Abstract
Complex interactions between periphery and the brain regulate food intake in mammals. Cannabinoid type-1 (CB1) receptor antagonists are potent hypophagic agents, but the sites where this acute action is exerted and the underlying mechanisms are not fully elucidated. To dissect the mechanisms underlying the hypophagic effect of CB1 receptor blockade, we combined the acute injection of the CB1 receptor antagonist rimonabant with the use of conditional CB1-knockout mice, as well as with pharmacological modulation of different central and peripheral circuits. Fasting/refeeding experiments revealed that CB1 receptor signaling in many specific brain neurons is dispensable for the acute hypophagic effects of rimonabant. CB1 receptor antagonist-induced hypophagia was fully abolished by peripheral blockade of β-adrenergic transmission, suggesting that this effect is mediated by increased activity of the sympathetic nervous system. Consistently, we found that rimonabant increases gastrointestinal metabolism via increased peripheral β-adrenergic receptor signaling in peripheral organs, including the gastrointestinal tract. Blockade of both visceral afferents and glutamatergic transmission in the nucleus tractus solitarii abolished rimonabant-induced hypophagia. Importantly, these mechanisms were specifically triggered by lipid-deprivation, revealing a nutrient-specific component acutely regulated by CB1 receptor blockade. Finally, peripheral blockade of sympathetic neurotransmission also blunted central effects of CB1 receptor blockade, such as fear responses and anxiety-like behaviors. These data demonstrate that, independently of their site of origin, important effects of CB1 receptor blockade are expressed via activation of peripheral sympathetic activity. Thus, CB1 receptors modulate bidirectional circuits between the periphery and the brain to regulate feeding and other behaviors.
Keywords: fear and anxiety, sympathetic system
Appropriate feeding responses are determined by complex cross-talks between the central nervous system and peripheral organs (1). The endocannabinoid system (ECS) is an important modulator of central and peripheral regulation of energy metabolism (2, 3). In the brain, endogenous and exogenous cannabinoids oppositely regulate food intake, according to the neuronal population involved (4). On the other hand, the cannabinoid type-1 (CB1) receptor antagonist rimonabant (SR141716) acutely induces hypophagia (5), but the sites and the mechanisms involved are not well defined yet.
CB1 receptors control the activity of many neurotransmitter systems involved in central regulation of food intake (2). However, the ECS also regulates food intake and energy balance via peripheral mechanisms (2, 3). Few studies have suggested that CB1 receptor signaling could regulate food intake at peripheral sites (2, 6, 7). Fasting triggers the synthesis of gastrointestinal endocannabinoids, and endocannabinoids produced in the gastrointestinal tract may regulate food (mainly fat) intake (6). The peripheral sympathetic nervous system (SNS) is one of the main mechanisms engaged by CB1 receptor-dependent signaling for the modulation of energy balance (8) and genetic or pharmacological CB1 receptor blockade increases plasma levels of norepinephrine (8, 9). In turn, SNS activity regulates meal patterns (10) and is oppositely regulated by the organism’s energy status, being decreased by fasting and increased by feeding (11). Finally, visceral afferents also play an important role in transmitting nutrient-derived intestinal signals to the brainstem, where glutamate and NMDA receptors modulate visceral sensory signaling pathways, ultimately regulating food intake (12). Interestingly, the interplay between SNS and brainstem glutamatergic activity also regulates other brain functions, such as fear and anxiety responses (13), and alterations in these functions represent the main side-effects of rimonabant use in humans (14).
In this study, we systematically investigated the potential sites and the mechanisms of the acute and rapid hypophagic action of rimonabant under fasting/refeeding, as well as its central effects on fear- and anxiety-like behaviors.
Results
CB1 Receptors in Different Brain Neuron Types Are Dispensable for the Rapid Hypophagic Effect of Rimonabant.
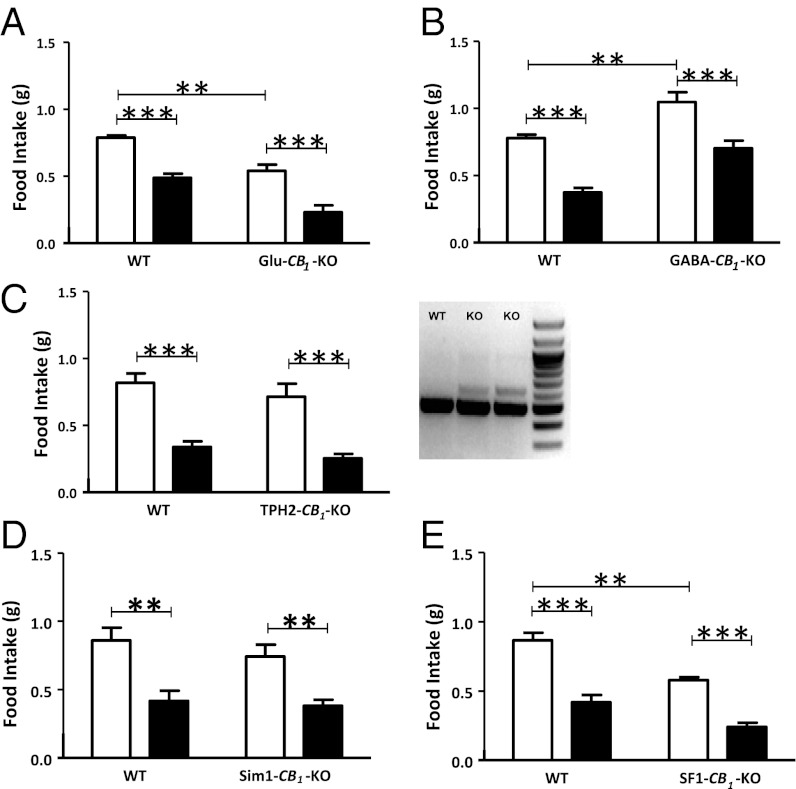
The acute hypophagic effect of rimonabant depends on CB1 receptors expressed in neurons (8). Thus, we tried to identify the exact neuronal population involved in this phenomenon. Rimonabant bore no effect on fasting-induced food intake in constitutive CB1-KO (5, 15) and in conditional mutant mice lacking CB1 receptor expression in forebrain and sympathetic neurons (8, 16) (CaMK-CB1-KO) (Fig. S1 A and B). Mice characterized by the deletion of CB1 receptors, either from cortical glutamatergic neurons (Glu-CB1-KO) or from GABAergic neurons (GABA-CB1-KO), respectively (17, 18), oppositely respond to fasting-induced food intake (4). Thus, CB1 receptors expressed on inhibitory or cortical excitatory brain neurons might mediate rimonabant hypophagic effect. Vehicle-treated Glu-CB1-KO ate less than their WT littermates (Fig. 1A), whereas GABA-CB1-KO displayed a hyperphagic phenotype (Fig. 1B) (4). Surprisingly, however, rimonabant administration similarly reduced food intake in WT, Glu-CB1-KO (Fig. 1A), and GABA-CB1-KO mice (Fig. 1B), with no significant interaction between the factors “genotype” and “treatment.”
