Abstract
Rapid antigenic variation of HA, the major virion surface protein of influenza A virus, remains the principal challenge to the development of broader and more effective vaccines. Some regions of HA, such as the stem region proximal to the viral membrane, are nevertheless highly conserved across strains and among most subtypes. A fundamental question in vaccine design is the extent to which HA stem regions on the surface of the virus are accessible to broadly neutralizing antibodies. Here we report 3D structures derived from cryoelectron tomography of HA on intact 2009 H1N1 pandemic virions in the presence and absence of the antibody C179, which neutralizes viruses expressing a broad range of HA subtypes, including H1, H2, H5, H6, and H9. By fitting previously derived crystallographic structures of trimeric HA into the density maps, we deduced the locations of the molecular surfaces of HA involved in interaction with C179. Using computational methods to distinguish individual unliganded HA trimers from those that have bound C179 antibody, we demonstrate that ∼75% of HA trimers on the surface of the virus have C179 bound to the stem domain. Thus, despite their close packing on the viral membrane, the majority of HA trimers on intact virions are available to bind anti-stem antibodies that target conserved HA epitopes, establishing the feasibility of universal influenza vaccines that elicit such antibodies.
Keywords: envelope glycoproteins, subvolume averaging, virus structure, hemagglutunin, cryo-electron microscopy
Influenza A virus (IAV) infects hundreds of millions of people worldwide annually. Although vaccines and drugs providing some level of protection against the virus are available, IAV persists due to the antigenic variability of hemagglutinin (HA), the most abundant virion surface protein (1, 2). HA is classified into 17 subtypes (H1–H17) based on antigenicity and sequence diversity (3, 4), with the various HAs classified phylogenetically into two distinct groups (5). The introduction of influenza viruses bearing novel HA variants from birds and pigs into the human population results in periodic global epidemics, such as the one in 1918 that claimed the lives of more than 50 million individuals (6). The most recent epidemic occurred in 2009, with the introduction of a swine origin virus that continues to circulate widely in human populations (7). Avian IAVs, such as H5N1 subtype strains, represent a potential pandemic risk if they become transmissible from person to person (8, 9).
HA is a trimeric glycoprotein, with each protomer formed from disulfide-linked subunits HA1 and HA2. The tip of the HA spike is formed from globular regions of HA1, and the membrane-proximal stem region is formed from portions of both HA1 and HA2 (10, 11). HA mediates viral entry via cellular attachment and membrane fusion activities (12, 13). The HA1 globular domain contains a binding site for the influenza cellular receptor sialic acid, whereas HA2 exerts membrane fusion activity via an N-terminal fusion peptide (14). A transmembrane region located close to the C terminus of HA2 anchors HA in the viral membrane. Although HA is the principal target for vaccines, viruses also display neuraminidase (NA), a less-abundant membrane protein that facilitates viral entry and release of virus particles from infected cells. Antibodies directed against the globular domain of HA, as well as the highly conserved stem region, efficiently neutralize the virus. The identification of broadly neutralizing antibodies that target highly conserved stem epitopes offers the exciting prospect of designing universal or pan-influenza vaccines that can protect against IAV expressing several or all subtypes of HA (10, 15–20).
A persistent question in IAV vaccine design has been the extent to which conserved stem regions of native HA trimers displayed on the virus surface are accessible to intact broadly neutralizing IgG antibodies. To address this question, we performed cryoelectron tomography of intact 2009 H1N1 pandemic influenza virus preparations in both the presence of and absence of the broadly neutralizing antibody C179. In contrast to viruses that display icosahedral symmetry, influenza virions are pleiomorphic and thus refractory to computational methods that have proven successful in structural determination of symmetrical viruses using X-ray crystallography or cryoelectron microscopy (21, 22). Our present knowledge of HA structure is thus derived largely from crystallographic analyses of soluble ectodomains of trimeric HA or EM analyses of chemically stained HA trimers (23, 24). However, the binding of Fab fragments to isolated HA ectodomains might not reflect antibody accessibility to native HA trimers displayed on intact viruses, or allow determination of the extent to which various neutralizing antibodies can access their epitopes in the context of intact virions.
To overcome these limitations, we used cryoelectron tomography and novel subvolume averaging techniques to obtain density maps of the HA trimers on the surface of the 2009 H1N1 pandemic influenza virus in the absence and presence of bound C179, a broadly neutralizing antibody (25, 26). This antibody binds the conserved stem region of HA near the viral membrane on virions with both spherical and filamentous morphologies. Finally, using methods to map antibody locations on individual virions, we derived the distribution of C179-bound HA spikes on the surface of viral membranes, which demonstrates that the majority of HA stem epitopes are accessible on H1N1 virions with both spherical and filamentous morphologies.
Results
Virus Morphology and Molecular Structure of Trimeric HA from Intact H1N1 Virus.
