Abstract
The function of the Munc18-1 protein hydrophobic pocket, which interacts with the syntaxin-1 N-terminal peptide, has been highly controversial in neurosecretion. Recent analysis of patients with familial hemophagocytic lymphohistiocytosis type 5 has identified the E132A mutation in the hydrophobic pocket of Munc18-2, prompting us to examine the role of this region in the context of immune cell secretion. Double knockdown of Munc18-1 and Munc18-2 in RBL-2H3 mast cells eliminates both IgE-dependent and ionomycin-induced degranulation and causes a significant reduction in syntaxin-11 without altering expressions of the other syntaxin isoforms examined. These phenotypes were effectively rescued on reexpression of wild-type Munc18-1 or Munc18-2 but not the mutants (F115E, E132A, and F115E/E132A) in the hydrophobic pocket of Munc18. In addition, these mutants show that they are unable to directly interact with syntaxin-11, as tested through protein interaction experiments. Our results demonstrate the crucial roles of the hydrophobic pocket of Munc18 in mast cell degranulation, which include the regulation of syntaxin-11. We also suggest that the functional importance of this region is significantly different between neuronal and immune cell exocytosis.
Keywords: membrane fusion, FHL5
The SNARE protein complex, which consists of isoforms of syntaxin, SNAP-25, and VAMP/synaptobrevin, orchestrates the central role in membrane fusion (1). However, the process is further modulated by indispensible proteins, such as Munc18 (2, 3) and Munc13 (4), through interactions with one or more SNARE proteins, particularly cognate syntaxins. The precise functions of these proteins and their structural determinants have been intensively studied in neuronal exocytosis (5, 6). Recent studies have revealed the critical roles of the SNARE proteins and their regulators in exocytosis from immune cells and platelets. In particular, genetic analysis of patients with familial hemophagocytic lymphohistiocytosis (FHL) types 3, 4, and 5 identified mutations in Munc13-4 (7-9), syntaxin-11 (10), and Munc18-2 (11, 12), respectively. In these diseases, there is markedly reduced degranulation of lytic granules in cytotoxic T lymphocytes (CTLs), natural killer (NK) cells, and platelets. In addition, patients with FHL5 have been shown to suffer from gastrointestinal symptoms and bleeding disorders, possibly from disrupted degranulation from mast cells and neutrophils, as well as platelets (13). Nonetheless, the underlying mechanisms, and particularly the structural determinants of the functions of these proteins, have not been investigated in a detailed manner.
The Munc18 family of proteins has been revealed to play multiple functions, which include both chaperoning cognate syntaxins (14–19) and priming/stimulating membrane fusion through interaction with the SNARE complex (20–23) (reviewed in ref. 24). The chaperoning function of Munc18 contributes to stabilizing the expression level of cognate syntaxins and, in some cases, trafficking them to the appropriate intracellular compartments. For instance, in Munc18-1-deficient neurons, the syntaxin-1 level is reduced by 50–75% (3), whereas in Munc18-1 single-knockdown (KD) and Munc18-1/2 double-knockdown (DKD) pheochromocytoma (PC) 12 cells, not only the reduction of syntaxin-1 expression level but also its localization at the plasma membrane are severely perturbed (14, 15). In some patients with FHL5 whose functional Munc18-2 protein is absent, a strong reduction in syntaxin-11 level was found in their immune cells (11, 12). The priming function of Munc18 is believed to be mediated in part through the interaction between the Munc18 hydrophobic pocket region and the syntaxin N-peptide. X-ray crystallography determined the structure of the hydrophobic pocket region of Munc18-1 and Munc18-3 bound with the N-peptide of their cognate syntaxins: syntaxin-1 and syntaxin-4, respectively (25, 26).
In Munc18-1, Phe115, together with Val119, Ala124, Ile127, and Leu130, forms a hydrophobic pocket that interacts with Leu8 of syntaxin-1, whereas Glu132 of Munc18-1 forms an electrostatic interaction with Arg4 of syntaxin-1 (25). The functional significance of this interaction was first proposed by Shen et al. (20). The authors have shown that through this binding mode, Munc18-1 interacts with the SNARE complex, which results in the stimulation of SNARE-dependent liposome fusion. As a consequence, several studies, including a recent rescue experiment using syntaxin KD neurons, have demonstrated the importance of the syntaxin-1 N-peptide in synaptic vesicle exocytosis (27–30). In contrast, the Munc18-1 hydrophobic pocket has been suggested to play limited or dispensable roles in exocytosis. For example, in the rescue experiments of Munc18-1 single-KD and Munc18-1/2 DKD PC12 cells as well as Munc18-1 knockout neurons, various mutants (F115E, E132A, F115E/E132A, and L130K) in the hydrophobic pocket region of Munc18-1 have exhibited either very mild or no impairment in their ability to rescue exocytosis in comparison with the wild type (15, 31, 32), leaving the function of the hydrophobic pocket region of Munc18 unclear.
Analysis of Munc18-2 in patients with FHL5 has identified an E132A mutation within the hydrophobic pocket region of Munc18-2 (33). We hypothesize that the hydrophobic pocket of Munc18-2 is indeed crucial for immune cell secretion and tested this hypothesis through analyses of the stable KD and rescue of Munc18 in mast cell model RBL-2H3 cells.
Results
Munc18 Essential for Mast Cell Degranulation.