Fig. 1.
The hypophagic effect of rimonabant does not depend on CB1 expression in brain neurons. Effect of rimonabant (3 mg/kg, i.p., black bars) or vehicle (white bars) in (A) mutant Glu-CB1-KO mice; (B) GABA-CB1-KO mice; (C) TPH2-CB1-KO mice; (D) Sim1-CB1-KO mice; (E) SF1-CB1-KO mice, and respective WT littermates. (C, Right) PCR on genomic DNA extracted from dorsal raphe of WT and TPH2-CB1-KO mice (KO). CB1-floxed allele (Lower); deleted CB1 allele (Upper). Data are means ± SEM. n = 6–9 mice per group. Statistics by two-way ANOVA followed by Bonferroni’s posttest. **P < 0.01, ***P < 0.001.
WT and TPH2-CB1-KO littermates (19), in which the CB1 gene is deleted in the raphe nucleus but not in other brain regions (Fig. 1C and Fig. S1C), consumed a comparable amount of food and showed a similar acute response to rimonabant under fasting-refeeding conditions (Fig. 1C), suggesting that blockade of CB1 receptor signaling on serotonergic neurons is dispensable for rimonabant-induced hypophagia.
The hypothalamus is a key brain region for the regulation of feeding (1), and the hypothalamic ECS activity is tightly controlled by the nutritional state of the organism (2). The paraventricular (PVN) and ventromedial (VMH) nuclei are among the hypothalamic areas where CB1 receptors likely modulate food intake (2).
Sim1-CB1-KO mice carry deletion of the CB1 gene in the PVN (Fig. S1D) and possibly in few scattered neurons located in other brain regions (e.g., basomedial amygdala, bed nucleus of the stria terminalis, ventral periaqueductal gray) and in some cells of the kidney (20). The mutant mice responded similarly to their WT littermates to fasting-induced food intake and to rimonabant treatment (Fig. 1D). Thus, CB1 receptor expression in the PVN is not necessary for the endogenous control of fasting-induced food intake, or for the pharmacological effect of CB1 receptor blockade.
To test the role of CB1 receptors in VMH neurons, we crossed CB1-floxed mice with SF1-Cre mice, characterized by the expression of the recombinase under the regulatory sequences of the steroidogenic factor-1 (SF1), leading to recombination in the VMH, pituitary gland, and gonads (21). SF1-CB1-KO mice displayed ∼80% CB1 receptor deletion in the VMH (Fig. S1E) and showed a reduced fasting-induced hyperphagia (Fig. 1E). However, the hypophagic effect of rimonabant was still present (Fig. 1E). Thus, VMH CB1 receptors are required for the endogenous control of fasting-induced food intake, but they are dispensable for the hypophagic effect of rimonabant.
Blockade of Peripheral β-Adrenergic Receptors Prevents Rimonabant-Induced Hypophagia in Fasting and Lipoprivic Conditions.
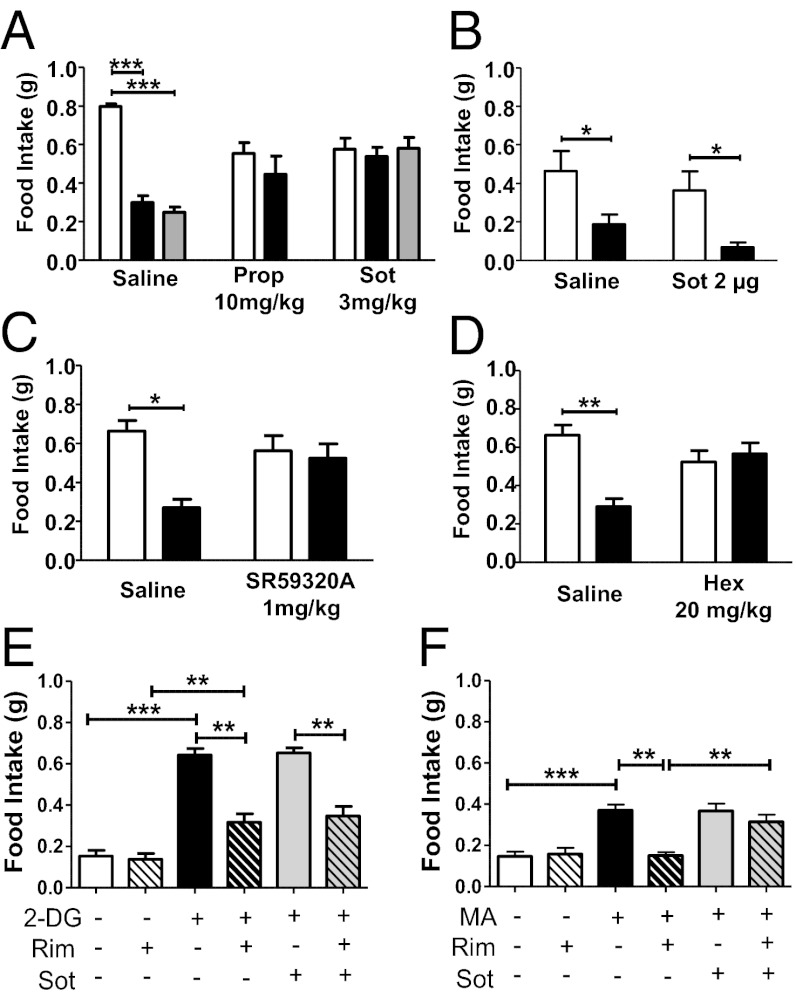
The SNS plays an important role in the regulation of food intake, particularly through the activation of peripheral β-adrenergic receptors (22, 23), and CB1 receptors can inhibit central and peripheral norepinephrine (NE) release (24, 25). The systemic injection of the generic β-blocker propranolol induced a slight and nondose-dependent reduction of food intake in fasted mice (Fig. 2A and Fig. S2A). Interestingly, however, propranolol (10 mg/kg) fully blocked the hypophagic effect of rimonabant (Fig. 2A), suggesting that activation of β-adrenergic receptors is necessary for the acute anorectic effect of rimonabant. To distinguish between central and peripheral effects of β-adrenoreceptors blockade, we systemically pretreated C57BL/6N mice with the peripherally restricted β-blocker sotalol (Fig. S2B) (26). Sotalol pretreatment (3 mg/kg) fully prevented the hypophagic effect of 3 and 10 mg/kg rimonabant (Fig. 2A). The central administration of sotalol (2 μg, intracerebroventricularly) did not affect rimonabant-induced hypophagia (Fig. 2B), confirming that the modulation of peripheral, but not central, β-adrenergic neurotransmission is involved in the hypophagic effect of rimonabant. Pretreatment with the selective β3-adrenoreceptor antagonist SR59230A (1 mg/kg) (27) or the ganglionic blocker hexamethonium (28) both abolished rimonabant-induced hypophagia (Fig. 2 C and D), suggesting that the activation of sympathetic ganglia and of β3-adrenoreceptors is necessary for rimonabant-induced hypophagia.