Our cryo-electron tomographic analysis of 2009 H1N1 suspensions indicated the presence of virions with spherical and elongated morphologies (Fig. 1 A and B). Slices through the center of the virions revealed the presence of several established features, including internal ribonucleoprotein particles, the internal matrix layer underneath the viral membrane, and glycoproteins protruding from the virus surface. Spherically shaped virions had an average diameter of ∼130 nm (n = 46), similar to the longer dimension of oval-shaped virions (length ∼137 nm; n = 38). Approximately 7% of the virions were filamentous, as defined by an elongated elliptical morphology, with lengths ranging from 170 nm to 1,300 nm and axial ratios ranging from ∼2 to ∼10. Lateral slices through the majority of the surface spikes were “peanut-shaped” in appearance (Fig. 1 C–E), with a small minority displaying a “mushroom-shaped” density, with a bulbous head emanating from the virus membrane (Fig. 1 F–H). The difference in shape between the two types of surface spikes are easily visualized in cylindrically symmetric averages in 3D (Fig. 1 E and H) and in the distinct 1D density profiles along the length of the spikes (Fig. S1A). Based on the distinct ectodomains of H1 HA and N1 NA already known from X-ray crystallography (10, 27), we assigned the peanut-shaped densities (blue map and lines) to trimeric HA and the mushroom-shaped densities (yellow map and lines) to NA (Fig. S1). We focused the rest of our structural analysis in this work on trimeric HA, which represents the majority of spikes seen on the surface of these virions.
Fig. 1.
Cryoelectron tomography of 2009 H1N1 pandemic influenza virus and shape based analysis of glycoproteins. Tomographic slices (∼5 nm thick) showing the interiors of several spherically shaped influenza virions (A) and a long filamentous virion (B). HA spikes, shown as a slice through a region rich in HA (C), as an expanded view highlighting their “peanut”-shaped appearance (D) and as a cylindrically symmetric average (E). NA spikes, which are much rarer than HA spikes, shown as a slice through a region rich in NA (F), as an expanded view highlighting their “mushroom”-shaped appearance (G), and as a cylindrically symmetric average (H). In D and G, the selected spikes are in the center of each panel, and arrows denote representative spike morphologies. (Scale bars: 100 nm.)
Through classification and 3D averaging of densities from individual glycoprotein spikes, we obtained 3D structures for the HA trimer displayed on both spherical virions (Fig. 2 A and B) and filamentous virions (Fig. 2 C and D) at resolutions of ∼25 Å and ∼30 Å, respectively (Fig. S2). In both cases, the ectodomain of trimeric HA was ∼16 nm long and ∼9 nm wide at the region farthest from the viral membrane, as determined by the dimensions of the density profile across the averaged structure. A short, narrow stalk connected the base of the ectodomain to the membrane. By docking the crystal structure of trimeric H1 HA [Protein Data Bank (PDB) ID code 3LZG, which includes coordinates for both HA1 and HA2] into the density maps, we obtained molecular models for native trimeric HA on spherical virions (Fig. 2B) and filamentous virions (Fig. 2D). The density maps and molecular models of HA spikes on both spherical and filamentous virions are closely comparable.
Fig. 2.
Three-dimensional structure of HA trimer. (A and B) Side views of the 3D structure of HA embedded in the viral membrane (gray) of spherically shaped virions. (B) Docking of HA1 (red) and HA2 (blue) ectodomain coordinates (PDB ID code: 3LZG) into the density map of trimeric HA, shown as a transparent isosurface at two different thresholds. The red asterisk in B indicates the cavity in the ectodomain. Side views of the 3D structure of HA embedded in the viral membrane (gray) from filamentous virions, shown as a solid isosurface (C) and in a front slice (D) to illustrate the general shape of a single protomer in the spike.
As a test of the generality of the structure obtained by our methods, we also obtained density maps and fits for HA trimers displayed on H3N2 virions. HA trimers from both spherical and filamentous virus morphologies showed similar density maps and molecular structures (Figs. S3 and S4), which in turn are closely comparable to those seen for H1N1 2009 virus at the ∼25-Å resolution of our maps.
Three-Dimensional Structure of the HA-C179 Complex.
A persistent question in influenza vaccine design has been the extent to which conserved stem regions near the viral membrane are accessible to broadly neutralizing IgG antibodies. We carried out cryoelectron tomography studies of H1N1 virus suspensions incubated with the broadly neutralizing antibody C179, which binds and neutralizes viruses expressing a broad range of HA subtypes, including H1, H2, H5, and H9 (25, 28). In addition, C179 neutralizes avian H5N1 and swine-origin 2009 H1N1 viruses with escape mutants mapping to residues in the stem region of HA (25, 26). The overall morphology of C179-treated H1N1 appears similar to that of native virions, indicating that antibody binding does not result in gross structural changes in the virion. (Fig. 3 A and B). For analysis of the structure of C179-bound HA, spikes displayed on the surface were extracted and subjected to 3D classification, followed by subvolume averaging and threefold symmetrization, to derive the averaged 3D structure of C179-bound HA trimers.
Fig. 3.