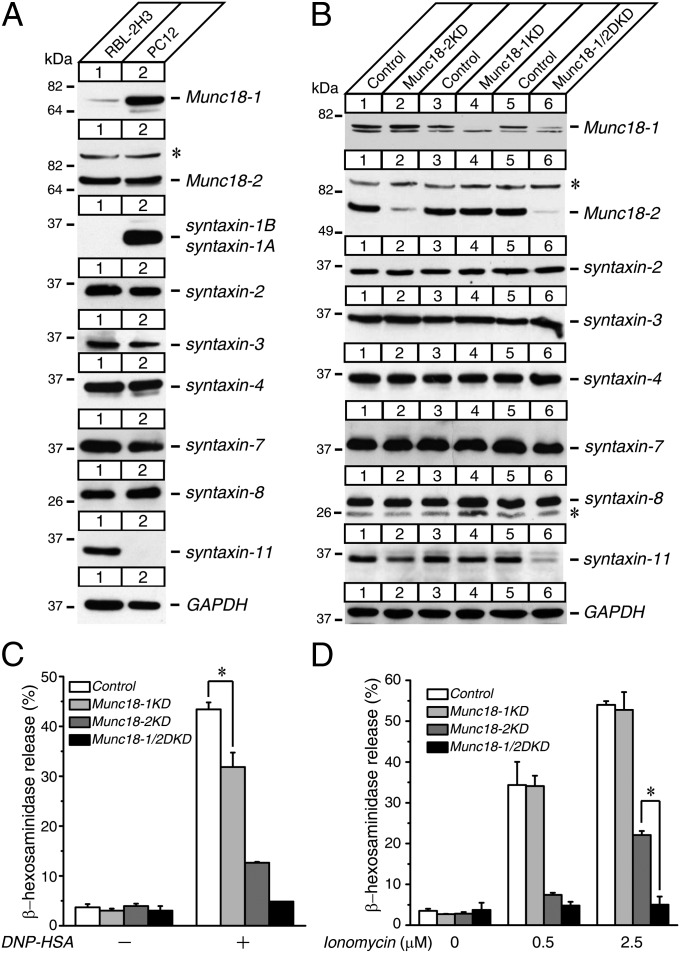
We first examined the protein expressions of Munc18 and syntaxin isoforms in RBL-2H3 cells by comparing them with neurosecretory PC12 cells as a reference (Fig. 1A). Because both PC12 cells and RBL-2H3 cells are derived from rat, a direct comparison is possible between the cell lines. We found that the expression of Munc18-1 in RBL-2H3 cells was substantially less than that in PC12 cells. In contrast, the expression of Munc18-2 was more abundant in RBL-2H3 cells than in PC12 cells. The neuronal syntaxin-1A/1B isoform was strongly expressed in PC12 cells but was completely absent in RBL-2H3 cells. Conversely, syntaxin-11 was expressed in RBL-2H3 cells but not in PC12 cells. The expressions of plasmalemmal syntaxins 2, 3, and 4, as well as endosomal syntaxins 7 and 8, were similar between these two cell lines. Selective expression of syntaxin-11 in RBL-2H3 cells is consistent with previous findings that this protein is enriched in tissues of the immune system, including the thymus, spleen, and lymph nodes, although it is largely absent in the brain (34, 35).
Fig. 1.
KD of both Munc18-1 and Munc18-2 results in dramatic reductions of syntaxin-11 expressions and striking degranulation defects in RBL-2H3 cells. (A) Expression profiles of Munc18-1 and Munc18-2, as well as various syntaxin proteins present in wild-type RBL-2H3 and PC12 cells. Twenty micrograms of homogenates from wild-type RBL-2H3 and PC12 cells were analyzed by SDS/PAGE and immunoblotting, using the antibodies indicated. (B) Stable Munc18-1 KD, Munc18-2 KD, and Munc18-1/2 DKD RBL-2H3 cells were generated by lentivirus-mediated shRNA. Twenty micrograms of cell homogenates were analyzed by SDS/PAGE and immunoblotting, using the antibodies indicated. (C and D) β-hexosaminidase release was stimulated by applying 0.01 μg/mL DNP-IgE and 50 ng/mL dinitrophenyl-human serum albumin (DNP-HSA) (C) and 0.5 μM and 2.5 μM ionomycin (D) for 1 h from control, Munc18-1 KD, Munc18-2 KD, and Munc18-1/2 DKD cells. Error bars, SEM (n = 5). The statistical significance of the differences in β-hexosaminidase release between control and Munc18-1 KD (C), Munc18-2 KD, and Munc18-1/2 DKD (D) is indicated. *P < 0.05 (Student t test). *Nonspecific band observed with rabbit polyclonal anti-Munc18-2 and anti-syntaxin-8 antibodies.
To elucidate the function of Munc18 in the degranulation of RBL-2H3 cells, we generated stable Munc18-1 and Munc18-2 single-KD as well as Munc18-1/2 DKD cells using lentivirus-mediated shRNA (Materials and Methods). We then examined whether the expression levels of the aforementioned syntaxin isoforms are altered by the KD of Munc18 through immunoblot analysis (Fig. 1B; quantification in Fig. S1). In Munc18-2-null patients with FHL5, the level of syntaxin-11 in NK cells, CTLs, and platelets was found to be dramatically decreased (11, 12, 36). We found that there were ∼20% reductions in syntaxin-11 level in Munc18-2 KD and more dramatic decreases in Munc18-1/2 DKD cells, at around 60%. However, we did not find changes in plasma membrane–localized syntaxin isoforms such as syntaxins 2, 3, and 4. Because syntaxin-11 has been shown to be localized on endosomal membranes, including late endosomes and lysosomes in macrophages, and to regulate trafficking steps between late endosomes, lysosomes, and the cell surface (37), we also tested other endosomal syntaxins such as syntaxin-7 and syntaxin-8 to see whether the reduction in expression is limited to syntaxin-11. We found a mild (∼15%) but statistically insignificant reduction of syntaxin-7 level in Munc18-1/2 DKD cells, but not in Munc18-2 single-KD cells, whereas syntaxin-8 levels were unchanged in all KD cells. Thus, Munc18-1/2 plays a critical role in maintaining the level of syntaxin-11 in mast cells.
