Abstract
The hippocampus and other medial temporal lobe structures have been linked to both memory and spatial cognition, but it has been unclear how these ideas are connected. We carried out parallel studies of path integration in patients with medial temporal lobe lesions and rats with hippocampal lesions. Subjects entered a circular arena without vision, searched for a target, and then attempted to return to the start location. Patients performed accurately, and as well as controls, so long as the outward path was relatively direct and the target was found within 20 s. In sharp contrast, rats with hippocampal lesions were impaired, even when the outward path was shorter than 1 m, involved no turns, and the target was found within 3 s. We suggest that patients succeeded because performance could be supported by working memory and that patients and rats differ after hippocampal lesions in their ability to construct a coherent working memory of spatial environments.
Keywords: amnesia, navigation
Two ideas have been central to recent discussions about the function of the hippocampus and other medial temporal lobe (MTL) structures. One idea emphasizes the role of these structures in memory (1–3) and the other emphasizes their role in spatial cognition, including spatial navigation and path integration (4–6). Path integration refers to the ability to use self-motion cues as one moves through space to keep track of a reference location (7, 8). These two ideas are compatible with each other to a large extent, because path integration requires memory, but there is potential mismatch as well, and it has been unclear how the two ideas relate to each other.
Discussion of the MTL and memory typically draws a fundamental distinction between working memory and long-term memory. Working memory (the limited amount of information that can be held in mind by active maintenance) is thought to be independent of the MTL and spared after MTL damage (9–12), whereas long-term memory is impaired (13). One might therefore expect that path integration should be intact after MTL damage whenever performance can be managed within working memory. In one study (14), memory-impaired patients with bilateral damage to the hippocampus or adjacent MTL structures were able to path integrate as well as controls in conditions when working memory likely supported performance (i.e., for paths involving only one or two turns and trial durations shorter than 35 s). In this study, however, the procedure was quite different from the standard methods traditionally used to test path integration in experimental animals.
Discussions about path integration in rodents emphasize the possible role of hippocampal place cells and entorhinal grid cells in computing information about spatial location (5, 6). If MTL structures are needed to carry out the computations needed for path integration, then MTL damage should impair path integration even in the case of short paths and short trial durations. That is, in the case of path integration, the distinction between working memory and long-term memory might be irrelevant. Most studies of path integration after hippocampal or entorhinal damage in rats have found impairment (15-18; but see ref. 19). However, it is notable that none of these studies reported how long it took to complete the trials. Accordingly, it remains possible that the animals in these studies might have performed well whenever trials were accomplished quickly, because in those instances performance might have been supported by working memory.
To address these issues, we carried out parallel experiments of path integration in humans and rodents. In both experiments, subjects searched for a target in a circular arena in the absence of vision and then tried to return to the start location. We assessed the accuracy of path integration as a function of three different measures: the distance traveled on the outward path, the time needed to find the target, and the number of turns taken on the outward path.
Results
Experiment 1: Path Integration in Humans.
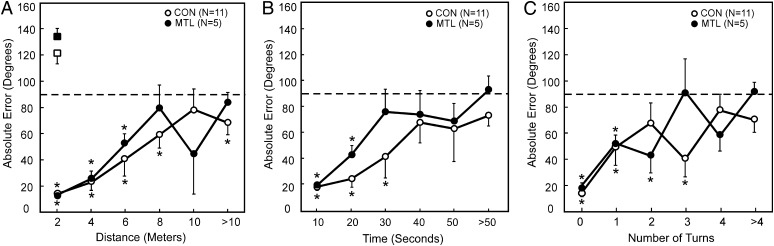
Overall performance across all trials was similar for the two groups [controls = 51.6 ± 4.2° error; patients = 57.8 ± 5.4° error; t(14) = 0.9, P > 0.1]. Both scores were better than chance (90°) (all P < 0.05). To assess variability in individual performance, the SD of each participant’s return scores was also calculated, and the individual SDs were then averaged for each group. These scores (68.6 ± 5.3° for controls and 77.2 ± 2.7° for patients) indicated that the two groups exhibited a similar dispersion in their return paths [t(14) = 1.0, P > 0.1].
For both groups, the accuracy of the return path was better and well above chance levels when the distance traveled on the outward path was short (Fig. 1A), when the tile was found quickly (Fig. 1B), and when only a small number of turns were taken on the outward path (Fig. 1C). The two groups performed similarly according to each of the three measures and at every bin size (all P > 0.15 with two exceptions; at three turns, Fig. 1C, P = 0.07; at 20 s, Fig. 1B, P = 0.08). For both groups, the accuracy of the return path gradually declined to chance levels because participants had more difficulty finding the tile. Even for controls, performance approached (or reached) chance levels when the outward path was > 8 m, when > 30 s was needed to find the tile, and when more than one turn was taken on the outward path.
