BIOCHEMISTRY Correction for “Confinement of caspase-12 proteolytic activity to autoprocessing,” by Sophie Roy, Jeffrey R. Sharom, Caroline Houde, Thomas P. Loisel, John P. Vaillancourt, Wei Shao, Maya Saleh, and Donald W. Nicholson, which appeared in issue 11, March 18, 2008, of Proc Natl Acad Sci USA (105:4133–4138; first published March 10, 2008; 10.1073/pnas.0706658105).
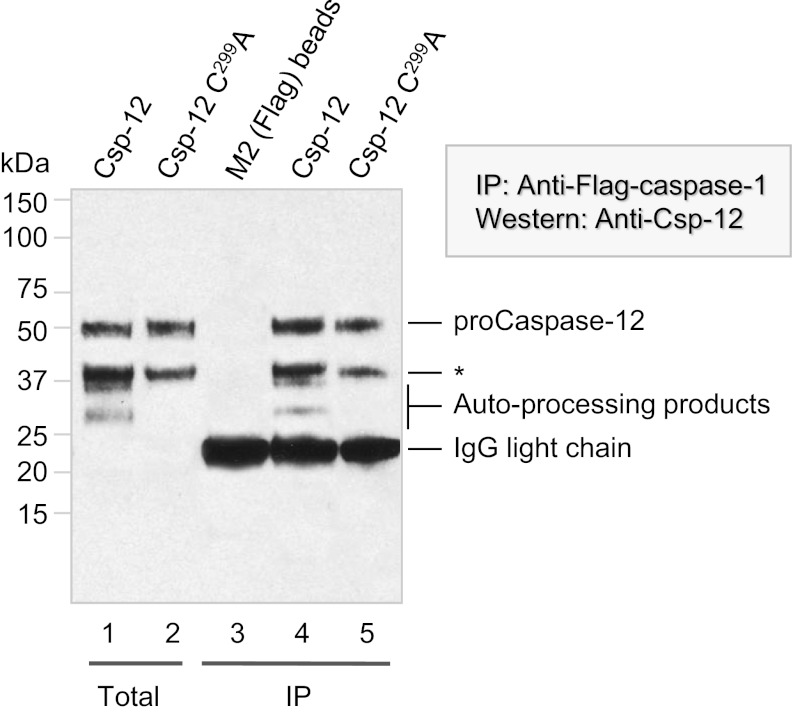
The authors note that Fig. 6 appeared incorrectly. There was an error in the alignment of the molecular mass markers, and minor adjustments have been made to the assignment of caspase-12 bands. The authors also note that the source of the anti-caspase-12 antibody for Fig. 6 was Sigma (clone 14F7). This error does not affect the conclusions of the article. The corrected figure and its corresponding legend appear below.
Fig. 6.
Procaspase-12 forms a complex with caspase-1 and is partially autoprocessed in the complex. HEK293T cells were cotransfected with expression vectors harboring Flag-tagged procaspase-1 (all lanes) plus either procaspase-12 (lanes 1 and 4) or the catalytically incapacitated C299A mutant (lanes 2 and 5). After 24 h, cells were harvested and lysed. One tenth of the lysate was directly applied to SDS/PAGE (lanes 1 and 2), and the remainder was immunoharvested with antibodies directed against the caspase-1 Flag epitope tag (lanes 4 and 5; lane 3 was processed in the same way, except that only lysis buffer was used). Immunoblotting for the large subunit (p20) of caspase-12 revealed that procaspase-12 and the resulting autocleavage product were both immunoharvested with caspase-1. The asterisk indicates a band of unknown identity that is detected by prebleed control serum.