Three-dimensional structure of HA bound to C179. (A and B) Tomographic slices (∼5 nm thick) through collections of influenza virions incubated with the neutralizing antibody C179. A long filamentous virion is visible in B. (Scale bars: 100 nm.) Gold fiducials used in image alignment appear as dark spots with white halos. Top (C) and side (D) views of the HA-C179 map represented as a solid isosurface. (E) Wire-mesh representation of front view of the density map of the complex formed by C179 antibody with trimeric HA. Coordinates for trimeric HA (with HA1 in red, highly conserved HA2 in blue, and helix A of HA2 in green) and the Fv portion of the antibody (cyan) were extracted from the structure of the complex of trimeric H5 HA with CR6261 (PDB ID code: 3GBM). Coordinates for the rest of the Fab (i.e., the non-Fv portion) were also extracted from the same set of coordinates, but were subjected to rigid body movement to accommodate the C179 density. Homologous residues corresponding to C179-selected escape mutant residues Val-52 (black spheres) and Thr-318 (yellow spheres) are highlighted. (F) Three-dimensional structure of HA-C179 derived from filamentous viruses shown superposed with the same coordinates used in E for spikes from virions with spherical morphology.
Top and side views of the density map of C179-bound HA trimers from spherically shaped virions show the presence of additional densities near the base of the HA spikes (Fig. 3 C and D). The map shows that the molecular contour of the HA component in the complex is similar to that of unliganded HA, suggesting that no major quaternary structural changes in HA are induced by C179 binding. Only density from the Fab arm that is bound to HA is visible in the averaged density maps; density from the other Fab and Fc portions is absent, because these portions are rotationally disordered with respect to the bound Fab arm. Crystallographic structures for the C179 Fab fragment alone or in complex with HA are not yet available, and we made use of the envelope of the HA-C179 3D map to provide shape restraints on molecular fitting of HA and Fab. For the purpose of fitting, we used the Fab fragment of CR6261, a stem region antibody, as a template for placing the coordinates into the density map (Fig. 3E), because this is an antibody for which a crystallographic structure for the entire Fab fragment is available, and because similar residues appear to be involved in the contact zone between HA and this antibody (25). The location of the Fab density is consistent with the position of stem region residues Val-52 in HA2 and Thr-318 in HA1, both of which are reportedly sites of mutations that allow escape from neutralization by C179 (25).
We generated molecular models for C179-bound HA trimers in three different ways. In one approach, we fit the HA trimer and CR6261 Fv fragment into the map as a single rigid body, and fit the non-Fv portion of the antibody separately (Fig. 3E). In a second approach, we obtained fits by treating the HA trimers and Fab fragments as separate rigid bodies; this method of fitting coordinates also produced a similar footprint of the antibody on HA, with a slightly shifted interface between the Fv fragment and HA (Fig. S5). Finally, we tested fits of the CR6261-HA trimer complex with inclusion of the entire Fab fragment. This led to a noticeably poorer fit of the non-Fv regions into the density map (Fig. 4A), but again without much difference in the location of the Fv fragment close to the stem region. Thus, we conclude that the fit shown in Fig. 3E is a good approximation of the general architecture of the HA-C179 complex.
Fig. 4.
Molecular comparisons of soluble group 1 and group 2 HA trimers complexed with anti-stem Fabs with HA-C179 on virus. (A) Structure obtained by fitting coordinates of the entire HA (H5)-CR6261 complex (PDB ID code: 3GBM) (10) as a single rigid body into the HA-C179 density map. (B–D) Front views of structures for complexes of other Fab fragments with trimeric HAs were placed into the experimentally obtained density map for the HA-C179 complex. Structures shown include (B) H1 (1918) HA bound to CR6261 Fab fragment (PDB ID code: 3GBN) (10), (C) H5 HA bound to scFv F10 (PDB ID code: 3FKU) (17), and (D) H3 HA bound to FI6V3 Fab fragment (PDB ID code: 3ZTJ) (15). Homologous C179-selected escape mutant residues Val-52 (black spheres) and Thr-318 (yellow spheres) are highlighted (25).
We also derived density maps of HA-C179 complexes displayed on filamentous virions to address the issue of whether primary isolates and certain strains of influenza that have a more filamentous population of virions (22, 29–31) can also bind to stem antibodies in the same manner as observed for spherical H1N1 virions. Density maps and fits obtained from spikes on filamentous H1N1 virions demonstrate that C179 binds HA trimers to form complexes that are essentially the same as those seen for spherical virions (Fig. 3F).
We further tested the generality of these observations by comparing the crystallographic structures of soluble ectodomain HA trimers complexed with other HA stem region-specific antibody complexes. In each case, the location of these antibodies relative to the C179 Fab density on native HA on intact H1N1 influenza virus was similar (Fig. 4). These structures included complexes of antibodies with HA trimers from both group 1 (H1 and H5) and group 2 (H3) influenza viruses bound by anti-stem Fabs, and in each case the location of the antibody–HA interface closely matches that derived here for the C179-HA trimer complex displayed on intact influenza virus. Because C179 is not expected to bind to HA trimers of the H3 subtype, we performed cryoelectron tomography studies of H3N2 virus incubated with C179 antibody as a control experiment. Density maps for H3 HA spikes on these virions were essentially the same as those of unliganded H3 HA trimers, with no additional density observed at the base of the spikes (Figs. S3 and S4).