Fig. 1.
Experiment 1: Path integration by memory-impaired patients (MTL) and CON. (A) Performance as a function of the distance traveled to find the tile. When participants were disoriented by rotation (squares), they were no longer able to rely on self-motion cues and failed to path integrate. (B) Performance as a function of the time taken to find the tile. (C) Performance as a function of the number of turns made to find the tile. The dotted line indicates chance performance (90° error). *Denotes above-chance performance. Brackets show SEM.
In the rotation condition, participants were unable to return accurately to the start location (Fig. 1A). Because the perceived direction heading was shifted systematically by rotation, accuracy was even worse than chance levels (all P < 0.05). There was no difference between groups [t(14) = 0.9, P > 0.1]. This result confirmed that participants were relying on self-motion cues to accomplish the task and did not have available other external cues.
Both groups demonstrated a gradual and similar decline in their confidence about the accuracy of the return path as the time taken to find the tile increased (Fig. S1). The results were the same when confidence ratings were plotted as a function of distance traveled or number of turns taken. For the 10 factual questions asked at the end of the test session, controls averaged 8.0 correct answers. Patients performed more poorly than controls, averaging only 5.0 correct answers (P = 0.01). Thus, despite the fact that the patients performed as well as controls at path integration, their memory for the test session itself was markedly impaired.
Experiment 2: Path Integration in Rats.
Behavioral findings.
Vision test.
Rats took substantially more time to locate the object during dark trials than during light trials [43.9 ± 3.2 s vs. 8.1 ± 1.0 s; t(5) = 13.3, P < 0.01]. This finding indicates that rats were unable to use visual cues to guide their performance.
Odor test.
Control rats tended to return to the original start box location more often than to the displaced start box and its associated odor trail and more often than to the box opposite the odor trail (18.3 ± 1.8% vs. 8.9 ± 1.6% and 10.0 ± 2.0%; all P < 0.05). This finding indicates that rats used path integration rather than odor trails to return to the open box. The rats with complete hippocampal lesions (H group) did not discriminate among the three locations.
Standard trials.
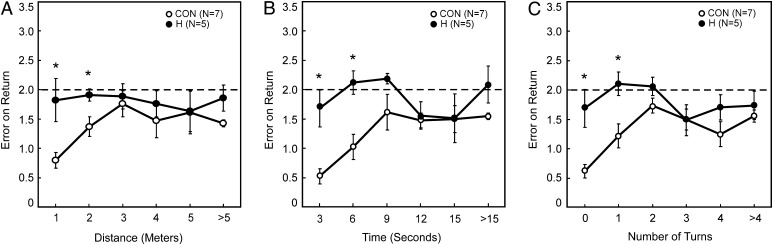
The control group performed best when the distance traveled to the food was short (Fig. 2A), when the food was found quickly (Fig. 2B), and when only a small number of turns were taken on the outward path (Fig. 2C). The accuracy of the return path declined as animals traveled further to find food, took more time, and made more turns. The animals with H lesions performed differently. For the H group, performance was poor even when rats traveled short distances to find food, when they found food quickly, and even when they made no turns. Specifically, compared with the control group, the H group was impaired when rats traveled 2 m or less on their outward path, when they took 6 s or less to find food, and when they made zero or one turn [Fig. 2; all t(10) > 2.5, all P < 0.05]. For longer distances, longer times, and greater number of turns, both groups performed poorly. Note that an animal performing entirely randomly should be expected to achieve a score of 2 on this task.
Fig. 2.
Experiment 2: Path integration by H group rats (H) and CON. The error in the return path was measured by which box the animal first returned to (start box = 0; the two boxes immediately adjacent to the start box = 1; the two boxes 90° removed from the start box = 2; the two boxes 135° removed from the start box = 3; the box 180° from the start box = 4). (A) Performance as a function of the distance traveled to find the food. (B) Performance as a function of the time taken to find the food. (C) Performance as a function of the number of turns made to find the food. The dotted line indicates chance performance. *Denotes group difference, P < 0.05. Brackets show SEM.
We also calculated how often rats returned to the correct start box before visiting other boxes (percent correct). The H group was impaired relative to its control group for seven comparisons (for 0–1 and 1–2 m; for 0–3, 3–6, and 6–9 s; and for zero and one turn). For these seven comparisons across all three measures, controls averaged 47.8% correct choices, and the H group averaged 12.9% correct choices [all t(10) > 2.4, all P < 0.05].
Neurohistological findings.