Fig. 2.
Blockade of peripheral β-adrenergic receptors prevents rimonabant-induced hypophagia. Effect of rimonabant (black bars, 3 mg/kg, i.p.; gray bars, 10 mg/kg, i.p.) or its vehicle (white bars) in fasted mice pretreated with (A) the β-blocker propranolol (Prop, 10 mg/kg, i.p.) or with the peripherally restricted β-blocker sotalol (Sot, 3 mg/kg, i.p.); (B) the β-blocker sotalol administered centrally (2 μg intracerebroventricularly); (C) the selective β3-blocker SR59320A (1 mg/kg, i.p.); (D) the ganglionic blocker hexamethonium (Hex, 20 mg/kg, i.p.). (E) Effect of β-blocker sotalol (3 mg/kg, i.p.) and rimonabant (Rim, 3 mg/kg, i.p.) in conditions of glucoprivation induced by 2-DG injection (250 mg/kg, i.p.). (F) Effect of β-blocker sotalol (3 mg/kg, i.p.) and rimonabant (3 mg/kg, i.p.) in conditions of lipoprivation induced by MA injection (68 mg/kg, i.p.). Data are means ± SEM. n = 5–8 mice per group. Statistics by two-way ANOVA followed by Bonferroni’s posttest. *P < 0.05, **P < 0.01, ***P < 0.001.
SNS activity affects the use of substrates and the metabolic pathways engaged in signaling nutrient availability (11). To investigate whether the SNS-dependent effect of rimonabant might modulate specific food intake induced by acute glucose or lipid-deprivation, we administered rimonabant together with 2-deoxy-glucose (2-DG), an inhibitor of glucose utilization (29), or mercaptoacetate (MA), an inhibitor of fatty acid oxidation (30). Rimonabant partially reversed 2-DG–induced hyperphagia (Fig. 2E) but it fully prevented MA-triggered feeding (Fig. 2F). Moreover, sotalol did not alter the effect of rimonabant on 2-DG–induced hyperphagia (Fig. 2E), but it did block the effect of rimonabant on MA-induced feeding (Fig. 2F). Thus, the activation of peripheral β-adrenergic receptors specifically mediates the CB1 receptor-dependent inhibition of hyperphagia induced by lipoprivation, but not that elicited by glucoprivation. According to this hypothesis, we measured the levels of plasma free fatty acids, which is a well-established marker of SNS-driven lypolisis (31). As observed in obese rats by others (9), rimonabant injection markedly increased this parameter only in conditions of food availability, such as free-feeding and refeeding (Fig. S2C).
CB1 Receptor Blockade Enhances Gastrointestinal Activity Through an Increase in β-Adrenergic Signaling.
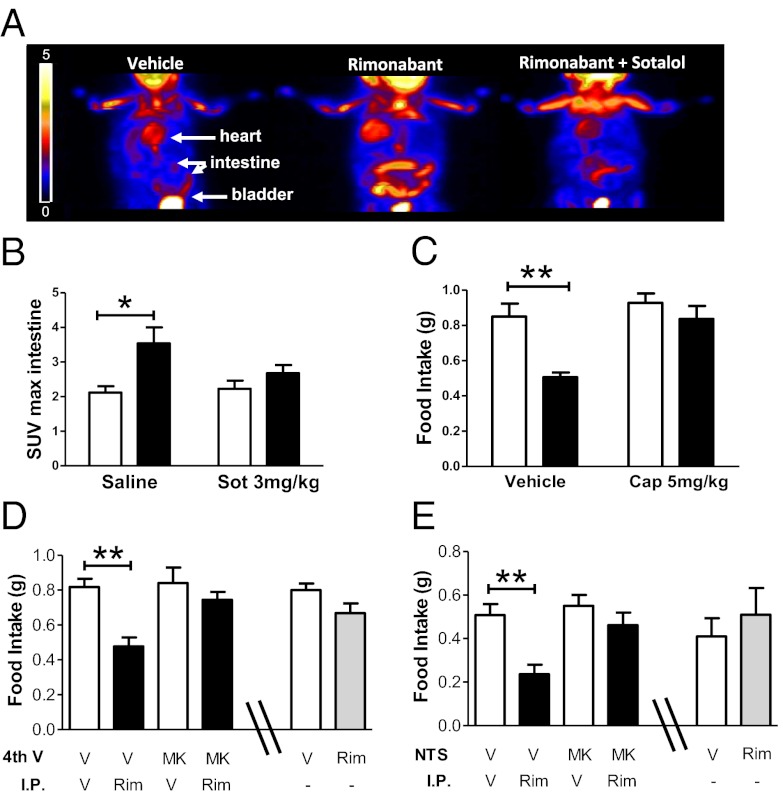
Gastrointestinal lipid-sensing mechanisms are mainly responsible for the hyperphagia induced by the inhibition of fatty-acid oxidation (30). Thus, we tested whether rimonabant-induced hypophagia is associated with functional metabolic changes in the gastrointestinal tract. Accordingly, rimonabant increased metabolism in the gut of food-deprived mice in a sotalol-dependent manner (Fig. 3 A and B). We further analyzed markers of the G protein-coupled β-adrenergic signaling (32), such as protein kinase A (PKA) activity and cAMP levels, in the duodenum. Western blot analysis for the phosphorylated substrates of PKA showed that rimonabant administration increases PKA activity (Fig. S3A). Despite the heterogeneity of the bands, semiquantitative evaluation revealed that rimonabant increased overall PKA-dependent phosphorylation by ∼70%, 120%, and 1,000% in fed, fasted, and refed conditions, respectively (Fig. S3A). These results were in agreement with increased cAMP levels in the same samples, as measured by ELISA (Fig. S3B). Quantitative RT-PCR analysis revealed that the expression levels of β1- and β2-adrenoreceptor subtypes were not altered by rimonabant treatment, whereas CB1 receptor blockade specifically reduced β3-adrenoreceptor mRNA expression in the duodenum (Fig. S3C). In line with several reports showing that increased sympathetic activity is able to rapidly and strongly decrease β3-adrenoreceptor mRNA both in vitro and in vivo (33–35), this effect has been proposed as a mechanism of receptor desensitization and is mediated by a cAMP-dependent process of transcriptional repression (36). Thus, increased β-adrenergic signaling in the duodenum seems to be the main event responsible for the β3-adrenoreceptor–mediated hypophagic effect of rimonabant. To further confirm the activation of peripheral SNS under our study conditions, we analyzed different biochemical