The 3D structures derived in Fig. 3 were obtained using threefold symmetrization of the HA-trimer–containing subvolumes, as in our previous structural analyses of antibody-bound envelope glycoprotein spikes on intact HIV (32, 33). Compared with HIV, in which the glycoproteins are sparsely packed on the viral membrane, HA trimers are much more densely packed, with an average spacing of ∼14 nm (Fig. 5A). Placing the space-filling coordinates for a whole antibody molecule at the location identified for C179 Fab binding suggests that steric constraints between whole antibody molecules bound to neighboring spikes (Fig. 5B) may limit occupancy and lower the stoichiometry of binding. We tested this possibility by performing 3D averaging of subvolumes in the absence of imposition of threefold symmetry. The absence of symmetry lowers the signal-to-noise ratio and introduces greater distortion from the presence of the missing wedge in limited-angle tomography, which can lead to underestimation of the actual occupancy. Despite these caveats, inspection of the 3D average is instructive, indicating that structures with a single C179 antibody bound to the HA trimer (Fig. 5 C and D) exist, consistent with the expectation that the close packing of the HA spikes may limit full occupancy on the viral membrane, with some HA molecules having one (Fig. 5C), two (Fig. S6) or three bound antibodies. Modeling of the HA packing arrangement indicates that three Fab moieties can be accommodated on each HA trimer without obvious steric constraints from neighboring HA trimers (Fig. 5B).
Fig. 5.
Molecular modeling of an IgG bound to C179 stem epitope between HA spikes and asymmetric HA-C179 reconstruction. (A) Glancing, 5-nm tomographic slice from a tomogram showing the top surface of several unliganded H1N1 influenza virions, the outline of which is indicated by the black circles. (Scale bar: 100 nm.) (B) Top view of HA bound to a surrogate IgG (PDB ID code: 1IGY) (50) on the C179 stem epitope via docking of HA coordinates and a Fab arm of the antibody into the Fab-shaped density of the 3D density map of the HA-C179 complex. The HA-C179 map is blue, with docked H1 HA molecules (PDB ID code: 3LZG) in red (HA1) and blue (HA2), the bound Fab arm of the IgG in cyan, the other Fab arm in magenta, and the Fc region in yellow. The neighboring unliganded H1 HA density map and docked H1 coordinates (PDB ID code: 3LZG) (11) are in green. Side (C) and top (D) views of the non–symmetry-imposed HA-C179 map class, represented as solid and mesh isosurfaces. The map was reconstructed with no symmetry imposed (i.e., asymmetric reconstruction). In D, HA and Fab coordinates are fitted into the asymmetric 3D map of HA-C179. In B, the membrane region (gray in C and D) is not shown for clarity.
HA and HA-C179 Distribution on Viral Surfaces.
To separate any unliganded HA spikes from antibody-bound HA spikes, we used a recently developed procedure for collaborative alignment (33). Our approach involves an iterative joint alignment-classification (JAC) technique, in which the classical similarity measure based on pairwise distances between two particles, or between a particle and a class average, is replaced by a one-to-many collaborative similarity function measured between a particle and a group of particles. This collaborative alignment framework enables robust alignment of correlated heterogeneous images. Mathematically, the collaborative similarity measure can be defined as follows: For n completely aligned and identical d-dimensional data elements (subvolumes), normalized and ordered in a vector form, the matrix obtained by stacking all of the vectors column-wise defined as V = [v1, …, vn] should have a rank of 1. When the subvolumes are not perfectly aligned, the rank of V grows. Thus, aligning all data vectors is equivalent to reducing the rank of V back to 1. The same argument also holds when the data are composed of m different shape classes, with m ≤ n. When the subvolumes are aligned, the rank of V is equal to m, which grows when the alignment is altered. This observation provides a collaborative reference frame for the alignment procedure; that is, the optimal alignment parameters are those that minimize the rank of V. This alignment scheme harnesses contributions from all particles collaboratively rather than using pairwise comparisons. Importantly, because each subvolume is uniquely identified by its location on the viral membrane, we can map unliganded and C179 antibody-bound spikes back to their positions on the viral membrane to determine their spatial distribution.