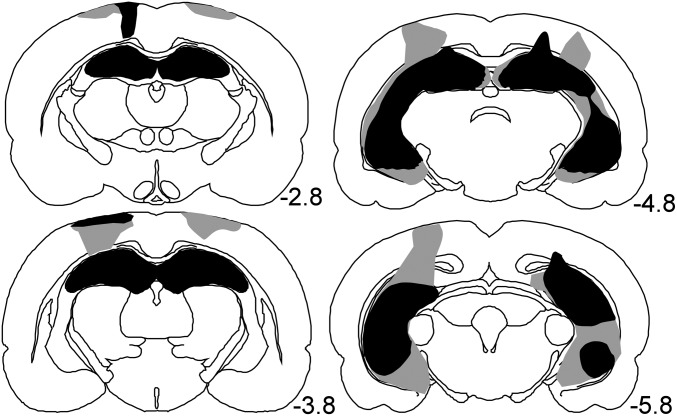
Fig. 3 shows reconstructions of coronal sections through the hippocampus of the lesion group; numbers represent the distance posterior to Bregma. All lesioned rats sustained bilateral damage to all cell fields of the hippocampus. The damage included 85–97% of the hippocampus (mean = 93%). Sparing occurred most frequently to the most medial aspect of the dorsal dentate gyrus and the dorsomedial CA1 cell field. The ventral-most region of the hippocampus was also spared in some animals. In all rats, there was some damage to the cortex and to the fimbria overlying the dorsal hippocampus, which was associated with the placement of the syringe during surgery and with spread of neurotoxin up the needle track. Two rats had minor damage to the posterior aspect of the lateral entorhinal cortex and posterior subiculum. There was no evidence of damage to the amygdala or thalamus in any animal. Fig. S2 shows histological images at three anteroposterior levels for each rat.
Fig. 3.
Reconstruction of coronal sections at four anteroposterior levels through the hippocampus showing the smallest (black) and largest (gray) lesion. Numbers to the right of each section represent the distance (in millimeters) posterior to Bregma. The upper left section is the most anterior section and the lower right section is the most posterior section.
Discussion
In two experiments, one with humans and one with rats, we assessed the capacity for path integration after bilateral damage to the hippocampus. In both studies, subjects entered a circular arena in the absence of vision, searched for a target, and then attempted to return to the start location at the perimeter of the arena. Experiment 1 demonstrated that patients with lesions to the hippocampus or larger MTL lesions returned to the start location accurately, and as well as controls, so long as the distance traveled on the outward path was short, the target was found quickly, and when only a small number of turns were taken on the outward path (Fig. 1). Patient and control groups also made similar confidence judgments about the accuracy of their returns (Fig. S1). Performance of both groups approached chance levels as participants had more difficulty finding the target. A control condition, in which path integration was disrupted by rotation, confirmed that performance depended on self-motion cues and not on other cues beyond experimental control (Fig. 1A). Last, despite the fact that path integration was intact when the path was short and direct, patients were impaired after the session at remembering facts about the tasks they had just completed.
It is often reported that controls outperform patients as a task becomes more difficult and as the material to be remembered comes to exceed what can be supported by working memory (see figure 3 in ref. 10). In that situation, controls can draw on their long-term memory, but patients cannot. In the present case, however, controls never outperformed the patients. Instead, their scores approached chance levels as the task became more difficult. It appeared that once participants traveled a sufficient distance and made a number of turns, they became lost. Working memory could support performance up to a point, but beyond that point it was not possible to transfer accurate information into long-term memory, presumably because of the interfering effects of additional distance, time, and turns, and the accumulation of errors. There is precedent for this idea that memory can be vulnerable to interference during the seconds after learning such that little long-term memory is formed. When humans or monkeys tried to memorize the pitch of a single tone or a synthetic sound, recognition accuracy deteriorated rapidly (within seconds) when intervening sounds were presented (20, 21).
In sharp contrast to the findings for humans, experiment 2 demonstrated that rats with complete hippocampal lesions were impaired at path integration relative to controls even when the outward path was shorter than 1 m, even when the target was found within 3 s, and even when animals made no turns on the outward path (Fig. 2). Both groups performed poorly for longer distances, longer times, and greater number of turns. Control conditions ruled out the relevance of visual or olfactory cues.
In earlier studies, rats with hippocampal lesions also exhibited impaired path integration (15–17). However, performance was not evaluated as a function of the time required to accomplish each trial (or as a function of distance traveled or number of turns taken). Accordingly, it remained possible that rats might succeed when trials were completed quickly and the paths to the target were short and direct. The present study, however, demonstrated impaired path integration after hippocampal lesions, even on trials when rats took short, direct paths to the target that required only a few seconds.