parameters also in the brown adipose tissue (BAT), an organ whose functions are critically under the control of the SNS (37). As in the gastrointestinal tract, rimonabant administration was able to increase glucose uptake and metabolic activity also in the BAT (Fig. S3D). Rimonabant action is most likely mediated by β-adrenergic receptor activation, as it was blocked by sotalol cotreatment (Fig. S3D). Furthermore, rimonabant increased PKA activity in this tissue when fasted animals were re-exposed to food (Fig. S3E). Direct measurement of NE uptake by PET imaging of 11C-metahydroxyephedrine, a NE analog (8, 38), revealed that rimonabant increased tracer uptake in the BAT of both free-fed and fasted animals (Fig. S3F). Taken together, these data show that rimonabant increases noradrenergic tone in peripheral tissues.
Fig. 3.
CB1 receptor blockade enhances gastrointestinal activity and increases glutamatergic transmission in the caudal brainstem trough vagal innervation to reduce food intake. (A) Three-dimensional whole-body small animal PET images showing 18F-FDG uptake in the intestine of mice treated with vehicle, rimonabant (10 mg/kg, i.p.), or rimonabant + sotalol (3 mg/kg, i.p.). Gradation bar, signal intensity expressed as radioactive counts. (B) Quantification [standard uptake value (SUV) max of 18F-FDG uptake] of data in A. Black bars, rimonabant; white bars, vehicle. (C) Effect of capsaicin-induced deafferentiation on rimonabant- (3 mg/kg, i.p., black bars) or vehicle- (white bars) induced changes in food intake. (D) Effect of the combination of fourth-ventricle injection of MK801 (MK, 2 µg in 2 µL) and rimonabant (Rim, 3 mg/kg, i.p., black bars) or their respective vehicles (white bars) on food intake. The effects of a direct injection of vehicle (V, white bar) or rimonabant (Rim, 10 µg, gray bar) in the fourth ventricle are presented in the last two bars of the graph. (E) Effect of the combination of NTS bilateral injection of MK801 (0.2 µg in 0.2 µL) and rimonabant (3 mg/kg, i.p., black bars) or their respective vehicles (white bars) on food intake. The effects of a direct bilateral injection of vehicle (V, white bar) or rimonabant (Rim 1 µg per site, gray bar) in the NTS are presented in the last two bars of the graph. Data are means ± SEM. n = 4–12 mice per group. Statistics by two-way ANOVA followed by Bonferroni’s posttest. *P < 0.05, **P < 0.01.
Capsaicin-sensitive fibers of the abdominal vagus nerve are known to contribute to both satiety and hyperphagia, particularly when the latter is induced by administration of fatty acid oxidation inhibitors, such as MA (30, 39, 40). To investigate whether vagal sensitive fibers may contribute to the anorectic effect of CB1 receptor blockade, we pretreated mice with a low dose of capsaicin (5 mg/kg) 1 wk before the experiment. This dose of capsaicin fully blocked cholecystokinin (CCK)-induced hypophagia, but did not alter eye wiping (Fig. S4 A and B) (41, 42). Thus, this treatment effectively disrupted vagal signaling, but spared primary sensory reflexes (41, 42). Interestingly, as previously shown with higher doses (7), capsaicin treatment blunted the hypophagia induced by rimonabant (Fig. 3C), suggesting a primary role of vagal transmission in the hypophagic effect of CB1 receptor antagonism.
Glutamatergic Transmission in the Nucleus Tractus Solitarii Mediates Rimonabant-Induced Hypophagia.
Glutamatergic transmission in the caudal brainstem is an important mechanism engaged by satiety signals derived from visceral afferents (12) and plays a specific role in feeding induced by lipoprivation (43, 44). Thus, it can be expected that glutamatergic transmission in the brainstem is critically involved in rimonabant-induced hypophagia.
Injection of the NMDA receptor antagonist MK801 (MK) into the fourth ventricle or directly into the nucleus tractus solitarii (NTS) fully prevented rimonabant effect on food intake (Fig. 3 D and E, and Fig. S4C). This effect was not a result of a local enhancing action of rimonabant on glutamatergic transmission, because the microinjection of the drug into the fourth ventricle or into the NTS failed to alter food intake (Fig. 3 D and E).
Peripheral β-Adrenergic Signaling Mediates the Effects of Rimonabant on Conditioned Freezing and Anxiety-Like Behaviors.
