Parsing a brain region’s disparate roles

Hippocampus and rodent path integration.
The hippocampus and related structures in the brain’s medial temporal lobe (MTL) are thought to mediate both long-term memory and spatial cognition, but the link between these seemingly disparate functions remains unclear. Soyun Kim et al. (pp. 4732–4737) explored the effects of MTL damage on path integration, the ability to return to a starting point after navigating an environment. Five patients with MTL damage and 11 healthy participants were blindfolded, given noise-cancelling earphones, and asked to find a square tile on the floor of a small circular arena before returning to the start site. A similar experiment was performed in rats with and without hippocampal lesions. Patients with MTL damage found their way back to the start site as efficiently as did healthy participants when the path was relatively straight and navigated quickly, the authors found. By contrast, rats with hippocampal lesions could not accurately return to the start site, no matter how short or direct the path. According to the authors, the findings suggest that rats may have difficulty constructing a coherent working memory of their spatial environments; alternatively, spatial working memory may be preserved after MTL damage in humans but not in rats. — A.G.
Immature human brain organizes early to decipher speech
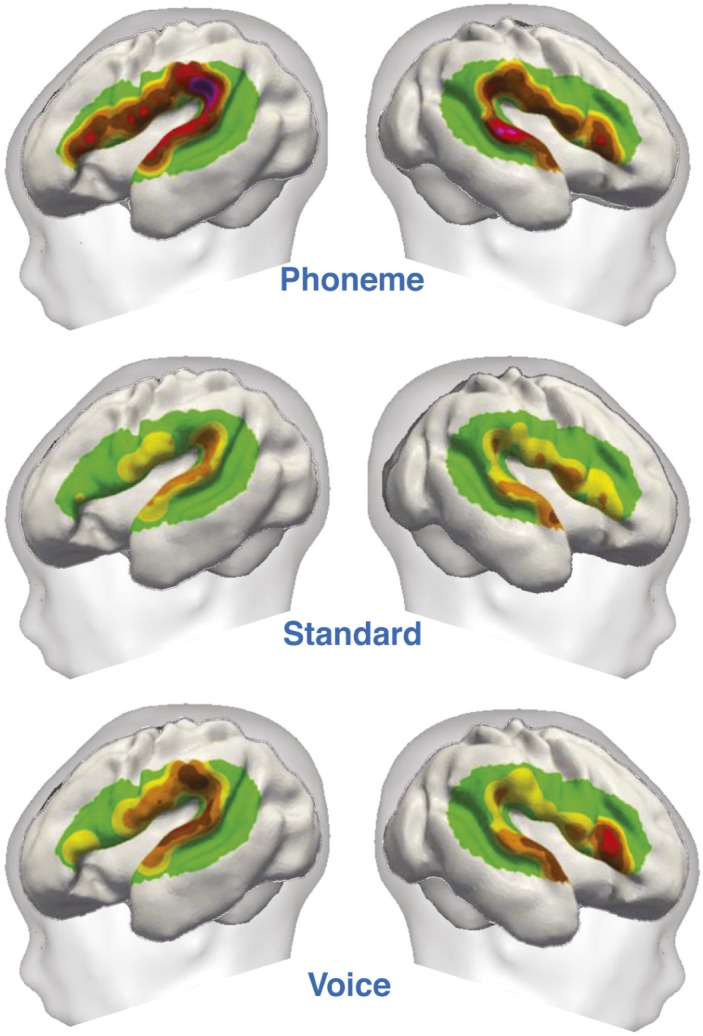
Functional organization of the perisylvian areas in preterm infants.
At birth, children can discriminate syllables and recognize human language. Although certain auditory capacities form prior to term, whether and how these immature cortical circuits process speech remains unclear. Using bedside functional optical imaging, Mahdi Mahmoudzadeh et al. (pp. 4846–4851) examined linguistic and nonlinguistic discrimination in 12 sleeping 28–32 week gestational-age preterm infants, the earliest developmental stage at which cortical responses to external stimuli can be recorded, to evaluate human cerebral responses to syllables at a time when many neurons in the brain are still migrating to their final location. The authors found similarities between the preterm and adult linguistic networks, including differentiable neural responses to changes between the “ba” to “ga” phoneme and from a male to female voice. In addition, although both tests elicited responses in the right frontal region, changes of phoneme elicited a response in the left frontal region, suggesting that certain linguistic areas of the brain have already organized to a sophisticated degree as early as 3 months prior to full term. The findings demonstrate that the immature human brain organizes early in development to establish neural functions that help recognize and decipher certain aspects of speech, according to the authors. — T.J.