We have considered two possible ways to understand these contrasting findings for humans and rats. One possibility is that humans and rats used different strategies to accomplish path integration. For example, rats may have used self-motion cues exclusively, and the impairment after hippocampal lesions then reflected the failure of the hippocampus to carry out computations necessary for spatial navigation. Perhaps humans found an alternative way to accomplish the same task that did not require the specific contribution to the task that is supported by the hippocampus. Although it is difficult to exclude this possibility, we cannot identify any particular strategy that participants used. Most participants simply described trying to visualize the environment and keep track of where they were (i.e., as if they were using self-motion cues). A few participants reported trying to count their steps, but these participants performed no differently than those who did not report counting. In any case, it is unclear how counting steps could aid performance, inasmuch as what is important to good performance is not only keeping track of the distance traveled but also the angles through which one moves. No participant reported performing verbal calculations for the turns that were made.
A second possibility turns on the organization of working memory in humans and rats. In an earlier study of path integration in patients with MTL damage (14), performance was also intact when path lengths and trial times were short. We supposed that performance in that case reflected the successful maintenance of spatial information within working memory. First, just as in the present study, participants were encouraged to hold actively in mind the paths they took as they moved so that they might later be able to point to their start location. Second, performance of patients was disrupted when efforts were made to interfere with the maintenance of working memory by introducing distraction. In the present case, we suggest that patients also relied on working memory to accomplish path integration when the path lengths and trial times were short. Working memory in humans is independent of the MTL and intact after MTL damage (9–12, 22).
If working memory can support path integration in patients with MTL lesions (so long as the path is simple), what accounts for the inability of rats with hippocampal lesions to path integrate even under the simplest of conditions? One possibility, which has been given little attention, is that rats may be limited in their ability to construct a coherent working memory of spatial environments. Under conditions where spatial working memory is effective, it is thought to depend importantly on medial prefrontal cortex (mPFC) (23–27). A related idea is that the mPFC works in collaboration with the hippocampus to accomplish spatial working memory (28–32). Specifically, successful performance has been related to synchronous activity of prefrontal neurons and hippocampal theta oscillations (see ref. 33 for a review).
Thus, there are two ways that the organization of working memory in rodents could account for the effect of hippocampal damage on path integration. First, poor path integration after hippocampal lesions may reflect a need to depend on long-term memory (because spatial working memory capacity in the rodent is limited). The situation would be analogous to patients with hippocampal lesions who are impaired at recalling 10 word pairs immediately after learning (34), because in humans remembering 10 word pairs exceeds the capacity of working memory. The point is that performance can depend on long-term memory even when memory is tested within seconds of learning (also see ref. 35), and performance after hippocampal lesions will be impaired within seconds after learning whenever working memory capacity is exceeded. Indeed, several studies have reported impairments in rats performing spatial tasks at short delays after hippocampal lesions: spontaneous or forced-choice alternation at delays of 0–5 s (36, 37) and matching to position at delays of 1–10 s (25, 38). Note however that for object recognition tasks, rats with hippocampal lesions have exhibited intact performance at short delays (and impaired performance at longer delays) (39, 40). In any case, impairments at short delays in spatial tasks could reflect a need to depend on long-term memory.
A second possible reason for impaired path integration after hippocampal lesions is that performance may reflect an impairment of working memory itself. For example, the rodent hippocampus could contribute to spatial working memory by providing essential spatial information to prefrontal cortex. A potentially important difference between humans and rodents is that the human hippocampus, in comparison with rodent hippocampus, makes a relatively weak contribution to cortical theta, and hippocampal and cortical theta are not reliably synchronized (33). Thus, the interaction between the hippocampus and mPFC in rats may be more critical for working memory than it is in humans. Specifically, a hippocampal lesion in rats might be expected to have a larger effect on mPFC function than a hippocampal lesion in humans. If so, spatial working memory and long-term memory may not be as sharply distinguished in the rodent as in humans.
In summary, in tests of path integration, fundamentally different findings were obtained after hippocampal lesions in humans and rats. The findings for humans may be understood in terms of the historic distinction between working memory and long-term memory and the idea that working memory is independent of MTL function. Specifically, path integration succeeded when the outward path was simple and direct and when the task could presumably be managed within working memory. In contrast, rats with hippocampal lesions failed to path integrate even under the simplest conditions (when they traveled less than 1 m within 3 s and made no turns). We considered two possible ways to understand these data. First, humans may have found an alternative strategy for path integration that did not depend exclusively on self-motion cues or a strategy different in some way from the spatial strategy thought to support path integration in the rat (and depend on the hippocampus) (5, 6). Second, we suggest that rats may have failed path integration because (unlike humans) they are limited in the kind of information that can be supported by working memory. Thus, after hippocampal lesions, rat prefrontal cortex may be unable to construct a coherent working memory for spatial environments, either because the capacity of working memory is exceeded or because prefrontal cortex does not have input that it needs from hippocampus.
Materials and Methods
Experiment 1.
Participants.