After determining that peripheral β-adrenergic signaling mediates the acute hypophagic effect of rimonabant, we wondered whether such a mechanism might also underpin other behavioral effects of CB1 antagonism. Indeed, brain functions, like fear and anxiety responses, are regulated by interactions between the SNS and brainstem glutamatergic activity (13), and alterations in these functions represent the main side-effects of rimonabant use in humans (14). For example, the increase in SNS activity induced by rimonabant may be partly responsible of the drug’s effect on conditioned freezing in fear-conditioning experiments (15). Rimonabant increased freezing of conditioned C57BL/6-N mice (Fig. 4A), and this effect was significantly impaired by sotalol pretreatment (Fig. 4A), suggesting that peripheral β-adrenergic signaling is involved in the effects of CB1 receptor blockade on fear-induced freezing responses. Given the proposed role of noradrenergic transmission in anxiety disorders (45), we wondered if the anxiogenic effect of rimonabant (46) also depends on SNS activity. Systemic sotalol pretreatment prevented the anxiogenic effect of both systemic (Fig. 4B and Fig. S5A) and central (Fig. 4C and Fig. S5B) CB1 receptor blockade in the elevated-plus-maze test, without altering locomotor activity (Fig. S5).
Fig. 4.
Peripheral β-adrenergic signaling mediates the effects of rimonabant on fear-induced freezing and anxiety. (A) Effects of sotalol (3 mg/kg) and rimonabant (10 mg/kg) on fear-induced freezing. (Left) Time-course (1-min bins) over the 8-min tone presentation; (Right) total freezing. (B) Effects of sotalol (Sot, 3 mg/kg) and systemic rimonabant (Rim, 10 mg/kg) on the elevated-plus-maze test. (C) Effects of sotalol (3 mg/kg) and central rimonabant (10 µg intracerebroventricularly) on the elevated-plus-maze test. Data are means ± SEM. n = 8–10 mice per group. Statistics by using one- or two-way ANOVA followed by Bonferroni’s posttest. *P < 0.05; **P < 0.01.
Discussion
An incessant cross-talk between periphery and brain guarantees proper control of energy balance. Our study reveals that the acute hypophagic effect of the CB1 receptor antagonist rimonabant in fasting-refeeding experiments does not primarily depend on CB1 receptors expressed in a wide range of brain neuron types. Conversely, modulation of SNS activity, activation of the gastrointestinal tract, and afferent stimulation of glutamatergic transmission in the NTS appear to be necessary mechanisms for the suppression of fasting- or lipoprivic-induced food intake caused by rimonabant. Furthermore, increase in peripheral β-adrenergic transmission seems to account for other behavioral effects of systemic and central pharmacological CB1 receptor blockade, such as increased fear-induced responses and anxiety-like behaviors.
Deletion of CB1 receptor in GABAergic or cortical glutamatergic neurons strongly affects the (endo)cannabinoid control of stimulated food intake (4). However, acute rimonabant injection maintained its effect in these two mutant lines, suggesting that CB1 receptor-dependent control of glutamatergic or GABAergic transmission is not involved in the hypophagia caused by pharmacological CB1 receptor blockade. Furthermore, although CB1 receptors are present in serotonergic neurons (47, 48), their expression is not required for the hypophagic effect of rimonabant, in line with the additive, but not synergistic effects of CB1 receptor blockade and serotonin reuptake inhibition (2, 49). The hypothalamus is a key structure for endocannabinoid-mediated control of energy balance and food intake (2). CB1 receptors are expressed in several hypothalamic nuclei where they can regulate both glutamatergic and GABAergic transmission (50), and expression and action of both orexigenic and anorexigenic hypothalamic neuropeptides (2, 51).
Among the hypothalamic nuclei, the VMH and the PVN are generally considered to exert food intake suppressant functions (1). The well-known inhibitory role of the ECS on neurotransmission would, therefore, find a logical mechanism of action in regulating the activity of PVN and VMH neurons to eventually increase food intake. Our data suggest that this finding holds true for VMH neurons, but not for PVN ones. Mice lacking CB1 receptors in the VMH displayed a reduced fasting-induced food intake, whereas mice lacking CB1 receptor in PVN neurons failed to show any phenotype. Nevertheless, CB1 receptor deletion in these nuclei did not alter rimonabant-induced acute hypophagia. The phenotype of SF1-CB1-KO mice is very similar to the one of Glu-CB1-KO one (4), as both these mutant mouse lines display reduced stimulated food intake, but normal response to rimonabant. We recently showed that virally induced partial deletion of the CB1 gene in the hypothalamus did not alter the rapid hypophagic effect of rimonabant 1 h after refeeding in fasted mice, although the drug did not decrease 24-h food intake (52). Thus, hypothalamic mechanisms may be involved in long-term effects of rimonabant, but not in the rapid hypophagic properties of acute rimonabant administration. A key difference between CaMK-CB1-KO mice (not responding to rimonabant) and all of the other mouse lines used in this study is the deletion of CB1 expression in a subset of peripheral sympathetic neurons (8). CB1 receptor activation inhibits peripheral noradrenergic transmission (24) and genetic deletion and pharmacological blockade of CB1 receptors increase the levels of circulating noradrenaline (8, 9). In turn, SNS activity inhibits food intake (22, 23), and specific blockade of peripheral, but not central, β-adrenoreceptors blunted rimonabant-induced hypophagia. Thus, we propose that rimonabant-induced reduction of food intake is because of an increase in SNS activity. At this stage, however, we cannot conclude whether this SNS activation is a result of direct blockade of CB1 receptors at SNS terminals or because of simultaneous upstream effects of the drug.
Activation of β-adrenergic receptors abolishes lipoprivic-induced hyperphagia (53) and enterocytes might specifically translate fatty-acid oxidation into a vagal afferent signal to control food intake (30). Here, we demonstrate that rimonabant blocks fasting- and lipoprivic-induced food intake by engaging sympathetic transmission in peripheral tissues, including the gastrointestinal tract.
