). Unlike CFH, these proteins are devoid of intrinsic complement regulatory activity under physiological conditions. However, their interaction with C3b prevents the binding of C3b to CFH and thereby prevents inactivation of C3b by CFH. This process we term deregulation. Whether or not C3b interacts with CFH or components of the CFHR family will be influenced by factors such as C3b density, surface polyanions, and the local concentrations of CFH and CFHR proteins (see text). In this way, CFHR proteins provide a sophisticated means through which complement activation can be modulated in vivo. (Inset) A general schematic for the functionally important portions of CFHR1, CFHR2, and CFHR5.
Structural and sequence analyses suggested that in addition to formation of homodimers, CFHR1, CFHR2, and CFHR5 were likely to form heterodimers. We speculate that dimerization is likely to occur intracellularly during synthesis of the proteins and the species seen in serum are therefore likely to reflect the different levels at which the proteins are made. Our data support the idea that each of the pairs CFHR1/CFHR2, CFHR2/CFHR5, and CFHR1/CFHR5 are simultaneously transcribed within certain cells, as we could observe these heterodimers in serum samples, although the major sites of physiological synthesis remain unknown. A priori we therefore predicted that homo- and heterodimers containing CFHR1 would predominate in sera from individuals without the ∆CFHR3-1 deletion polymorphism, because this protein is most abundant with a mean serum concentration equimolar to that of CFH [CFH = 116–562 μg/mL, 0.7–3.6 μM, mean 2.1 μM (13); CFHR1 = 70–100 μg/mL, 1.7–2.5 μM, mean 2.1 μM (11)]. In contrast, the median concentration of CFHR5 [3–6 μg/mL, 0.05–0.09 μM, mean 0.07μM (14)] is much lower. We are not aware of published estimates for the circulating concentration of CFHR2 but our data suggest its concentration is intermediate between CFHR1 and CFHR5 (Fig. 2A, Coomassie gel Inset). Consistent with the predominance of CFHR1-containing dimers, CFHR2-CFHR5 heterodimers were only readily detectable in sera from patients deficient in CFHR1 (those with the ∆CFHR3-1 deletion polymorphism).
We were unable to demonstrate C3 regulatory activity for CFHR5 and were unable to demonstrate an interaction between CFHR1 and C5. Interestingly, although CFHR3 has previously been reported as a regulator of complement (in nonphysiological conditions), other experiments reported in the same article demonstrate that, as shown here for CFHR1, CFHR2, and CFHR5, CFHR3 can also deregulate CFH (15). Recently, CFHR4 was shown to be devoid of intrinsic complement activity but able to act as a platform on which complement activation could proceed unhindered (16). Therefore, if CFHR4 was able to compete for CFH ligands, then it too has the potential to deregulate CFH activity.
Taken together, our data suggest that the CFHR1, CFHR2, and CFHR5 modulate complement activation by competing with CFH for C3b binding. In contrast to CFH-C3b interaction, which prevents further C3b generation (negative regulation), the interaction of these CFHR proteins with C3b enables C3b amplification to proceed unhindered. The ability of CFHR proteins to deregulate CFH would be predicted to be influenced by many factors, including: (i) the concentration and composition of the CFHR proteins relative to CFH in the vicinity of complement activation; (ii) the spatial density of deposited C3 (for example, we speculate that the action of large dimers such as CFHR5-CFHR5 may be important when spatial density is low); (iii) the polyanion composition of the surface upon, which complement is activated because the polyanion affinities of the different CFHR proteins may vary; and (iv) the flow rate across the site of complement activation in surfaces in contact with blood (the enhanced avidity of dimeric species would favor their interaction with ligand relative to CFH under high flow), such as within the kidney.
Our data had obvious implications for how we now consider the impact of the C3 glomerulopathy-associated CFHR5 mutation in which there is duplication of the dimerization domain (duplication of SCR1 and SCR2, CFHR51212–9) (8). Theoretically, this duplication would result in trimeric or higher-order complexes. However, because CFHR1 is abundant in vivo, we speculate that the most common species would be trimeric and composed of two molecules of CFHR1 complexed with CFHR51212–9. When we purified CFHR1, CFHR2, CFHR5, and CFHR51212–9 from an affected individual, this serum fraction was more potent in deregulation than serum fractions from healthy controls. We observed the same phenomenon using a serum preparation derived from a C3 glomerulopathy patient carrying a hybrid CFHR3-1 protein in addition to the normal CFHR3 and CFHR1 proteins (9). If it is assumed that CFH plays a physiological role in protecting the GBM from C3 activation, our data would suggest that C3 glomerulopathy develops in these individuals because the presence of CFHR51212–9 or the CFHR3-1 hybrid protein results in a greater degree of CFHR-mediated deregulation.
CFH serum levels are not actively regulated in an individual, varying only under extreme conditions, such as meningococcal sepsis, where tight interactions with the bacterium deplete CFH. We speculate that fine-tuning of complement activation (complement modulation) could be achieved by altering CFHR levels. It is notable that in otitis media with effusion, where complement is strongly activated in the middle ear effusion fluid, CFHR5 levels were noted to be high (17) and it was proposed that competition between CFHR5 and CFH might be relevant in this circumstance. This theory requires further study but our data would predict that a local increase in CFHR protein concentration would, through enhanced CFH deregulation, enable rapid enhancement of complement activation. The opposite might be achieved by down-regulating CFHR concentrations thereby reducing deregulation.
In summary, our observations have revealed an exciting and unique function of the CFHR proteins. We propose that these molecules have evolved to enable complement to be modulated at a sophisticated level under diverse circumstances. Understanding how these proteins modulate activation during infection, tissue injury, and inflammation will enable us not only to gain further understanding of the role of complement in disease but also to devise novel strategies to increase or decrease complement activation therapeutically.