Five memory-impaired patients participated (Table 1), four with bilateral lesions thought to be limited to the hippocampus (CA fields, dentate gyrus, and subicular complex) and one with larger MTL lesions. Patients G.W. and D.A. became amnesic in 2001 and 2011, respectively, following a drug overdose and associated respiratory failure. Patient K.E. became amnesic in 2004 after an episode of ischemia associated with kidney failure and toxic shock syndrome. Patient L.J. (the only female) became amnesic in 1988 during a 6-mo period with no known precipitating event. Her memory impairment has been stable since that time. Estimates of MTL damage were based on quantitative analysis of MRIs from 19 age-matched, healthy males for G.W., K.E., and G.P., 8 younger healthy males for D.A., and 11 age-matched, healthy females for patient L.J. (41). G.W., K.E., L.J., and D.A. have an average bilateral reduction in hippocampal volume of 48%, 49%, 46%, and 35%, respectively. All values are more than 2.9 SDs from the control mean. On the basis of two patients (L.M. and W.H.) with similar bilateral volume loss in the hippocampus for whom detailed postmortem neurohistological information was obtained (42), the degree of volume loss in these four patients likely reflects nearly complete loss of hippocampal neurons. The volume of the parahippocampal gyrus includes temporopolar, perirhinal, entorhinal, and parahippocampal cortices. G.W., K.E., L.J., and D.A. have an average bilateral reduction in the volume of parahippocampal gyrus by 10%, 11%, −17%, and −5%, respectively (all values within 2 SDs of the control mean). The volumes for parahippocampal gyrus differ a little from the volumes reported previously for these patients and are based on newly published, more detailed guidelines for identifying the caudal border of the gyrus (43).
Table 1.
Characteristics of memory-impaired patients
| WMS-R |
|||||||||
| Patient | Sex | Age, y | Education, y | WAIS-III IQ | Attention | Verbal | Visual | General | Delay |
| D.A. | M | 30 | 12 | 95 | 104 | 90 | 91 | 90 | 56 |
| K.E. | M | 70 | 13.5 | 108 | 114 | 64 | 84 | 72 | 55 |
| L.J. | F | 74 | 12 | 101 | 105 | 83 | 60 | 69 | <50 |
| G.W. | M | 53 | 12 | 108 | 105 | 67 | 86 | 70 | <50 |
| G.P. | M | 61 | 16 | 90 | 102 | 79 | 62 | 66 | 50 |
The WMS-R does not provide numerical scores for individuals who score < 50. IQ score for D.A. is from the WAIS-IV. WAIS-III, Wechsler Adult Intelligence Scale-III; WMS-R, Wechsler Memory Scale-Revised.
One patient (G.P.) has severe memory impairment resulting from viral encephalitis. He has demonstrated virtually no new learning since the onset of his amnesia, and during repeated testing over many weeks he does not recognize that he has been tested before (44). G.P. has an average bilateral reduction in hippocampal volume of 96%. The volume of the parahippocampal gyrus is reduced by 94%. Eight coronal MRIs from each of the five patients are available in Fig. S3.
Eleven healthy volunteers also participated (three females; mean age, 61.3 y; range = 25–76 y; mean education, 14.8 y). All procedures were approved by the Institutional Review Board at the University of California at San Diego, and participants gave written informed consent before participation.
Apparatus.
The experiment was carried out in an indoor circular space (4-m diameter). A string was laid out on the floor and marked every 5° to describe the perimeter of the circle (arc length = 17.4 cm). Participants wore a blindfold and noise-cancelling earphones; a walker was provided for safety.
Path integration.
The task was to start from one of eight equidistant locations on the perimeter of the circle, find a square tile (19 cm) placed on the floor within the circle, and return to the start location. On each trial, the tile was equally likely to be within one of the six 45° segments of the circle that were most remote from the start location. In addition, the tile could be in any of four positions along a radius within a segment: near the origin, 0.75 m from the origin, 1.5 m from the origin, and near the perimeter. Of these 24 possible tile locations, 16 different locations were selected for each session.
Participants could detect the tile with their feet or with the wheels of the walker. If the participant reached the perimeter of the circle while searching for the tile, he or she was guided back into the circle. If the participant could not locate the tile within 5 min (on 2.3% of the trials), he or she was guided to the tile and then allowed to return to the start point. Participants were instructed to actively maintain the start location in mind as they proceeded, so that they could be successful at returning to the start point. Immediately after completing each trial, participants provided a rating of 1–5 to indicate their confidence that they had returned to a point within one arm’s length of where they had started from.