Deafferentiation of vagal afferents by capsaicin pretreatment abolishes satiety signals (refs. 12 and 42, and present results), likely through increase of NMDA signaling in the NTS (12). Similar mechanisms are likely involved in rimonabant-induced hypophagia, which requires intact abdominal capsaicin-sensitive fibers (ref. 7 and present results) and NMDA receptor activation in the NTS (present results). Vagal afferents contain CB1 receptors (54). However, it is unlikely that rimonabant directly acts on vagal terminals in the NTS to reduce fasting-induced food intake. First, local injection of rimonabant into the NTS and the fourth ventricle did not acutely alter food intake. Second, CaMK-CB1-KO mice, where rimonabant effects are abolished, still contain normal levels of CB1 receptors in the vagal nodose ganglion (8). Conversely, our data suggest that the increased NMDA receptor signaling in the NTS is indirectly mediated by the rimonabant-induced increase of peripheral sympathetic adrenergic transmission. Interestingly, surgical and chemical lesions of vagal afferents, and lesions of sensory terminals in the NTS specifically abolish lipoprivic-induced feeding and Fos expression in the NTS (40, 43, 55). Thus, rimonabant likely exerts its hypophagic action by specifically interfering with a periphery-to-brain mechanism engaged to encode signals related to the use of fatty-acid substrates and availability. This conclusion is in agreement with recent evidence suggesting that gut-derived endocannabinoids exert a critical control over fat intake (6).
Our data suggest that systemic pharmacological CB1 receptor blockade under fasting or lipoprivic conditions (i) directly or indirectly increases SNS activity (particularly in the gastrointestinal tract), eventually leading to (ii) activation of afferent fibers sending glutamatergic projections to the NTS, where (iii) increased NMDA neurotransmission triggers the decrease of food intake (27, 56). At this stage, we cannot determine which downstream events follow the rimonabant-induced activation of NTS neurons to decrease food intake. However, NTS signaling is known to engage several brain regions to modulate food intake, including hypothalamic circuits and dopaminergic transmission in limbic systems controlling food-related reward (2, 57–59).
Apart from the rapid modulation of food intake, rimonabant administration induces many additional behavioral and neuropsychiatric effects in animals and humans (14, 26). In particular, rimonabant increases freezing responses to conditioned cued fear stimuli in rodents (15). Interestingly, conditioned freezing shares similar β-adrenergic–dependent mechanisms (13). Our findings surprisingly suggest that rimonabant-induced freezing requires increased peripheral SNS activation. Interestingly, blockade of peripheral β-adrenergic transmission is also able to prevent the anxiogenic effect of systemic rimonabant injections. In addition, when rimonabant treatment is restricted to the central nervous system (intracerebroventricularly), its anxiogenic action still requires active peripheral β-adrenergic transmission. These data indicate that CB1 receptor blockade is able to act centrally to increase peripheral noradrenaline release, and this may in turn lead to increased freezing and anxiety. This theory can be explained by two main sets of observations: (i) increased SNS activity is positively correlated with an increased anxiety status in both humans and laboratory animals (45); and (ii) the nutritional status strongly influences mood and behavior, in particular anxiety and fear. For example, the vagal satiety mediator CCK is also able to increase anxiety via vagal signaling (60). This result is likely because of the widespread expression of CCK receptors in the central nervous system (61), as well as to an increase in noradrenergic-projection signaling, after vagal activation, from the NTS to key brain regions involved in the control of emotional behaviors, such as the PVN, the bed nucleus of stria terminalis, and the basolateral amygdala (62). Hence, rimonabant action in the central nervous system may be a means of increasing peripheral noradrenergic tone and of CCK-mediated vagal transmission (63), thus changing the perception of the nutritional status. The synergistic actions of these two mediators likely result in increased fear and anxiety behavior, as observed after acute rimonabant administration in our experimental setting. Thus, this finding leads us to conclude that the behavioral and hypophagic actions of rimonabant described herein share strong functional connections.
These data reveal a specific CB1 receptor-dependent circuit linking peripheral lipid-sensing mechanisms to brain activity to modulate food intake in mammals through the SNS. Other behavioral effects of rimonabant, such as those on fear and anxiety, share similar neurobiological substrates. Taken together, the present findings pinpoint a unique CB1 receptor-dependent bidirectional cross-talk between brain and periphery for the regulation of feeding and anxiety-related behaviors.
Experimental Procedures
Experimental procedures are described in SI Experimental Procedures. Procedures discussed are the conditional CB1R mutant mice used, behavioral procedures, local and systemic pharmacological injections, biochemical experiments, and data analysis (for statistics, see Table S1). All animal procedures were conducted in accordance with standard ethical guidelines (European Communities Directive 86/60-EEC) and were approved by the Committee on Animal Health and Care of Institut National de la Santé et de la Recherche Médicale and French Ministry of Agriculture and Forestry (authorization A3310035).
Supplementary Material
Acknowledgments
We thank N. Aubailly, D. Gonzales and the personnel of the Animal and Genotyping Facilities of the NeuroCentre Magendie for mouse caring and genotyping; S. K. Nave, J. Rubenstein, M. Ekker, G. Schütz, T. Lemberger for providing Cre-expressing mice; and F. Georges and all the members of the G.M. laboratory for valuable suggestions. This work was supported in part by the European Research Council ENDOFOOD, ERC-2010-StG-260515 (to G.M.); Institut National de la Santé et de la Recherche Médicale (G.M. and D.C.); the European Foundation for the Study of Diabetes-Sanofi Aventis (D.C. and G.M.); Aquitaine Region (D.C. and G.M.); EU-FP7 REPROBESITY, HEALTH-F2-2008-223713 (to G.M., B.L., and U.P.); EU-FP7 NEUROFAST FPVII-KBBE-2009-3-245009) (to U.P.); Ministero dell'Istruzione, dell'Università e della Ricerca--Progetti di Rilevante Interesse Nazionale (MIUR–PRIN) 2007 (to U.P.); FP7-People2009-IEF-251494 (to D.C. and E.B.); Institut National de la Santé et de la Recherche Médicale/Aquitaine Region PhD Fellowship Programme (to P.C.); the Fondation pour la Recherche Medicale (G.M.); Spanish Ministerio de Economía y Competitividad (MINECO), SAF2010-22198-C02-01 (to M.M.-F.) and SAF2012-35759 (to M.G.); Comunidad de Madrid S2010/BMD-2353 (to M.M.-F.) and S2010/BMD-2308 (to M.G.); and an European Molecular Biology Organization Long-Term Fellowship (to L.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218573110/-/DCSupplemental.