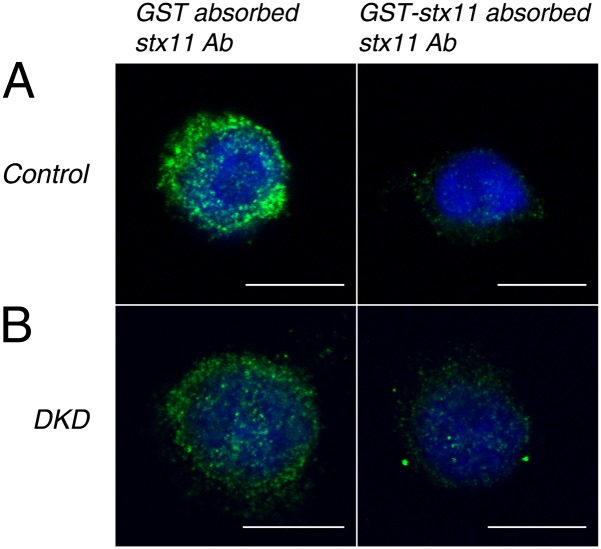
We previously found that Munc18-1/2 DKD causes not only a strong reduction in syntaxin-1 level but also perturbation of its plasmalemmal localization in PC12 cells (15, 16). We therefore examined whether the subcellular localization of syntaxin-11 can be significantly altered by Munc18-1/2 DKD in RBL-2H3 cells (Fig. 2; low-magnification data available in Fig. S2). Confocal immunofluorescence microscopy using rabbit polyclonal anti-syntaxin-11 antibody showed punctuate stainings, which indicated the enrichment of syntaxin-11 on lysosomal granules. This staining was specific to syntaxin-11 because the signal was strongly reduced on preabsorption of the antibody with the antigen, GST-syntaxin-11 but not with GST alone. Importantly, the syntaxin-11 immunostaining was not changed in Munc18-1/2 DKD cells (Fig. 2 and Fig. S2). Thus, we conclude that although the syntaxin-11 expression level is dramatically reduced, its localization is not affected by Munc18-1/2 DKD in mast cells.
Fig. 2.
Confocal immunofluorescence microscopy revealing that the subcellular localization of syntaxin-11 is unaltered on Munc18-1/2 DKD. Control and stable Munc18-1/2 DKD RBL-2H3 cells were permeabilized and stained with either GST or GST-syntaxin-11–absorbed rabbit polyclonal anti-syntaxin-11 antibody followed by Alexa488-conjugated goat anti-rabbit antibody (Invitrogen) and DAPI (see SI Materials and Methods for more detail). Green indicates syntaxin-11 and blue indicates DAPI. (A) Control and (B) Munc18-1/2 DKD cells. Note that there is substantially diminished green intensity (Right) where GST-syntaxin-11 absorbed the anti-syntaxin-11 antibody was used to stain. (Scale bar, 10 μm.)
To measure the secretion capability of the control and the KD cells, we examined the degranulation by measuring release of β-hexosaminidase (Fig. 1 C and D) (38). This protein is an endogenous enzyme stored in and released from secretory granules/lysosomes in mast cells as well as RBL-2H3 cells. We confirmed that the control RBL-2H3 cells exhibit robust IgE-dependent as well as ionomycin-induced β-hexosaminidase release. Ionomycin is a Ca2+ ionophore that directly induces an increase in intracellular Ca2+ concentration, triggering degranulation. We found severe reductions in β-hexosaminidase release in Munc18-2 single-KD cells and an almost complete abolishment in Munc18-1/2 DKD cells. We also observed a significant, yet smaller, reduction in Munc18-1 single-KD cells. These reductions in β-hexosaminidase release were observed in both IgE-dependent and ionomycin-induced secretion, suggesting that the impairment lies in secretory machinery itself. The strong reduction in β-hexosaminidase release in Munc18-2 KD cells is consistent with a previous report that antisense-mediated downregulation of Munc18-2 caused ∼50% reduction in β-hexosaminidase release (38).
Munc18-1 and Munc18-2 Can Effectively Support Mast Cell Degranulation.
The greater impairment of degranulation by Munc18-2 KD than Munc18-1 KD could be a result of either the difference in their expression levels (i.e., more abundant expression of Munc18-2 than Munc18-1) in RBL-2H3 cells or the functional specificity between these two isoforms. To examine whether any functional specificity exists between Munc18-1 and Munc18-2, we performed the rescue experiments using both isoforms.
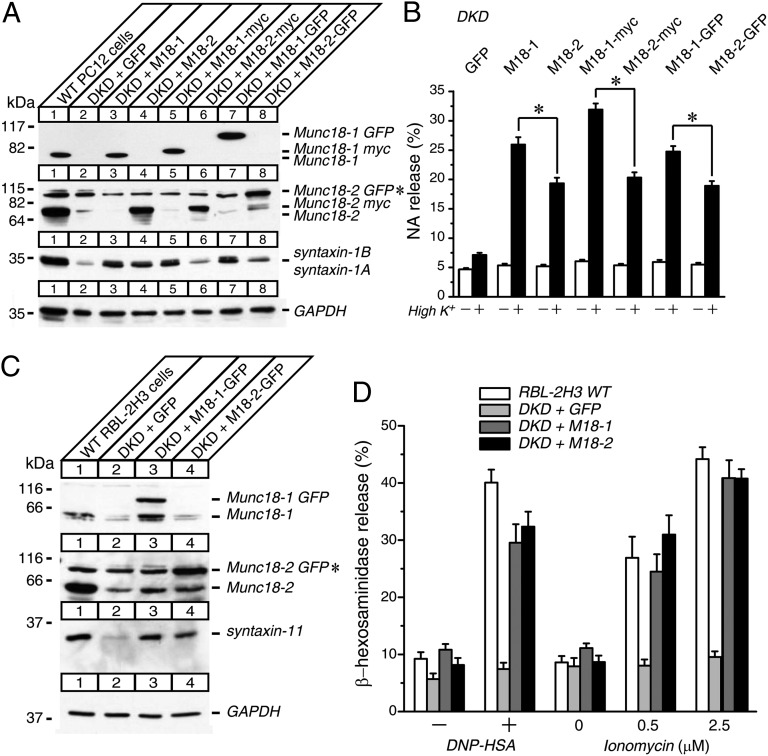
Previous study has shown that Munc18-1 is more effective in rescuing neurosecretion from Munc18-1-deficient chromaffin cells than Munc18-2 (39). We first examined whether stable expression of Munc18-1 rescues neurosecretion of Munc18-1/2 DKD PC12 cells better than that of Munc18-2 (Fig. 3). In these experiments, Munc18-1 and Munc18-2 were expressed in three different configurations: without any tag, with myc, or with an Emerald GFP (EmGFP; Invitrogen) tag (Fig. 3A). In all cases, Munc18-1 rescued defective secretion of Munc18-1/2 DKD cells significantly better than Munc18-2 (Fig. 3B). Interestingly, the recovery of the syntaxin-1 level was also higher in Munc18-1 rescue than that of Munc18-2 (Fig. 3A). Thus, our results confirm that functional specificity exists between the two isoforms in neuronal secretion and that this difference seems to be related with their ability to restore syntaxin-1 expression. This experiment also ensures that the addition of EmGFP tag to Munc18 does not affect the folding or function of the protein.