Two practice trials were given, first without the blindfold and then with the blindfold. Sixteen trials (two from each start location) were then given in which the blindfolded participant searched for the tile and then tried to return to the start location. Controls were tested in a single session. Patients were given two sessions separated by1–10 wk (2 practice trials, 16 test trials, and confidence ratings). To confirm that participants were indeed relying only on self-motion cues rather than using external cues beyond experimental control, four rotation trials were also given after the first session (14). For the rotation condition, participants were led from a start location directly to a platform without making any turns (average duration, 5.3 s). After stepping onto the platform, participants were slowly rotated 190° by a remotely controlled motor (∼14°/s) and then tried to return to the start point. If participants were relying only on self-motion cues and were unable to use external cues, their performance should be disrupted by the rotation. After the rotation condition, participants were asked 10 factual questions (four free recall, six true-false) to assess their memory for the entire test session. The 10 factual questions about the testing session yielded a score from 0 to 10. Chance performance was estimated to be 35%.
During testing, one experimenter followed the participant with a measuring wheel to record the distance traveled on the outward path. Another experimenter traced onto a map of the arena the path taken by the participant and also recorded the time taken to find the tile. Two raters independently recorded the point on the perimeter to which participants returned (mean interrater error = 1.9°). In addition, the number of turns taken on the outward path (changes in heading direction ≥ 90°) was later counted by two raters, based on the drawings (mean interrater error = 0.3 turns).
Data analysis.
The accuracy of the return path (absolute difference in degrees between the return location and the start location) was measured as a function of the distance traveled on the outward path (0–2, 2–4, 4–6, 6–8, 8–10, > 10 m), the time needed to find the tile (0–10, 10–20, 20–30, 30–40, 40–50, > 50 s), and the number of turns taken on the outward path (0, 1, 2, 3, 4, > 4 turns). Participants distributed their trials rather evenly across these values, and a minimum of seven observations contributed to each of the 18 bins (three measures × six bins). Also, of the 11 controls, 9.4 on average contributed scores to each of the 18 bins. Of the 5 patients, 4.8 on average contributed scores to each of the 18 bins.
Experiment 2.
Subjects.
Subjects were 18 male Long Evans rats weighing between 300 and 350 g at the beginning of the study. Rats were individually housed and maintained on a 12:12 h light:dark cycle and tested in the light phase of the cycle. Six rats were used to verify that visual cues could not be used to guide performance (Vision Test). Five rats were prepared with complete hippocampal lesions (H group), and seven rats served as controls (CON). All procedures were approved by the University of California at San Diego, Institutional Animal Care and Use Committee.
Apparatus.
The apparatus was a 2-m diameter circular Plexiglas table painted white and elevated 64 cm above the floor. Eight (12-cm diameter) holes were placed equidistant around the perimeter with centers 13 cm from the edge. Start boxes attached below each of the holes were filled with used rodent bedding to distribute odor cues. A movable mesh screen could block access to and from the boxes. The apparatus was mounted on a central bearing that allowed it to be rotated. In addition, a fixed central platform (45.5-cm diameter) was mounted flush to the table surface. In this way, the main table could be rotated while a rat on the central platform remained stationary. The apparatus could be illuminated by fluorescent lights and could also be insulated from visible light. A camera mounted above the center of the table and attached to a video tracking system (Smart Tracking, San Diego Instruments) allowed animals to be tracked in the dark by an infrared camera with the aid of infrared lights.
Vision and odor tests.
Methods used to test for the possible role of vision or odor trails are described in SI Materials and Methods.
Path integration.
Preoperative training.
Pretraining began after rats were food deprived to ∼80% of their free-feeding weight. First, rats explored the illuminated table for 10 min with no food present and all boxes blocked. After 2 d of exploration, five food pellets (750-mg rodent pellets, Bio-Serv) were placed on the table, and rats were given 10 min to eat three or more pellets. After a rat had done this on two consecutive days, similar trials began with the rat inside a start box. After a rat completed three trials within 10 min for two consecutive days (exit start box, return with food to start box), training then continued with the lights off. After two successful days in the dark (three or more pellets eaten within the time limit), the final phase of training was introduced. In this phase (four trials per day), the rat was required to exit a start box in the dark, locate a single pellet on the table, and return to the same box (all other box entrances were blocked). Preoperative training was complete when a rat successfully completed four trials in a day on two consecutive days (5-min time limit). On average, pretraining required 18 d.
Surgery.
Surgical methods for removing the hippocampus bilaterally are described in SI Materials and Methods.
Postoperative testing.
Rats were given four standard trials (see the following paragraph) and one odor probe trial each day until they accumulated 50 standard trials. All trials were conducted with the lights off and with a 5-min time limit. Trials were discarded if the rat consumed the food on the testing table rather than returning to the start box (this occurred on fewer than 5% of the trials). The food pellet could be located in any of 12 locations distributed across the table. The order in which these locations were used was determined pseudo-randomly. In addition, each start box was equally likely to be used each day. No box was repeated until all eight boxes were used. The table was rotated between trials, and each start box was equally likely to be placed in each of eight possible locations in the testing room.