References
- 1.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 2.Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27(1):73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- 3.Tam J, et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16(2):167–179. doi: 10.1016/j.cmet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellocchio L, et al. Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci. 2010;13(3):281–283. doi: 10.1038/nn.2494. [DOI] [PubMed] [Google Scholar]
- 5.Di Marzo V, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410(6830):822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 6.DiPatrizio NV, Astarita G, Schwartz G, Li X, Piomelli D. Endocannabinoid signal in the gut controls dietary fat intake. Proc Natl Acad Sci USA. 2011;108(31):12904–12908. doi: 10.1073/pnas.1104675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gómez R, et al. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22(21):9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quarta C, et al. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 2010;11(4):273–285. doi: 10.1016/j.cmet.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Mølhøj S, et al. Effect of the cannabinoid receptor-1 antagonist rimonabant on lipolysis in rats. Eur J Pharmacol. 2010;646(1-3):38–45. doi: 10.1016/j.ejphar.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Fu J, et al. Sympathetic activity controls fat-induced oleoylethanolamide signaling in small intestine. J Neurosci. 2011;31(15):5730–5736. doi: 10.1523/JNEUROSCI.5668-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landsberg L. Feast or famine: The sympathetic nervous system response to nutrient intake. Cell Mol Neurobiol. 2006;26(4-6):497–508. doi: 10.1007/s10571-006-9010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritter RC. A tale of two endings: Modulation of satiation by NMDA receptors on or near central and peripheral vagal afferent terminals. Physiol Behav. 2011;105(1):94–99. doi: 10.1016/j.physbeh.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King SO, 2nd, Williams CL. Novelty-induced arousal enhances memory for cued classical fear conditioning: interactions between peripheral adrenergic and brainstem glutamatergic systems. Learn Mem. 2009;16(10):625–634. doi: 10.1101/lm.1513109. [DOI] [PubMed] [Google Scholar]
- 14.Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: A meta-analysis of randomised trials. Lancet. 2007;370(9600):1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 15.Marsicano G, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418(6897):530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 16.Marsicano G, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302(5642):84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 17.Monory K, et al. Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice. PLoS Biol. 2007;5(10):e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monory K, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51(4):455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubreucq S, et al. Genetic dissection of the role of cannabinoid type-1 receptors in the emotional consequences of repeated social stress in mice. Neuropsychopharmacology. 2012;37(8):1885–1900. doi: 10.1038/npp.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubreucq S, et al. Cannabinoid type 1 receptors located on single-minded 1-expressing neurons control emotional behaviors. Neuroscience. 2012;204:230–244. doi: 10.1016/j.neuroscience.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 21.Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44(9):419–424. doi: 10.1002/dvg.20231. [DOI] [PubMed] [Google Scholar]
- 22.Wellman PJ. Modulation of eating by central catecholamine systems. Curr Drug Targets. 2005;6(2):191–199. doi: 10.2174/1389450053174532. [DOI] [PubMed] [Google Scholar]
- 23.White CL, et al. Effect of a beta-3 agonist on food intake in two strains of rats that differ in susceptibility to obesity. Physiol Behav. 2004;82(2-3):489–496. doi: 10.1016/j.physbeh.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 24.Ishac EJ, et al. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118(8):2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzavara ET, Perry KW, Rodriguez DE, Bymaster FP, Nomikos GG. The cannabinoid CB(1) receptor antagonist SR141716A increases norepinephrine outflow in the rat anterior hypothalamus. Eur J Pharmacol. 2001;426(3):R3–R4. doi: 10.1016/s0014-2999(01)01228-6. [DOI] [PubMed] [Google Scholar]
- 26.Arendt RM, Greenblatt DJ, deJong RH, Bonin JD, Abernethy DR. Pharmacokinetics, central nervous system uptake, and lipid solubility of propranolol, acebutolol, and sotalol. Cardiology. 1984;71(6):307–314. doi: 10.1159/000173684. [DOI] [PubMed] [Google Scholar]
- 27.Emorine LJ, et al. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989;245(4922):1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- 28.Wanke E, et al. Activation of a muscarinic receptor selectively inhibits a rapidly inactivated Ca2+ current in rat sympathetic neurons. Proc Natl Acad Sci USA. 1987;84(12):4313–4317. doi: 10.1073/pnas.84.12.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: Location in the hindbrain. Science. 1981;213(4506):451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- 30.Langhans W, Leitner C, Arnold M. Dietary fat sensing via fatty acid oxidation in enterocytes: Possible role in the control of eating. Am J Physiol Regul Integr Comp Physiol. 2011;300(3):R554–R565. doi: 10.1152/ajpregu.00610.2010. [DOI] [PubMed] [Google Scholar]
- 31.Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol. 2010;318(1–2):34–43. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science. 2003;300(5625):1530–1532. doi: 10.1126/science.1079206. [DOI] [PubMed] [Google Scholar]
- 33.Bengtsson T, Redegren K, Strosberg AD, Nedergaard J, Cannon B. Down-regulation of beta3 adrenoreceptor gene expression in brown fat cells is transient and recovery is dependent upon a short-lived protein factor. J Biol Chem. 1996;271(52):33366–33375. doi: 10.1074/jbc.271.52.33366. [DOI] [PubMed] [Google Scholar]
- 34.Fève B, Piétri-Rouxel F, el Hadri K, Drumare MF, Strosberg AD. Long term phorbol ester treatment down-regulates the beta 3-adrenergic receptor in 3T3-F442A adipocytes. J Biol Chem. 1995;270(18):10952–10959. doi: 10.1074/jbc.270.18.10952. [DOI] [PubMed] [Google Scholar]
- 35.Granneman JG, Lahners KN. Regulation of mouse beta 3-adrenergic receptor gene expression and mRNA splice variants in adipocytes. Am J Physiol. 1995;268(4 Pt 1):C1040–C1044. doi: 10.1152/ajpcell.1995.268.4.C1040. [DOI] [PubMed] [Google Scholar]