Fig. 3.
Stable reexpression of wild-type Munc18-1 or Munc18-2 restores the syntaxin-11 expressions and the degranulation defects of Munc18-1/2 DKD RBL-2H3 cells while functional specificities exist in PC12 cells. (A) Twenty micrograms of homogenates of Munc18-1/2 DKD PC12 cells rescued with EmGFP, wild-type Munc18-1, and Munc18-2 (without tag, with myc, and with EmGFP) were analyzed by SDS/PAGE and immunoblotting, using the antibodies indicated. (B) NA release was stimulated by 70 mM KCl for 15 min in the rescued cells. Error bars, SEM (n = 9). The statistical significance of the differences in NA release between wild-type Munc18-1 and Munc18-2 rescued PC12 cells in each tag configuration are indicated. *P < 0.05 (paired t test). (C) Munc18-1/2 DKD RBL-2H3 cells were infected with lentiviruses that express EmGFP, wild-type Munc18-1-EmGFP, and wild-type Munc18-2-EmGFP. Twenty micrograms of cell homogenates were analyzed by SDS/PAGE and immunoblotting, using the antibodies indicated. (D) β-Hexosaminidase release was stimulated by applying 0.01 μg/mL DNP-IgE and 50 ng/mL DNP-HSA, as well as 0.5 μM and 2.5 μM ionomycin for 1 h from wild-type RBL-2H3 cells and rescued cells. Error bars, SEM (n = 9). *Nonspecific band observed with rabbit polyclonal anti-Munc18-2 antibody that comigrated with Munc18-2-EmGFP.
We then performed the rescue of Munc18-1/2 DKD RBL-2H3 cells by stably expressing Munc18-1 or Munc18-2 fused with EmGFP (Fig. 3C). On reexpression of Munc18-1 or Munc18-2, the level of syntaxin-11 was rescued in both cases (Fig. 3C). In addition, we found that defective β-hexosaminidase release was comparably rescued by reexpressions of either Munc18-1 or Munc18-2 (Fig. 3D). The rescued cells exhibited a level of degranulation similar to that of control cells, indicating that reexpressed Munc18-1 or Munc18-2 could fully recover the degranulation. These results suggest that there is no clear functional specificity between these two isoforms in regulating mast cell degranulation, as well as stabilizing the expression of syntaxin-11.
Mutations in the Hydrophobic Pocket of Munc18 Attenuate the Interaction with Syntaxin-11 and Cause Severe Impairment in Their Rescuing Activity.
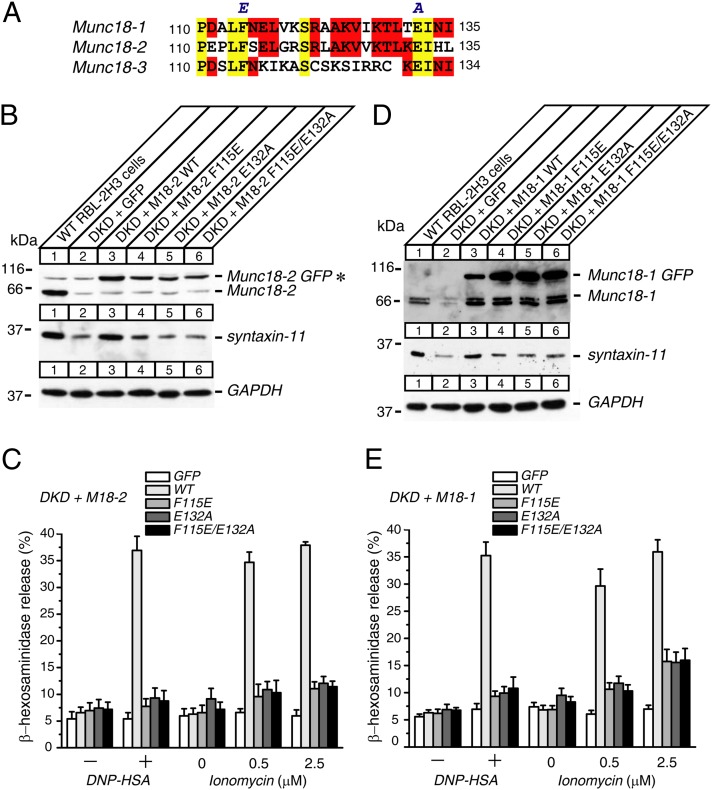
To elucidate the role for the hydrophobic pocket of Munc18-2 in mast cell degranulation, we introduced point mutations (F115E, E132A, and F115E/E132A) in this region that include the mutation discovered in the patient with FHL5 (E132A) (Fig. 4A). We expressed these mutants in DKD RBL-2H3 cells and examined their ability to restore degranulation as well as syntaxin-11 expression levels in comparison with the wild type (Fig. 4B). The expression level of all mutants was similar to that of the wild type, implying that these mutations do not cause major problems in protein folding or expression. Unlike the wild-type Munc18-2, however, these mutants did not restore the level of syntaxin-11.
Fig. 4.
Hydrophobic pocket mutants of either Munc18-1 or Munc18-2 do not restore the syntaxin-11 expressions or the degranulation defects of Munc18-1/2 DKD RBL-2H3 cells. (A) Sequence alignment among Munc18-1, Munc18-2, and Munc18-3, indicating that F115 and E132 are conserved residues in Munc18 isoforms. (B and D) Munc18-1/2 DKD RBL-2H3 cells were infected with lentiviruses that express EmGFP, wild-type Munc18-EmGFP, and hydrophobic pocket mutant Munc18-EmGFP (F115E, E132A, and F115E/E132A) (B) Munc18-2 and (D) Munc18-1. Infected cells were selected with blasticidin (20 μg/mL). Twenty micrograms of homogenates of surviving cells were analyzed by SDS/PAGE and immunoblotting, using the antibodies indicated. *Nonspecific band observed with rabbit polyclonal anti-Munc18-2 antibody that comigrated with Munc18-2-EmGFP. (C and E) β-hexosaminidase release was stimulated by applying 0.01 μg/mL DNP-IgE and 50 ng/mL DNP-HSA, as well as 0.5 μM and 2.5 μM ionomycin for 1 h from rescued cells (C) Munc18-2 and (E) Munc18-1. Error bars, SEM (n = 5 for C, n = 6 for E).