Standard trials.
For the first four trials of each day, rats were placed in a start box with a food pellet placed on the table. The trial began when the rat left the start box and ended when the rat located the food and returned to the open start box. The rat was allowed to eat the pellet in the box before being removed.
Data analysis.
Performance was measured by how accurately the rat returned to the start box after locating the food. The animal could return to the start box itself (a score of zero), one of the boxes 45° on either side of the start box (a score of 1), one of the boxes 90° on either side of the start box (a score of 2), one of the boxes 135° on either side of the start box (a score of 3), or the box that was 180° from the start box (a score of 4). We also included a second performance measure (percent correct). This measure referred to the proportion of trials in which the rat returned to the start box before visiting any other boxes.
Performance accuracy (score of 0–4) was recorded as a function of the distance traveled on the outward path (0–1, 1–2, 2–3, 3–4, 4–5, > 5 m), the time needed to find the food (0–3, 3–6, 6–9, 9–12, 12–15, > 15 s), and the number of turns taken on the outward path (0, 1, 2, 3, 4, > 4 turns). Rats distributed their trials across these values, and a minimum of 20 observations contributed to each of the 18 bins (three measures × six bins). Also, all 7 control rats and all 5 H group rats contributed scores to each of the 18 bins.
Histology.
Histological methods used to evaluate the lesions are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Jennifer Frascino, Erin Light, Laura Johnson, Ian Crane, Chris Fascenda, Nareg Kalajian, Nour al-Timim, Patrick Simpson, Caitlin Barlow, Kathleen Kuo, Stephanie Liu, Claudia Hernandez, Devin Christopher, and Raymond Branch for assistance. This work was supported by the Medical Research Service of the Department of Veterans Affairs, National Science Foundation Grant 1120395, and National Institute of Mental Health Grant 24600.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300869110/-/DCSupplemental.
References
- 1.Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 3.Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. Upper Saddle River, NJ: Oxford Univ; 2001. [Google Scholar]
- 4.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon Press; 1978. [Google Scholar]
- 5.Whitlock JR, Sutherland RJ, Witter MP, Moser MB, Moser EI. Navigating from hippocampus to parietal cortex. Proc Natl Acad Sci USA. 2008;105(39):14755–14762. doi: 10.1073/pnas.0804216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map.’. Nat Rev Neurosci. 2006;7(8):663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- 7.Etienne AS, Jeffery KJ. Path integration in mammals. Hippocampus. 2004;14(2):180–192. doi: 10.1002/hipo.10173. [DOI] [PubMed] [Google Scholar]
- 8.Benhamou S. Path integration by swimming rats. Anim Behav. 1997;54(2):321–327. doi: 10.1006/anbe.1996.0464. [DOI] [PubMed] [Google Scholar]
- 9.Drachman DA, Arbit J. Memory and the hippocampal complex. II. Is memory a multiple process? Arch Neurol. 1966;15(1):52–61. doi: 10.1001/archneur.1966.00470130056005. [DOI] [PubMed] [Google Scholar]
- 10.Jeneson A, Squire LR. Working memory, long-term memory, and medial temporal lobe function. Learn Mem. 2012;19(1):15–25. doi: 10.1101/lm.024018.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baddeley AD, Warrington EK. Amnesia and distinction between long-and short-term memory. J Verb Learn Verb Be. 1970;9(2):176–189. [Google Scholar]
- 12.Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- 13.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 14.Shrager Y, Kirwan CB, Squire LR. Neural basis of the cognitive map: Path integration does not require hippocampus or entorhinal cortex. Proc Natl Acad Sci USA. 2008;105(33):12034–12038. doi: 10.1073/pnas.0805414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maaswinkel H, Jarrard LE, Whishaw IQ. Hippocampectomized rats are impaired in homing by path integration. Hippocampus. 1999;9(5):553–561. doi: 10.1002/(SICI)1098-1063(1999)9:5<553::AID-HIPO9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Whishaw IQ, Hines DJ, Wallace DG. Dead reckoning (path integration) requires the hippocampal formation: Evidence from spontaneous exploration and spatial learning tasks in light (allothetic) and dark (idiothetic) tests. Behav Brain Res. 2001;127(1-2):49–69. doi: 10.1016/s0166-4328(01)00359-x. [DOI] [PubMed] [Google Scholar]
- 17.Save E, Guazzelli A, Poucet B. Dissociation of the effects of bilateral lesions of the dorsal hippocampus and parietal cortex on path integration in the rat. Behav Neurosci. 2001;115(6):1212–1223. doi: 10.1037//0735-7044.115.6.1212. [DOI] [PubMed] [Google Scholar]