- 36.Bengtsson T, Cannon B, Nedergaard J. Differential adrenergic regulation of the gene expression of the beta-adrenoceptor subtypes beta1, beta2 and beta3 in brown adipocytes. Biochem J. 2000;347(Pt 3):643–651. [PMC free article] [PubMed] [Google Scholar]
- 37.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond) 2010;34(Suppl 1):S36–S42. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thackeray JT, Beanlands RS, Dasilva JN. Presence of specific 11C-meta-Hydroxyephedrine retention in heart, lung, pancreas, and brown adipose tissue. J Nucl Med. 2007;48(10):1733–1740. doi: 10.2967/jnumed.107.043570. [DOI] [PubMed] [Google Scholar]
- 39.Ritter R, Brenner L, Yox D. Participation of vagal sensory neurons in putative satiety signals from upper gastrointestinal tract. In: Ritter S, Ritter R, Barnes CD, editors. Neuroanatomy and Physiology of Abdominal Vagal Afferents. Boca Raton, FL: CRC; 1992. pp. 221–248. [Google Scholar]
- 40.Ritter S, Taylor JS. Capsaicin abolishes lipoprivic but not glucoprivic feeding in rats. Am J Physiol. 1989;256(6 Pt 2):R1232–R1239. doi: 10.1152/ajpregu.1989.256.6.R1232. [DOI] [PubMed] [Google Scholar]
- 41.Dogan MD, et al. Lipopolysaccharide fever is initiated via a capsaicin-sensitive mechanism independent of the subtype-1 vanilloid receptor. Br J Pharmacol. 2004;143(8):1023–1032. doi: 10.1038/sj.bjp.0705977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garami A, Balaskó M, Székely M, Solymár M, Pétervári E. Fasting hypometabolism and refeeding hyperphagia in rats: Effects of capsaicin desensitization of the abdominal vagus. Eur J Pharmacol. 2010;644(1–3):61–66. doi: 10.1016/j.ejphar.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Calingasan NY, Ritter S. Lateral parabrachial subnucleus lesions abolish feeding induced by mercaptoacetate but not by 2-deoxy-D-glucose. Am J Physiol. 1993;265(5 Pt 2):R1168–R1178. doi: 10.1152/ajpregu.1993.265.5.R1168. [DOI] [PubMed] [Google Scholar]
- 44.Duva MA, Siu A, Stanley BG. The NMDA receptor antagonist MK-801 alters lipoprivic eating elicited by 2-mercaptoacetate. Physiol Behav. 2005;83(5):787–791. doi: 10.1016/j.physbeh.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 45.Pervanidou P, Chrousos GP. Neuroendocrinology of post-traumatic stress disorder. Prog Brain Res. 2010;182:149–160. doi: 10.1016/S0079-6123(10)82005-9. [DOI] [PubMed] [Google Scholar]
- 46.Moreira FA, Crippa JA. The psychiatric side-effects of rimonabant. Rev Bras Psiquiatr. 2009;31(2):145–153. doi: 10.1590/s1516-44462009000200012. [DOI] [PubMed] [Google Scholar]
- 47.Häring M, Marsicano G, Lutz B, Monory K. Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience. 2007;146(3):1212–1219. doi: 10.1016/j.neuroscience.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 48.Nakazi M, Bauer U, Nickel T, Kathmann M, Schlicker E. Inhibition of serotonin release in the mouse brain via presynaptic cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;361(1):19–24. doi: 10.1007/s002109900147. [DOI] [PubMed] [Google Scholar]
- 49.Tallett AJ, Blundell JE, Rodgers RJ. Effects of acute low-dose combined treatment with rimonabant and sibutramine on appetite and weight gain in rats. Pharmacol Biochem Behav. 2010;97(1):92–100. doi: 10.1016/j.pbb.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 50.Reguero L, et al. GABAergic and cortical and subcortical glutamatergic axon terminals contain CB1 cannabinoid receptors in the ventromedial nucleus of the hypothalamus. PLoS ONE. 2011;6(10):e26167. doi: 10.1371/journal.pone.0026167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cota D, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112(3):423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cardinal P, et al. Hypothalamic CB1 cannabinoid receptors regulate energy balance in mice. Endocrinology. 2012;153(9):4136–4143. doi: 10.1210/en.2012-1405. [DOI] [PubMed] [Google Scholar]
- 53.Nisoli E, Garosi V, Blundell JE, Carruba MO. Salbutamol antagonizes insulin- and sodium mercaptoacetate-induced but not 2-deoxy-D-glucose-induced hyperphagia. Pharmacol Biochem Behav. 1996;54(2):409–413. doi: 10.1016/0091-3057(95)02091-8. [DOI] [PubMed] [Google Scholar]
- 54.Burdyga G, et al. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci. 2004;24(11):2708–2715. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langhans W, Scharrer E. Evidence for a vagally mediated satiety signal derived from hepatic fatty acid oxidation. J Auton Nerv Syst. 1987;18(1):13–18. doi: 10.1016/0165-1838(87)90129-9. [DOI] [PubMed] [Google Scholar]
- 56.Gu Q, Lin YS, Lee LY. Epinephrine enhances the sensitivity of rat vagal chemosensitive neurons: Role of beta3-adrenoceptor. J Appl Physiol. 2007;102(4):1545–1555. doi: 10.1152/japplphysiol.01010.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grill HJ, Hayes MR. The nucleus tractus solitarius: A portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int J Obes (Lond) 2009;33(Suppl 1):S11–S15. doi: 10.1038/ijo.2009.10. [DOI] [PubMed] [Google Scholar]
- 58.Kim EM, Quinn JG, Spanswick D, O’Hare E. Feeding association between the nucleus of the solitary tract and the ventral tegmental area. Appetite. 2009;53(3):457–460. doi: 10.1016/j.appet.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Sinnayah P, et al. Feeding induced by cannabinoids is mediated independently of the melanocortin system. PLoS ONE. 2008;3(5):e2202. doi: 10.1371/journal.pone.0002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daugé V, Léna I. CCK in anxiety and cognitive processes. Neurosci Biobehav Rev. 1998;22(6):815–825. doi: 10.1016/s0149-7634(98)00011-6. [DOI] [PubMed] [Google Scholar]
- 61.Dockray GJ. Cholecystokinin and gut-brain signalling. Regul Pept. 2009;155(1–3):6–10. doi: 10.1016/j.regpep.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 62.Esteban S, García-Sevilla JA. Effects induced by cannabinoids on monoaminergic systems in the brain and their implications for psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(1):78–87. doi: 10.1016/j.pnpbp.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 63.Roux J, Wanaverbecq N, Jean A, Lebrun B, Trouslard J. Depolarization-induced release of endocannabinoids by murine dorsal motor nucleus of the vagus nerve neurons differentially regulates inhibitory and excitatory neurotransmission. Neuropharmacology. 2009;56(8):1106–1115. doi: 10.1016/j.neuropharm.2009.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.