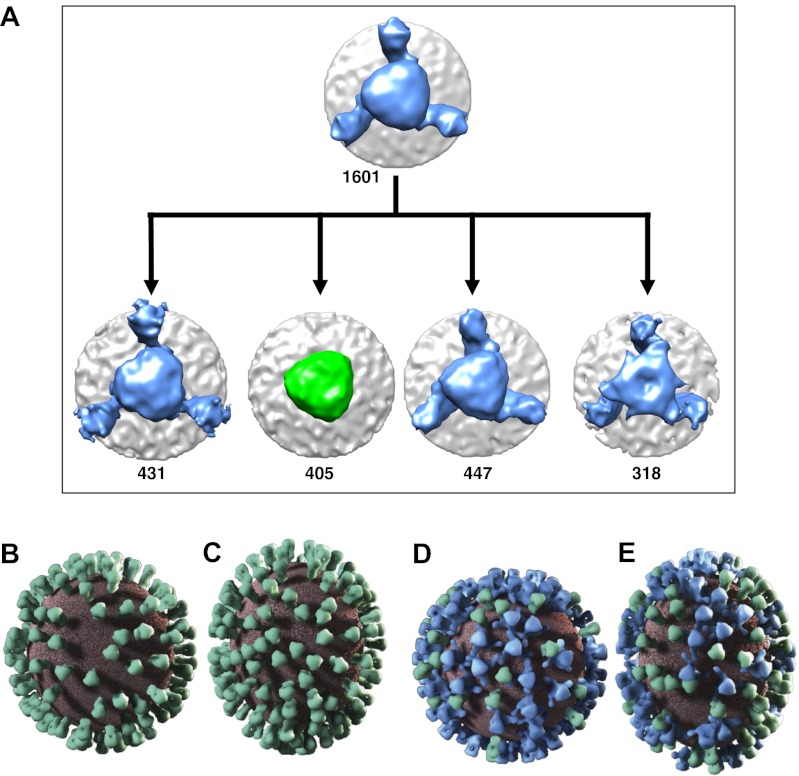
Our analysis revealed that ∼75% of all HA trimers were complexed with the antibody (Fig. 6A), and that both antibody-bound and unliganded HA trimers were dispersed randomly throughout the surface of the viral membrane (Fig. 6 B–E). Our collaborative alignment method invokes threefold averaging, which is not sensitive to substoichiometric occupancy. Thus, we include the illustration of spikes with three bound Fab fragments solely to show their location on the viral membrane and to establish that the majority of HA trimers (>75%) can be bound by antibody. Models for influenza entry into target cells postulate that the contact zone between virus and cell membranes is likely to involve numerous (more than six) HA trimers, with all three protomers in each trimer undergoing a pH-induced conformational change to expose the fusogenic domain in HA2 (34). Thus, the binding of >75% trimers by one or more C179 antibodies could be sufficient to substantially reduce formation of the required constellation of fusion-competent spikes at the virus–cellular membrane interface.
Fig. 6.
Sorting of C179-bound HA molecules and visualization of glycoprotein distribution on viral surfaces. (A) Computational separation of H1N1-C179 dataset spikes into four principal classes, showing that ∼75% of all trimeric HA spikes selected from the surface of the viral membrane are bound to C179 (blue), and ∼25% are unliganded (green). Spikes were selected using automated procedures equally likely to select unliganded and liganded spikes. The actual number of spikes contributing to each class is shown. Three-dimensional models of the distribution of unliganded trimeric HA (green) on spherically shaped virions, with minimal (B) and noticeable (C) levels of elongation from the H1N1 dataset. (D and E) From the H1N1-C179 dataset, 3D models of the distribution of the C179-bound (blue) and unliganded (green) HA spikes on the membrane (brick red), shown at their actual locations on two representative virions. The structures displayed are those of the average density maps for unliganded and C179-bound spikes, respectively.
Discussion
The first 3D structure of the soluble trimers of the HA ectodomain was determined by X-ray crystallography more than 3 decades ago (14). Subsequent studies established that HA architecture and structural features are conserved among HA subtypes (10, 11). Genetic, biochemical, epitope mapping, and vaccine studies with HA are often done in the context of intact influenza virions, yet the results are interpreted in the context of HA ectodomain structures derived by X-ray crystallography. Thus, the extent to which the structure of the soluble, fragmented HA, which lacks transmembrane and cytosolic domains, represents the native, membrane-bound HA trimer has remained an unresolved question. Although the cryoelectron tomography studies that we present here are limited to a resolution of ∼2–3 nm, our determination of the structure of native HA trimers displayed on intact H1N1 virions answers this question by establishing the overall molecular similarity between the quaternary structures of virus-bound and soluble ectodomain HA structures. Our finding that the footprint of the regions on native HA trimers that interact with C179 closely matches the footprints identified by X-ray crystallography for the binding of other stem region antibodies suggests a conserved strategy of different stem antibodies to access this region of HA on native virions. The demonstration of similar structures of the complex on spherical and filamentous virions suggests that stem region antibodies elicited using spherical vaccine platforms (e.g., virus-like particles) are able to target stem regions on filamentous influenza forms that have been observed in influenza isolates for almost 70 years (35–37).