Strikingly, these mutants also showed severe impairments in the rescue of defective IgE-dependent and ionomycin-induced β-hexosaminidase release of DKD RBL-2H3 cells (Fig. 4C). In addition, we did not see any additive deteriorating effects of the double mutant (F115E/E132A) over single mutants. Our results indicate that the hydrophobic pocket of Munc18-2 plays a crucial role in mast cell degranulation, at least in part via its regulation of syntaxin-11.
The lack of rescue by the hydrophobic pocket mutants in Munc18-2 is surprising, as we previously found that the expressions of Munc18-1 with the identical mutations showed 70–80% of the rescue ability compared with the wild type, using Munc18-1 KD and Munc18-1/2 DKD PC12 cells (15, 31). Furthermore, the same (F115E) and a similar (L130K) mutant have shown a complete rescue ability in autaptic neurotransmission of Munc18-1-deficient neurons (32). By taking advantage of the fact that Munc18-1 can also rescue degranulation of the DKD RBL-2H3 cells (Fig. 3D), we examined whether the same hydrophobic pocket mutants of Munc18-1 can restore mast cell degranulation and syntaxin-11 level (Fig. 4 D and E). We found that these mutants also show strong impairment in restoring degranulation and syntaxin-11 level. Therefore, the hydrophobic pocket mutations cause similar functional impairment for both Munc18 isoforms. Our results also suggest that the effects of hydrophobic pocket mutations in Munc18-1 are dramatically different between neuronal and immune cell exocytosis.
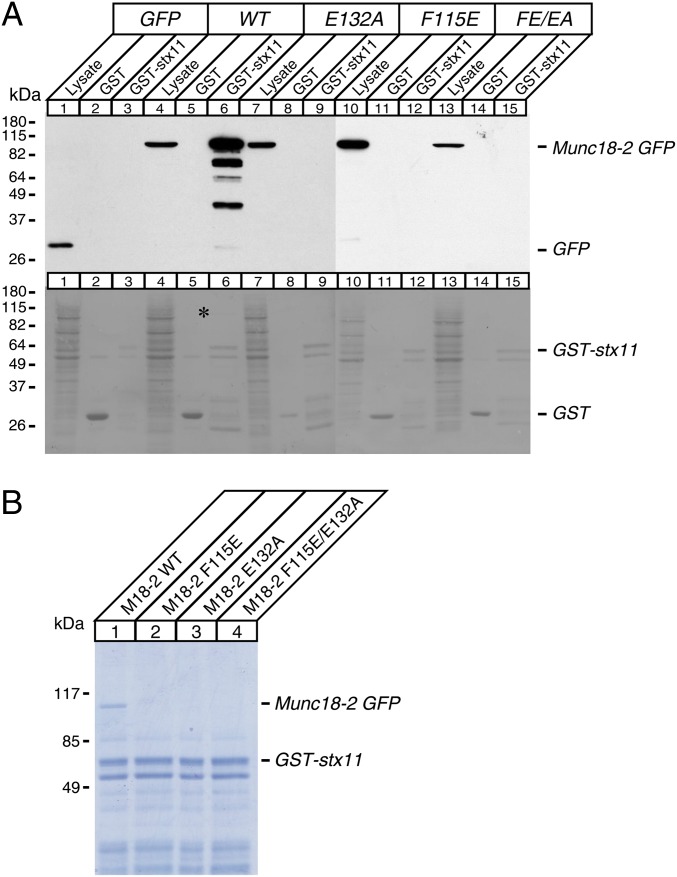
To test whether the impairment of syntaxin-11 restoration by hydrophobic pocket mutants is a result of reduced interactions between two proteins, we performed protein interaction experiments using GST-fused syntaxin-11 and potassium glutamate buffer solubilized lysates of human embryonic kidney (HEK)-293FT cells that were transfected with wild-type Munc18-2 and mutants fused with EmGFP. We then analyzed the pull-down mixtures by immunoblotting with mouse monoclonal anti-EmGFP antibody and Coomassie Blue staining (Fig. 5 A, Upper, and B). We found that wild-type Munc18-2 directly binds to syntaxin-11, as a strong Munc18-2-EmGFP band was seen in both immunoblotting and Coomassie Blue staining. However, syntaxin-11 failed to pull down all hydrophobic pocket mutants of Munc18-2. This was not a result of unequal loadings or aberrant expressions of mutants, as there were similar loadings between lanes (Fig. 5A, Lower) and comparable expressions of the mutants to that of wild type were seen in lysate lanes (Fig. 5A, Upper). These data are in agreement with the previous finding that the E132A mutant of EGFP-Munc18-2 fails to coimmunoprecipitate FLAG-syntaxin-11 in HEK-293 cells (33). Collectively, our data suggest that Munc18 works as a stabilizing regulator for syntaxin-11 and that the hydrophobic pocket of Munc18 is the essential region that governs the function in mast cell degranulation.
Fig. 5.
Hydrophobic pocket mutants of Munc18-2 abolish the direct interaction with syntaxin-11. (A, Upper) Solubilized lysates of HEK-293FT cells that were transfected with EmGFP, wild-type Munc18-2-EmGFP, F115E, E132A, and F115E/E132A of Munc18-2-EmGFP were incubated with 10 μg GST or GST-syntaxin-11 attached to glutathione agarose beads. The mixtures were washed extensively, and the proteins were analyzed on SDS/PAGE and immunoblotting, using mouse monoclonal anti-EmGFP antibody. Solubilized lysates of HEK-293FT cells were loaded in lysate lanes. (A, Lower) Ponceau S staining of the above membrane showing similar loadings between lanes. *Wild-type Munc18-2-EmGFP band that was pulled down by GST-syntaxin-11. (B) The binding mixture between solubilized lysates of HEK-293FT cells that were transfected with wild-type Munc18-2-EmGFP, F115E, E132A, F115E/E132A, and 10 μg of GST-syntaxin-11 was analyzed on SDS/PAGE, followed by Coomassie Blue staining. Note that only wild-type Munc18-2-EmGFP was pulled down by GST-syntaxin-11, not any of the mutants.