- 18.Parron C, Save E. Evidence for entorhinal and parietal cortices involvement in path integration in the rat. Exp Brain Res. 2004;159(3):349–359. doi: 10.1007/s00221-004-1960-8. [DOI] [PubMed] [Google Scholar]
- 19.Alyan S, McNaughton BL. Hippocampectomized rats are capable of homing by path integration. Behav Neurosci. 1999;113(1):19–31. doi: 10.1037//0735-7044.113.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Deutsch D. Tones and numbers: Specificity of interference in immediate memory. Science. 1970;168(3939):1604–1605. doi: 10.1126/science.168.3939.1604. [DOI] [PubMed] [Google Scholar]
- 21.Scott BH, Mishkin M, Yin PB. Monkeys have a limited form of short-term memory in audition. Proc Natl Acad Sci USA. 2012;109(30):12237–12241. doi: 10.1073/pnas.1209685109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrager Y, Levy DA, Hopkins RO, Squire LR. Working memory and the organization of brain systems. J Neurosci. 2008;28(18):4818–4822. doi: 10.1523/JNEUROSCI.0710-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuster JM. The Prefrontal Cortex. 4th Ed. Boston: Elsevier; 2008. [Google Scholar]
- 24.Horst NK, Laubach M. The role of rat dorsomedial prefrontal cortex in spatial working memory. Neuroscience. 2009;164(2):444–456. doi: 10.1016/j.neuroscience.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kesner RP, Hunt ME, Williams JM, Long JM. Prefrontal cortex and working memory for spatial response, spatial location, and visual object information in the rat. Cereb Cortex. 1996;6(2):311–318. doi: 10.1093/cercor/6.2.311. [DOI] [PubMed] [Google Scholar]
- 26.Kolb B, Nonneman AJ, Singh RK. Double dissociation of spatial impairments and perseveration following selective prefrontal lesions in rats. J Comp Physiol Psychol. 1974;87(4):772–780. doi: 10.1037/h0036970. [DOI] [PubMed] [Google Scholar]
- 27.Le Marec N, Ethier K, Rompré PP, Godbout R. Involvement of the medial prefrontal cortex in two alternation tasks using different environments. Brain Cogn. 2002;48(2-3):432–436. [PubMed] [Google Scholar]
- 28.Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Working memory performance correlates with prefrontal-hippocampal theta interactions but not with prefrontal neuron firing rates. Front Integr Neurosci. 2010;4:2. doi: 10.3389/neuro.07.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3(12):2187–2199. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang GW, Cai JX. Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behav Brain Res. 2006;175(2):329–336. doi: 10.1016/j.bbr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Lee I, Kesner RP. Differential roles of dorsal hippocampal subregions in spatial working memory with short versus intermediate delay. Behav Neurosci. 2003;117(5):1044–1053. doi: 10.1037/0735-7044.117.5.1044. [DOI] [PubMed] [Google Scholar]
- 32.Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46(1):141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 33.Hyman JM, Hasselmo ME, Seamans JK. What is the functional relevance of prefrontal cortex entrainment to hippocampal theta rhythms? Front Neurosci. 2011;5:24. doi: 10.3389/fnins.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manns JR, Hopkins RO, Squire LR. Semantic memory and the human hippocampus. Neuron. 2003;38(1):127–133. doi: 10.1016/s0896-6273(03)00146-6. [DOI] [PubMed] [Google Scholar]
- 35.Baddeley A, Allen R, Vargha-Khadem F. Is the hippocampus necessary for visual and verbal binding in working memory? Neuropsychologia. 2010;48(4):1089–1095. doi: 10.1016/j.neuropsychologia.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Gross CG, Black P, Chorover SL. Hippocampal lesions - Effects on memory in rats. Psychon Sci. 1968;12(5):165–166. [Google Scholar]
- 37.Racine RJ, Kimble DP. Hippocampal lesions and delayed alternation in the rat. Psychon Sci. 1965;3:285–286. [Google Scholar]
- 38.Steele RJ, Morris RG. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9(2):118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 39.Clark RE, West AN, Zola SM, Squire LR. Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus. 2001;11(2):176–186. doi: 10.1002/hipo.1035. [DOI] [PubMed] [Google Scholar]
- 40.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20(23):8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gold JJ, Squire LR. Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus. 2005;15(1):79–85. doi: 10.1002/hipo.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci. 1996;16(16):5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frankó E, Insausti AM, Artacho-Pérula E, Insausti R, Chavoix C. Identification of the human medial temporal lobe regions on magnetic resonance images. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bayley PJ, Frascino JC, Squire LR. Robust habit learning in the absence of awareness and independent of the medial temporal lobe. Nature. 2005;436(7050):550–553. doi: 10.1038/nature03857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.