An important conclusion that can be drawn from our structural analysis results is that the majority of virion-bound HA is accessible to a broadly neutralizing anti-stem antibody. Thus, despite the high virion surface density of HA trimers, there is sufficient space for anti-stem antibodies to bind and neutralize the virus by preventing conformational changes essential for mediating entry. Antibody flexibility would promote orientation of the Fc outward and away from the membrane (38, 39), suggesting a possible link between Fc accessibility on native virions and Fc-mediated immune responses that require the binding of Fc receptor and complement proteins to Fc regions of antibody bound to membrane-embedded HA (Fig. S7) (40). One model for HA-mediated influenza entry includes more than six HA trimers under a pH-induced conformational change to expose the fusion peptide of HA2, causing viral and cellular membrane fusion (34). Previous work has indicated that C179 antibody can inhibit cell–cell membrane fusion mediated by influenza HA (25). Thus, even in the absence of complete saturation of (more than six) viral HA trimer groups with antibodies, the randomly distributed binding of antibodies such as C179 to a significant fraction of viral HA trimers will likely prevent formation of a constellation of fusion-competent spikes that need to be oriented toward the virus–cellular membrane interface required for infection. C179 was the first broadly neutralizing antibody identified for influenza HA subtypes (25). Although other stem-specific antibodies were not available for this study, these antibodies share an epitope footprint on helix A of HA2 (Fig. 4). This suggests a relatively conserved strategy for targeting the HA stem. In addition, supplemental analyses of the epitope footprints of different classes of stem antibodies, such as 12D1, FI6V3, and CR8020 (15, 16, 41) that localize to different heights along HA from H3 reinforce the idea that universal influenza vaccines that elicit stem region antibodies could be effective (Fig. S8).
For the most part, structure-based epitope mapping on viral particles has been limited to icosahedral virus particles labeled with Fabs, because standard structural techniques make use of icosahedral symmetry or the assumption that all virus particles are identical (42–44). Fabs have generally been used in structural studies, because intact antibodies tend to cause virus aggregation; however, 3D structural information on the molecular arrangement of viral antigens, such as viral surface glycoproteins, and the disposition of epitopes on intact nonsymmetrical viruses, such as influenza, lags behind that for icosahedral viruses. Here we have used cryoelectron tomography combined with 3D image processing and averaging to investigate 3D structures of trimeric HA on native influenza virions and from immune complexes between neutralizing antibody and virus particles. Our results demonstrate that the locations of conserved regions of HA displayed on the virus can be mapped by structural analysis of virus–antibody complexes. Similar studies with tetrameric NA also may contribute to future efforts to identify and map conserved epitopes within viral NA (Fig. S1). Another exciting prospect is the extension of the cryoelectron tomographic studies reported here to antibodies targeting conserved and variable region epitopes in both stem and head regions of the various HA subtypes on native virions, thereby expanding the mapping of HA epitopes across strains and subtypes.
In summary, our results provide a structural basis for understanding the neutralizing activity of a stem-specific antibody that targets conserved regions between various HA subtypes, and supports vaccine strategies aimed at eliciting robust anti-stem antibody responses against influenza.
Materials and Methods
Virus preparation, antibody purification, and sample preparation for cryoelectron tomography are described in detail in SI Materials and Methods. Tilt series were aligned using automated fiducial-based alignment (45) and reconstructed with dimensions of 2,048 × 2,048 × 512 voxels through R-weighted back-projection as implemented in IMOD (46). Spherical virions and filamentous virion segments within the tomograms were extracted into 4803 voxels. Spike subvolumes (1003 voxels) were automatically extracted from viral surfaces as described previously (47). Alignment, classification, and 3D averaging of the extracted spike volumes were carried out using essentially the same principles as described previously (32, 48). For separation of unliganded and liganded spikes, we used a collaborative alignment and clustering algorithm based on the concept of minimizing matrix rank and its convex surrogate, the nuclear norm (33), to derive the structures of liganded HA complexes. In this approach, the problem of low signal-to-noise ratios is addressed using an algorithm that uses a “collaborative” measure of similarity within the dataset based on the nuclear norm, which retains all data details essential for the alignment without resorting to the use of class averages. A more detailed description of the principles of collaborative alignment and its application to distinguish distinct conformations of HIV envelope glycoproteins is reported in Kuybeda et al. (33). The resolutions of the maps presented here are in the range of ∼25 Å to ∼30 Å (Fig. S2).
Docking of atomic coordinates into density maps was done using UCSF Chimera (49). Molecular models were derived from coordinates of the ectodomain of HA 2009 H1 (PDB ID code: 3LZG) (11) and the ectodomain of HA H5 in complex with Fab CR6261 (PDB ID code: 3GBM) (10) docked into 3D density maps for the unliganded HA and HA-C179 complex, respectively. Residue positions of C179-selected escape mutants from H2 [C179, A/Okuda/57 (H2N2) (25)] were positioned onto the 2009 HA ectodomain structure. Residues were localized by sequence alignment between HAs via GCG-Lite and then by structural marking of residues as spheres on HA crystal structures docked into the 3D density maps of HA-antibody complexes.