We found a positive correlation between the syntaxin-11 level and the degranulation level in our Munc18 KD RBL-2H3 cells, as well as their rescued cells. However, we also observed strong reductions of degranulation in Munc18-2 single-KD cells, even though their syntaxin-11 level is reduced by a mere 20% (Fig. 1 B–D and Fig. S1). This implies that the secretion defect of Munc18-2 single-KD is unlikely to be a result of the reduction of syntaxin-11 alone. Therefore, we finally investigated whether syntaxin-11 down-regulation can account for a dramatic degranulation defect in Munc18-1/2 DKD cells.
To determine this, we examined the secretion capability of stable syntaxin-11 KD RBL-2H3 cells that exhibited an ∼80% reduction in syntaxin-11 level (Fig. S3). This reduction is more than the reduction (∼60%) in syntaxin-11 level caused by Munc18-1/2 DKD (Fig. S1). However, we did not see a significant reduction in β-hexosaminidase release in syntaxin-11 KD cells compared with control (Fig. S3). At first, this was unexpected because mutations in syntaxin-11 cause severe reductions in secretion from CTLs, NK cells, neutrophils, and platelets in patients with FHL4, as well as recently established syntaxin-11 knockout mice (10, 40–42). However, our result is consistent with that seen in syntaxin-11 knockout mice, in which mast cell degranulation was not significantly impaired (42). Thus, it is likely that other syntaxin isoforms can compensate for the reduced level of syntaxin-11 in mast cell degranulation. Taken together, the striking impairment of degranulation by Munc18 KD in mast cells is not entirely caused by the reductions in syntaxin-11 level.
Discussion
The structure versus function relationship of the SNARE proteins and their regulators has been intensively studied in neuronal secretion using genetically engineered neurons, adrenal chromaffin cells, and PC12 cells. However, there have been significantly fewer studies conducted based on immune cell exocytosis (36, 40, 43, 44).
In this study, we have established the method to perform stable KD and rescue of the protein of interest, using RBL-2H3 mast cells. This system allowed us to study the structure versus function relationship of Munc18 in IgE-dependent as well as ionomycin-induced degranulation in mast cells. Importantly, the stable KD of Munc18-1/2 in RBL-2H3 cells not only abolished degranulation but also caused a significant reduction in syntaxin-11 level without affecting its localization. Thus, our KD RBL-2H3 cells have recapitulated the phenotype of CTLs, NK cells, and platelets from some of the patients with FHL5 in whom loss of Munc18-2 expression induces striking reductions in the level of syntaxin-11 (11, 12, 36). Furthermore, we demonstrated that reexpression of Munc18-1 or Munc18-2 effectively restores degranulation and the expression level of syntaxin-11.
These results strongly indicate that Munc18 is crucial for mast cell degranulation in part through its chaperoning function for syntaxin-11. We suggest that RBL-2H3 cells would be ideal model systems to study the structure versus function relationship of the protein of interest and that the derived findings from this approach would be applicable in many aspects to immune cell exocytosis in general.
Since the finding of the interaction between the Munc18 hydrophobic pocket and the N-peptide of syntaxin (25, 26), which has been shown to support the binding of Munc18-1 to the SNARE complex and facilitates the SNARE-mediated liposome fusion (20), many efforts have been made to identify the crucial role of this interaction in physiological membrane fusion. Several studies suggest the important role of syntaxin-1 N-peptide (27, 28, 30). In particular, Zhou et al. demonstrated that the deletion of the N-peptide of syntaxin-1A abolishes its ability to rescue exocytosis of syntaxin-1 KD neurons (30).
In contrast, the crucial roles for the hydrophobic pocket of Munc18 have not been revealed in neurotransmitter release, as the mutants in this region have been illustrated to be as equally effective in rescuing the exocytosis as the wild type (32). Although it is difficult to explain the difference between the last two studies, we suggest the following possibilities: Either the striking effect of N-peptide deletion on syntaxin-1 function could be because the N-peptide may exert functions other than interacting with the hydrophobic pocket of Munc18-1, or the lack of the phenotype in the hydrophobic pocket region of Munc18-1 could be because the point mutations tested (F115E and L130K) may not be strong enough to disrupt its binding with the N-peptide of syntaxin-1. Despite the latter possibility, in this study we found that the same mutation (F115E) as the one tested in synaptic vesicle exocytosis strongly impairs the rescuing ability of Munc18-1 and Munc18-2 in mast cell degranulation (Fig. 4). Therefore, the functional significance of the hydrophobic pocket of Munc18 seems to be strikingly different between neuronal and immune cell exocytosis.
The reason the hydrophobic mutations in Munc18 cause substantially different outcomes in exocytosis between neuronal cells and mast cells awaits further study. We found that all hydrophobic pocket mutants of Munc18-1 or Munc18-2 do not restore the syntaxin-11 level in the DKD RBL-2H3 cells. In contrast, our previous study showed that the level of syntaxin-1 is significantly restored by the same Munc18-1 mutants in the DKD PC12 cells, although at a slightly lower level than that achieved by wild-type Munc18-1 (15). Thus, the functional significance of the Munc18 hydrophobic pocket in different cell types seems to be explained at least in part by the differential degree of involvement of this region in regulation of the cognate syntaxins.
We propose the following hypothesis to explain the difference: The relative importance of the binding between Munc18 hydrophobic pocket and syntaxin N-peptide (binding mode 1) is inversely related to the degree of tightness of the binding between the cleft formed by domain 1 and domain 3a of Munc18-1 and “closed” syntaxin-1A (binding mode 2). Binding mode 2 is the major binding mode for the binary interaction between Munc18-1 and syntaxin-1A (15, 25, 45), whereas binding mode 1 alone cannot support the binary interaction but can further secure the binary interaction (20, 25). For example, Munc18-1 and syntaxin-1A bind each other at low nanomolar concentrations even when the hydrophobic pocket of Munc18-1 is mutated or the N-peptide of syntaxin-1A is removed (25, 31).