Antibacterial peptides and drug design
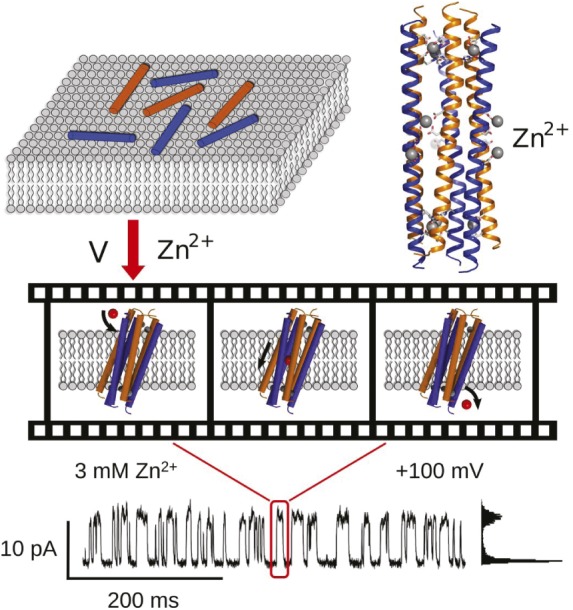
The human host-defense peptide dermcidin (DCD) can form helices and bind to the surface of membranes (Upper Left). In the presence of Zn2+ ions, it assembles into a hexameric structure, which was crystallized (Upper Right) and found to form tilted ion channels in membranes (Middle). Driven by transmembrane potential, ions traverse the pore by permeating into and out of channel eyelets at its side. The distinct channel activity of DCD was recorded by electrophysiology (Bottom panel).
Antimicrobial peptides (AMPs) are tools of host defense that kill bacteria by disrupting their cell membranes. The peptides’ broad spectrum of activity, coupled with the low risk of inciting resistance, make AMPs attractive candidates for rational drug design, but mammalian AMPs are insufficiently understood on a molecular level to serve as a template for such an endeavor. Chen Song et al. (pp. 4586–4591) dissected the structure and mechanism of action of the human AMP dermcidin (DCD). X-ray crystallography of DCD revealed a tight barrel consisting of three antiparallel peptide dimers enclosing a hydrophilic channel, with several lateral openings or eyelets. When DCD was added to a membrane bilayer in the presence of a membrane potential and Zn2+ ions, the peptide exhibited channel activity. Molecular dynamics simulations, which were highly consistent with observations from electrophysiology, showed DCD crossing the membrane at a lipid-dependent angle and forming a highly permeable water and ion channel with a conductance of 80–110 pS, sufficient to dissipate the transmembrane potential of a bacterium on a millisecond time scale. The tilting of DCD allows ions to enter the channel through the eyelets, speeding up the process. According to the authors, the findings may provide a foundation for the rational design of AMP-based drugs. — C.B.
EEG signatures to detect loss and recovery of consciousness
General anesthesia is required for most surgical and many nonsurgical procedures, but anesthesiologists do not yet have a reliable way to be certain that a patient given general anesthesia is unconscious. To identify electroencephalogram (EEG) signatures associated with loss and recovery of consciousness, Patrick Purdon et al. (pp. E1142–E1151) recorded high-density EEG in 10 healthy volunteers while administering increasing and decreasing levels of propofol. The volunteers’ level of consciousness was assessed based on their ability to respond to words and click sounds every 4 seconds. The authors identified highly structured EEG signatures associated with different states of unconsciousness and sedation induced by propofol. During profound unconsciousness, the amplitudes of alpha and beta EEG waves were largest at the peaks of low-frequency oscillations, whereas during loss and recovery of consciousness these amplitudes were largest at the troughs of low-frequency oscillations. The transition between these two states could be used to predict when individuals lose and recover consciousness, the authors suggest. The findings provide insights into the mechanisms of propofol-induced unconsciousness and may present a way to precisely monitor the brain states of patients receiving general anesthesia, according to the authors. — S.R.
Molecular signatures of sleep deficiency
Sleep deficiency can lead to a host of health conditions including obesity, heart disease, and cognitive impairment, but the molecular mechanisms linking sleep to health remain largely elusive. Carla Möller-Levet et al. (pp. E1132–E1141) explored how inadequate sleep alters gene expression in human blood. The authors studied 26 volunteers who were allowed to sleep less than 6 hours, or as long as 10 hours, every night for 1 week. After each week, the volunteers stayed awake for about 40 hours and provided blood samples during this time. Analysis of RNA from the samples revealed the effects of insufficient sleep on the expression of 711 genes and numerous biological processes including metabolism, inflammation, immunity, and various cell stress responses. Furthermore, inadequate sleep reduced the number of genes that normally peak and wane in expression throughout the day from 1,855 to 1,481, and reduced the amplitude of expression for the remaining genes. The authors found that the number of genes affected by total sleep deprivation was seven times higher after a week of insufficient, compared with sufficient, sleep. According to the authors, the findings reveal the effects of sleep deficiency on gene expression in human blood and how sleep could influence human health. — A.G.