To derive the spatial distribution of unliganded and antibody-bound HA trimers, viral membranes were approximated as ellipsoid surfaces based on best fit of XYZ voxel coordinate sets near the viral membrane from the locations of base regions of the spikes. The resulting ellipsoidal coordinate systems were used to produce normal positional vectors for each spike subvolume. Spike subvolumes were subjected to 3D classification, and positions were annotated back onto the ellipsoidal surfaces as either unbound or antibody-bound based on the class identity of each spike. The average HA and HA-C179 maps were placed via positional vectors at unbound and antibody-bound positions, respectively, to produce 3D surface distributions.
Supplementary Material
Acknowledgments
We thank S. Fellini and S. Chacko and their colleagues for continued support of our use of the Biowulf computing cluster at the National Institutes of Health (NIH) (http://biowulf.nih.gov). This work was supported by funds from the intramural program of the National Cancer Institute (to S.S.), the intramural program of the National Institute of Allergy and Infectious Diseases (to K.S.), the Department of Defense (to G.S.), and an NIH Intramural AIDS Research Fellowship (to J.R.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214913110/-/DCSupplemental.
References
- 1.Gething MJ, Bye J, Skehel J, Waterfield M. Cloning and DNA sequence of double-stranded copies of haemagglutinin genes from H2 and H3 strains elucidates antigenic shift and drift in human influenza virus. Nature. 1980;287(5780):301–306. doi: 10.1038/287301a0. [DOI] [PubMed] [Google Scholar]
- 2.Laver WG, Gerhard W, Webster RG, Frankel ME, Air GM. Antigenic drift in type A influenza virus: peptide mapping and antigenic analysis of A/PR/8/34 (HON1) variants selected with monoclonal antibodies. Proc Natl Acad Sci USA. 1979;76(3):1425–1429. doi: 10.1073/pnas.76.3.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell RJ, et al. H1 and H7 influenza haemagglutinin structures extend a structural classification of haemagglutinin subtypes. Virology. 2004;325(2):287–296. doi: 10.1016/j.virol.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 4.Tong S, et al. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci USA. 2012;109(11):4269–4274. doi: 10.1073/pnas.1116200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gamblin SJ, Skehel JJ. Influenza hemagglutinin and neuraminidase membrane glycoproteins. J Biol Chem. 2010;285(37):28403–28409. doi: 10.1074/jbc.R110.129809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taubenberger JK, Morens DM. 1918 Influenza: The mother of all pandemics. Emerg Infect Dis. 2006;12(1):15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garten RJ, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325(5937):197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imai M, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature. 2012;486(7403):420–428. doi: 10.1038/nature10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada S, et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature. 2006;444(7117):378–382. doi: 10.1038/nature05264. [DOI] [PubMed] [Google Scholar]
- 10.Ekiert DC, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324(5924):246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu R, et al. Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science. 2010;328(5976):357–360. doi: 10.1126/science.1186430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bullough PA, Hughson FM, Skehel JJ, Wiley DC. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371(6492):37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 13.Skehel JJ, et al. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci USA. 1982;79(4):968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson IA, Skehel JJ, Wiley DC. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- 15.Corti D, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333(6044):850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 16.Ekiert DC, et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science. 2011;333(6044):843–850. doi: 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sui J, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16(3):265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang TT, et al. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci USA. 2010;107(44):18979–18984. doi: 10.1073/pnas.1013387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei CJ, et al. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science. 2010;329(5995):1060–1064. doi: 10.1126/science.1192517. [DOI] [PubMed] [Google Scholar]
- 20.Whittle JR, et al. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc Natl Acad Sci USA. 2011;108(34):14216–14221. doi: 10.1073/pnas.1111497108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng RH, et al. Nucleocapsid and glycoprotein organization in an enveloped virus. Cell. 1995;80(4):621–630. doi: 10.1016/0092-8674(95)90516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris A, et al. Influenza virus pleiomorphy characterized by cryoelectron tomography. Proc Natl Acad Sci USA. 2006;103(50):19123–19127. doi: 10.1073/pnas.0607614103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Böttcher C, Ludwig K, Herrmann A, van Heel M, Stark H. Structure of influenza haemagglutinin at neutral and at fusogenic pH by electron cryo-microscopy. FEBS Lett. 1999;463(3):255–259. doi: 10.1016/s0014-5793(99)01475-1. [DOI] [PubMed] [Google Scholar]