This offers an explanation for why the role of the hydrophobic pocket of Munc18 would be of limited significance in neurosecretion. In contrast, our data found that mutations in the hydrophobic pocket region of Munc18-2 failed to interact with syntaxin-11 (Fig. 5). Therefore, we speculate that binding mode 2 between Munc18 and syntaxin-11 is much weaker than that between Munc18 and syntaxin-1A. Indeed, a recent study has demonstrated that the crucial role of syntaxin N-peptide in monomeric interaction with Munc18 is isoform-dependent; deletion of N-peptide in syntaxin-1 has little effect on its binding to Munc18-1, whereas a similar deletion in syntaxin-4 dramatically reduces its binding with Munc18-3 (46). Mechanistically, we suggest that these two binding modes work together in chaperoning the cognate syntaxins in both neuronal and immune cell exocytosis; however, the relative contributions of these two binding modes vary depending on cell types.
Although there is a positive correlation between the syntaxin-11 level and the degranulation level in our Munc18 KD RBL-2H3 cells, as well as their rescued cells (Figs. 1–4), the striking impairment of degranulation by Munc18 KD in mast cells is not accounted entirely by the reductions in syntaxin-11 level. For example, syntaxin-11 KD alone does not cause severe defects in degranulation (Fig. S3). Thus, Munc18 plays crucial roles other than the regulation of syntaxin-11, which may include the regulation of other syntaxins, such as syntaxin-7 (Fig. 2), and the direct stimulation/priming of SNARE-mediated fusion of lysosomal granules (22). Our results suggest that the hydrophobic pocket region is likely involved in these other crucial functions of Munc18 in immune cell exocytosis.
Materials and Methods
Parental pLKO-puro plasmid for lentivirus-mediated KD was purchased from Sigma-Aldrich. pLVX-IRES-puro plasmid for lentivirus-mediated Munc18-1 and Munc18-2 expression was purchased from Clontech Laboratories. psPAX2 was purchased from Addgene, and pMD.G was a kind gift from Tomoyuki Mashimo from the University of Texas Southwestern Medical Center at Dallas. We obtained mouse monoclonal antibodies against syntaxin-1A and syntaxin-1B (clone HPC-1) (47) from Sigma-Aldrich; Munc18-1 and syntaxin-8 from BD Biosciences; GAPDH (clone 6C5) from Millipore; EmGFP from Clontech Laboratories; rabbit polyclonal antibodies against syntaxins 2, 3, and 4 from Synaptic Systems; and syntaxins 7 and 11 from Proteintech. Rabbit anti-Munc18-2 antibody (48) was a kind gift from Vesa Olkkonen from the National Public Health Institute in Helsinki, Finland. More details are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Thomas C. Südhof for providing cDNA of rat Munc18-1 and Munc18-2. This research was supported by the Canada Research Chair Program, the Heart and Stroke Foundation (Grants NA6217 and T6700), and the Canadian Institute of Health Research (MOP-93665).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214887110/-/DCSupplemental.
References
- 1.Söllner T, et al. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 2.Hata Y, Slaughter CA, Südhof TC. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993;366(6453):347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- 3.Verhage M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287(5454):864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 4.Augustin I, Rosenmund C, Südhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400(6743):457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- 5.Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490(7419):201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizo J, Südhof TC. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices—guilty as charged? Annu Rev Cell Dev Biol. 2012;28:279–308. doi: 10.1146/annurev-cellbio-101011-155818. [DOI] [PubMed] [Google Scholar]
- 7.Feldmann J, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115(4):461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto K, et al. Identification of novel MUNC13-4 mutations in familial haemophagocytic lymphohistiocytosis and functional analysis of MUNC13-4-deficient cytotoxic T lymphocytes. J Med Genet. 2004;41(10):763–767. doi: 10.1136/jmg.2004.021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santoro A, et al. Novel Munc13-4 mutations in children and young adult patients with haemophagocytic lymphohistiocytosis. J Med Genet. 2006;43(12):953–960. doi: 10.1136/jmg.2006.041863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryceson YT, et al. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110(6):1906–1915. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Côte M, et al. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. 2009;119(12):3765–3773. doi: 10.1172/JCI40732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.zur Stadt U, et al. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. Am J Hum Genet. 2009;85(4):482–492. doi: 10.1016/j.ajhg.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meeths M, et al. Spectrum of clinical presentations in familial hemophagocytic lymphohistiocytosis (FHL) type 5 patients with mutations in STXBP2. Blood. 2010;116(15):2635–2643. doi: 10.1182/blood-2010-05-282541. [DOI] [PubMed] [Google Scholar]
- 14.Arunachalam L, et al. Munc18-1 is critical for plasma membrane localization of syntaxin1 but not of SNAP-25 in PC12 cells. Mol Biol Cell. 2008;19(2):722–734. doi: 10.1091/mbc.E07-07-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han L, et al. Rescue of Munc18-1 and -2 double knockdown reveals the essential functions of interaction between Munc18 and closed syntaxin in PC12 cells. Mol Biol Cell. 2009;20(23):4962–4975. doi: 10.1091/mbc.E09-08-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han GA, et al. Munc18-1 domain-1 controls vesicle docking and secretion by interacting with syntaxin-1 and chaperoning it to the plasma membrane. Mol Biol Cell. 2011;22(21):4134–4149. doi: 10.1091/mbc.E11-02-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medine CN, Rickman C, Chamberlain LH, Duncan RR. Munc18-1 prevents the formation of ectopic SNARE complexes in living cells. J Cell Sci. 2007;120(Pt 24):4407–4415. doi: 10.1242/jcs.020230. [DOI] [PubMed] [Google Scholar]
- 18.Rowe J, et al. Blockade of membrane transport and disassembly of the Golgi complex by expression of syntaxin 1A in neurosecretion-incompetent cells: Prevention by rbSEC1. J Cell Sci. 1999;112(Pt 12):1865–1877. doi: 10.1242/jcs.112.12.1865. [DOI] [PubMed] [Google Scholar]