Materials and Methods
Protein expression and purification, crystallization and X-ray data collection, structure solution and refinement were performed using standard protocols and are described in SI Materials and Methods. All functional assays, detection of heterodimers by immunoprecipitation and ELISAs, in vivo studies, MALS, and surface plasmon resonance are discussed in SI Materials and Methods. See Table S2 for primers used in this study.
Supplementary Material
Acknowledgments
We thank Dr. J. McRae for the rabbit anti-human complement factor H-related 5 antibody. This work was supported in part by a Medical Research Council United Kingdom studentship (to J.J.E.C.); Medical Research Council Grant G0701298 (to C.L.H.); and Wellcome Trust Senior Fellowship in Clinical Science WT098476MA (to M.C.P.). E.G.d.J. is an Imperial College Junior Research Fellow, and S.J. is supported by Medical Research Council Programme Grant RWIX0 (to S.M.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3ZD2 and 3ZD1).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219260110/-/DCSupplemental.
References
- 1.Pickering MC, Cook HT. Translational mini-review series on complement factor H: renal diseases associated with complement factor H: Novel insights from humans and animals. Clin Exp Immunol. 2008;151(2):210–230. doi: 10.1111/j.1365-2249.2007.03574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Cordoba SR, Tortajada A, Harris CL, Morgan BP. Complement dysregulation and disease: From genes and proteins to diagnostics and drugs. Immunobiology. 2012;217(11):1034–1046. doi: 10.1016/j.imbio.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Hageman GS, et al. AMD Clinical Study Group Extended haplotypes in the complement factor H (CFH) and CFH-related (CFHR) family of genes protect against age-related macular degeneration: characterization, ethnic distribution and evolutionary implications. Ann Med. 2006;38(8):592–604. [PMC free article] [PubMed] [Google Scholar]
- 4.Abarrategui-Garrido C, Martínez-Barricarte R, López-Trascasa M, de Córdoba SR, Sánchez-Corral P. Characterization of complement factor H-related (CFHR) proteins in plasma reveals novel genetic variations of CFHR1 associated with atypical hemolytic uremic syndrome. Blood. 2009;114(19):4261–4271. doi: 10.1182/blood-2009-05-223834. [DOI] [PubMed] [Google Scholar]
- 5.Gharavi AG, et al. Genome-wide association study identifies susceptibility loci for IgA nephropathy. Nat Genet. 2011;43(4):321–327. doi: 10.1038/ng.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes AE, et al. A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat Genet. 2006;38(10):1173–1177. doi: 10.1038/ng1890. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, et al. BIOLUPUS Network GENLES Network Association of genetic variants in complement factor H and factor H-related genes with systemic lupus erythematosus susceptibility. PLoS Genet. 2011;7(5):e1002079. doi: 10.1371/journal.pgen.1002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gale DP, et al. Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet. 2010;376(9743):794–801. doi: 10.1016/S0140-6736(10)60670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malik TH, et al. A hybrid CFHR3-1 gene causes familial C3 glomerulopathy. J Am Soc Nephrol. 2012;23(7):1155–1160. doi: 10.1681/ASN.2012020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickering MC, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31(4):424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 11.Heinen S, et al. Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood. 2009;114(12):2439–2447. doi: 10.1182/blood-2009-02-205641. [DOI] [PubMed] [Google Scholar]
- 12.Hellwage J, et al. Functional properties of complement factor H-related proteins FHR-3 and FHR-4: Binding to the C3d region of C3b and differential regulation by heparin. FEBS Lett. 1999;462(3):345–352. doi: 10.1016/s0014-5793(99)01554-9. [DOI] [PubMed] [Google Scholar]
- 13.Esparza-Gordillo J, et al. Genetic and environmental factors influencing the human factor H plasma levels. Immunogenetics. 2004;56(2):77–82. doi: 10.1007/s00251-004-0660-7. [DOI] [PubMed] [Google Scholar]
- 14.McRae JL, et al. Human factor H-related protein 5 has cofactor activity, inhibits C3 convertase activity, binds heparin and C-reactive protein, and associates with lipoprotein. J Immunol. 2005;174(10):6250–6256. doi: 10.4049/jimmunol.174.10.6250. [DOI] [PubMed] [Google Scholar]
- 15.Fritsche LG, et al. An imbalance of human complement regulatory proteins CFHR1, CFHR3 and factor H influences risk for age-related macular degeneration (AMD) Hum Mol Genet. 2010;19(23):4694–4704. doi: 10.1093/hmg/ddq399. [DOI] [PubMed] [Google Scholar]
- 16.Hebecker M, Józsi M. Factor H-related protein 4 activates complement by serving as a platform for the assembly of alternative pathway C3 convertase via its interaction with C3b protein. J Biol Chem. 2012;287(23):19528–19536. doi: 10.1074/jbc.M112.364471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Närkiö-Mäkelä M, Hellwage J, Tahkokallio O, Meri S. Complement-regulator factor H and related proteins in otitis media with effusion. Clin Immunol. 2001;100(1):118–126. doi: 10.1006/clim.2001.5043. [DOI] [PubMed] [Google Scholar]
- 18.Wu J, et al. Structure of complement fragment C3b-factor H and implications for host protection by complement regulators. Nat Immunol. 2009;10(7):728–733. doi: 10.1038/ni.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan HP, et al. Structural basis for engagement by complement factor H of C3b on a self surface. Nat Struct Mol Biol. 2011;18(4):463–470. doi: 10.1038/nsmb.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kajander T, et al. Dual interaction of factor H with C3d and glycosaminoglycans in host-nonhost discrimination by complement. Proc Natl Acad Sci USA. 2011;108(7):2897–2902. doi: 10.1073/pnas.1017087108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.