- 24.Dreyfus C, et al. Highly conserved protective epitopes on influenza B viruses. Science. 2012;337(6100):1343–1348. doi: 10.1126/science.1222908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okuno Y, Isegawa Y, Sasao F, Ueda S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J Virol. 1993;67(5):2552–2558. doi: 10.1128/jvi.67.5.2552-2558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakabe S, et al. A cross-reactive neutralizing monoclonal antibody protects mice from H5N1 and pandemic (H1N1) 2009 virus infection. Antiviral Res. 2010;88(3):249–255. doi: 10.1016/j.antiviral.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, et al. The 2009 pandemic H1N1 neuraminidase N1 lacks the 150-cavity in its active site. Nat Struct Mol Biol. 2010;17(10):1266–1268. doi: 10.1038/nsmb.1909. [DOI] [PubMed] [Google Scholar]
- 28.Smirnov YA, et al. An epitope shared by the hemagglutinins of H1, H2, H5, and H6 subtypes of influenza A virus. Acta Virol. 1999;43(4):237–244. [PubMed] [Google Scholar]
- 29.Calder LJ, Wasilewski S, Berriman JA, Rosenthal PB. Structural organization of a filamentous influenza A virus. Proc Natl Acad Sci USA. 2010;107(23):10685–10690. doi: 10.1073/pnas.1002123107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontana J, Cardone G, Heymann JB, Winkler DC, Steven AC. Structural changes in influenza virus at low pH characterized by cryo-electron tomography. J Virol. 2012;86(6):2919–2929. doi: 10.1128/JVI.06698-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee KK. Architecture of a nascent viral fusion pore. EMBO J. 2010;29(7):1299–1311. doi: 10.1038/emboj.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartesaghi A, et al. Classification and 3D averaging with missing wedge correction in biological electron tomography. J Struct Biol. 2008;162(3):436–450. doi: 10.1016/j.jsb.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuybeda O, et al. A collaborative framework for 3D alignment and classification of heterogeneous subvolumes in cryo-electron tomography. J Struct Biol. 2012 doi: 10.1016/j.jsb.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blumenthal R, Sarkar DP, Durell S, Howard DE, Morris SJ. Dilation of the influenza hemagglutinin fusion pore revealed by the kinetics of individual cell-cell fusion events. J Cell Biol. 1996;135(1):63–71. doi: 10.1083/jcb.135.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choppin PW, Murphy JS, Tamm I. Studies of two kinds of virus particles which comprise influenza A2 virus strains, III: Morphological characteristics: Independence to morphological and functional traits. J Exp Med. 1960;112:945–952. doi: 10.1084/jem.112.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosley VM, Wyckoff RW. Election micrography of the virus of influenza. Nature. 1946;157:263. doi: 10.1038/157263a0. [DOI] [PubMed] [Google Scholar]
- 37.Lakdawala SS, et al. Eurasian-origin gene segments contribute to the transmissibility, aerosol release, and morphology of the 2009 pandemic H1N1 influenza virus. PLoS Pathog. 2011;7(12):e1002443. doi: 10.1371/journal.ppat.1002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saphire EO, et al. Contrasting IgG structures reveal extreme asymmetry and flexibility. J Mol Biol. 2002;319(1):9–18. doi: 10.1016/S0022-2836(02)00244-9. [DOI] [PubMed] [Google Scholar]
- 39.Tran EE, et al. Structural mechanism of trimeric HIV-1 envelope glycoprotein activation. PLoS Pathog. 2012;8(7):e1002797. doi: 10.1371/journal.ppat.1002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terajima M, et al. Complement-dependent lysis of influenza a virus-infected cells by broadly cross-reactive human monoclonal antibodies. J Virol. 2011;85(24):13463–13467. doi: 10.1128/JVI.05193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang TT, et al. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog. 2010;6(2):e1000796. doi: 10.1371/journal.ppat.1000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker TS, Olson NH, Fuller SD. Adding the third dimension to virus life cycles: Three-dimensional reconstruction of icosahedral viruses from cryo-electron micrographs. Microbiol Mol Biol Rev. 1999;63(4):862–922. doi: 10.1128/mmbr.63.4.862-922.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris A, et al. Epitope diversity of hepatitis B virus capsids: quasi-equivalent variations in spike epitopes and binding of different antibodies to the same epitope. J Mol Biol. 2006;355(3):562–576. doi: 10.1016/j.jmb.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 44.Prasad BV, Burns JW, Marietta E, Estes MK, Chiu W. Localization of VP4 neutralization sites in rotavirus by three-dimensional cryo-electron microscopy. Nature. 1990;343(6257):476–479. doi: 10.1038/343476a0. [DOI] [PubMed] [Google Scholar]
- 45.Amat F, et al. Markov random field based automatic image alignment for electron tomography. J Struct Biol. 2008;161(3):260–275. doi: 10.1016/j.jsb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116(1):71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 47.White TA, et al. Molecular architectures of trimeric SIV and HIV-1 envelope glycoproteins on intact viruses: Strain-dependent variation in quaternary structure. PLoS Pathog. 2010;6(12):e1001249. doi: 10.1371/journal.ppat.1001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frank GA, et al. Computational separation of conformational heterogeneity using cryo-electron tomography and 3D sub-volume averaging. J Struct Biol. 2012;178(2):165–176. doi: 10.1016/j.jsb.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 50.Harris LJ, Skaletsky E, McPherson A. Crystallographic structure of an intact IgG1 monoclonal antibody. J Mol Biol. 1998;275(5):861–872. doi: 10.1006/jmbi.1997.1508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.