- 19.Rowe J, Calegari F, Taverna E, Longhi R, Rosa P. Syntaxin 1A is delivered to the apical and basolateral domains of epithelial cells: The role of munc-18 proteins. J Cell Sci. 2001;114(Pt 18):3323–3332. doi: 10.1242/jcs.114.18.3323. [DOI] [PubMed] [Google Scholar]
- 20.Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128(1):183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Tareste D, Shen J, Melia TJ, Rothman JE. SNAREpin/Munc18 promotes adhesion and fusion of large vesicles to giant membranes. Proc Natl Acad Sci USA. 2008;105(7):2380–2385. doi: 10.1073/pnas.0712125105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Südhof TC, Rothman JE. Membrane fusion: Grappling with SNARE and SM proteins. Science. 2009;323(5913):474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen J, Rathore SS, Khandan L, Rothman JE. SNARE bundle and syntaxin N-peptide constitute a minimal complement for Munc18-1 activation of membrane fusion. J Cell Biol. 2010;190(1):55–63. doi: 10.1083/jcb.201003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han GA, Malintan NT, Collins BM, Meunier FA, Sugita S. Munc18-1 as a key regulator of neurosecretion. J Neurochem. 2010;115(1):1–10. doi: 10.1111/j.1471-4159.2010.06900.x. [DOI] [PubMed] [Google Scholar]
- 25.Burkhardt P, Hattendorf DA, Weis WI, Fasshauer D. Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. EMBO J. 2008;27(7):923–933. doi: 10.1038/emboj.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu SH, Latham CF, Gee CL, James DE, Martin JL. Structure of the Munc18c/Syntaxin4 N-peptide complex defines universal features of the N-peptide binding mode of Sec1/Munc18 proteins. Proc Natl Acad Sci USA. 2007;104(21):8773–8778. doi: 10.1073/pnas.0701124104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khvotchev M, et al. Dual modes of Munc18-1/SNARE interactions are coupled by functionally critical binding to syntaxin-1 N terminus. J Neurosci. 2007;27(45):12147–12155. doi: 10.1523/JNEUROSCI.3655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McEwen JM, Kaplan JM. UNC-18 promotes both the anterograde trafficking and synaptic function of syntaxin. Mol Biol Cell. 2008;19(9):3836–3846. doi: 10.1091/mbc.E08-02-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rathore SS, et al. Syntaxin N-terminal peptide motif is an initiation factor for the assembly of the SNARE-Sec1/Munc18 membrane fusion complex. Proc Natl Acad Sci USA. 2010;107(52):22399–22406. doi: 10.1073/pnas.1012997108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou P, et al. Syntaxin-1 N-peptide and H(abc)-domain perform distinct essential functions in synaptic vesicle fusion. EMBO J. 2013;32(1):159–171. doi: 10.1038/emboj.2012.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malintan NT, et al. Abrogating Munc18-1-SNARE complex interaction has limited impact on exocytosis in PC12 cells. J Biol Chem. 2009;284(32):21637–21646. doi: 10.1074/jbc.M109.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meijer M, et al. Munc18-1 mutations that strongly impair SNARE-complex binding support normal synaptic transmission. EMBO J. 2012;31(9):2156–2168. doi: 10.1038/emboj.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cetica V, et al. STXBP2 mutations in children with familial haemophagocytic lymphohistiocytosis type 5. J Med Genet. 2010;47(9):595–600. doi: 10.1136/jmg.2009.075341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prekeris R, Klumperman J, Scheller RH. Syntaxin 11 is an atypical SNARE abundant in the immune system. Eur J Cell Biol. 2000;79(11):771–780. doi: 10.1078/0171-9335-00109. [DOI] [PubMed] [Google Scholar]
- 35.Valdez AC, Cabaniols JP, Brown MJ, Roche PA. Syntaxin 11 is associated with SNAP-23 on late endosomes and the trans-Golgi network. J Cell Sci. 1999;112(Pt 6):845–854. doi: 10.1242/jcs.112.6.845. [DOI] [PubMed] [Google Scholar]
- 36.Al Hawas R, et al. Munc18b/STXBP2 is required for platelet secretion. Blood. 2012;120(12):2493–2500. doi: 10.1182/blood-2012-05-430629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Offenhäuser C, et al. Syntaxin 11 binds Vti1b and regulates late endosome to lysosome fusion in macrophages. Traffic. 2011;12(6):762–773. doi: 10.1111/j.1600-0854.2011.01189.x. [DOI] [PubMed] [Google Scholar]
- 38.Tadokoro S, Kurimoto T, Nakanishi M, Hirashima N. Munc18-2 regulates exocytotic membrane fusion positively interacting with syntaxin-3 in RBL-2H3 cells. Mol Immunol. 2007;44(13):3427–3433. doi: 10.1016/j.molimm.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Gulyás-Kovács A, et al. Munc18-1: Sequential interactions with the fusion machinery stimulate vesicle docking and priming. J Neurosci. 2007;27(32):8676–8686. doi: 10.1523/JNEUROSCI.0658-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye S, et al. Syntaxin-11, but not syntaxin-2 or syntaxin-4, is required for platelet secretion. Blood. 2012;120(12):2484–2492. doi: 10.1182/blood-2012-05-430603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kögl T, et al. Hemophagocytic lymphohistiocytosis (HLH) in Syntaxin-11 deficient mice: T-cell exhaustion limits fatal disease. Blood. 2013;121(4):604–613. doi: 10.1182/blood-2012-07-441139. [DOI] [PubMed] [Google Scholar]
- 42.D'Orlando O, et al. Syntaxin 11 is required for NK and CD8(+) T-cell cytotoxicity and neutrophil degranulation. Eur J Immunol. 2013;43(1):194–208. doi: 10.1002/eji.201142343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren Q, et al. Munc13-4 is a limiting factor in the pathway required for platelet granule release and hemostasis. Blood. 2010;116(6):869–877. doi: 10.1182/blood-2010-02-270934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elstak ED, et al. The munc13-4-rab27 complex is specifically required for tethering secretory lysosomes at the plasma membrane. Blood. 2011;118(6):1570–1578. doi: 10.1182/blood-2011-02-339523. [DOI] [PubMed] [Google Scholar]
- 45.Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404(6776):355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- 46.Christie MP, et al. Low-resolution solution structures of Munc18:Syntaxin protein complexes indicate an open binding mode driven by the Syntaxin N-peptide. Proc Natl Acad Sci USA. 2012;109(25):9816–9821. doi: 10.1073/pnas.1116975109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnstable CJ, Hofstein R, Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985;352(2):286–290. doi: 10.1016/0165-3806(85)90116-6. [DOI] [PubMed] [Google Scholar]
- 48.Riento K, et al. Interaction of Munc-18-2 with syntaxin 3 controls the association of apical SNAREs in epithelial cells. J Cell Sci. 1998;111(Pt 17):2681–2688. doi: 10.1242/jcs.111.17.2681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.