Abstract
Caspase-1 is a cysteine protease that can be activated by both endogenous and exogenous inflammatory stimuli and has been shown to have important functions in processes as diverse as proteolytic activation of cytokines, cell death, and membrane repair. Caspase-1–dependent production of the inflammatory cytokines IL-1 and IL-18 has also been implicated in the regulation of appetite, body weight, glucose homeostasis, and lipid metabolism. Consistent with the emerging views of caspase-1 in metabolic regulation, we find that caspase-1–deficient mice have dramatically accelerated triglyceride clearance, without alteration in lipid production or absorption, and resultant decrease in steady-state circulating triglyceride and fatty acid levels. Surprisingly, this effect is independent of IL-1-family signaling, supporting the concept that caspase-1 influences lipid metabolism through multiple mechanisms, not limited to cytokines.
Keywords: inflammasome, inflammation, lipoprotein
Inflammation affects almost every aspect of physiology, ranging from metabolism to behavior. Chronic inflammation is also increasingly implicated in a variety of pathological processes and diseases of homeostasis, including atherosclerosis, obesity, and type 2 diabetes (1, 2). Studies of critically ill patients or animals have shown that multiple metabolic fluxes are altered by inflammation. Septic patients may go through periods of profound hyperglycemia or hypoglycemia, experience muscle wasting in cachexia, and have disordered lipid metabolism (3, 4). Simultaneously, studies in endocrinology and metabolism have revealed that obesity and its accompanying diseases—atherosclerosis, type 2 diabetes, and nonalcoholic steatohepatitis, for example—are accompanied by a state of chronic, low-grade inflammation most prominently observed in white adipose tissue (WAT) (5–7). These observations suggested that inflammation induced by overfed and distressed WAT might lead to cytokine elaboration and that these cytokines may disrupt normal metabolism. Proof of concept emerged in the mid-1990s with the discovery that the inflammatory cytokine TNF-α could cause insulin resistance in cultured 3T3-L1 adipocytes (8), that expression of this cytokine was increased in adipose tissue of obese animals and humans (5), and that suppression of TNF-α signaling improved insulin resistance in a model of diet-induced obesity (9). Recent studies have also revealed an important role played by caspase-1 in a variety of inflammatory conditions (10). The function of caspase-1 is best understood in the context of inflammasomes—multiprotein complexes involved in regulated processing and secretion of leaderless cytoplasmic proteins, including members of the IL-1 family (11). The inflammasomes containing nucleotide binding oligomerization domain-like receptor (NLR) family, pyrin domain containing 3 (NLRP3) protein have been reported to sense a vast array of nonmicrobial molecules such as hyaluronan, extracellular ATP, crystals of cholesterol, monosodium urate, silica, or asbestos (12), and even fatty acids (13) or glucose (14, 15), many of which are found endogenously.
One function of the inflammasome is the proteolytic activation of IL-1β and IL-18 from their respective propeptide forms. However, many additional functions of the inflammasome have now been described, including unconventional secretion of proteins lacking endoplasmic reticulum-targeting signal (“leader”) sequences (16) and an inflammatory form of programmed cell death called “pyroptosis.” Caspase-1 has also been reported to have effects on metabolic function. Specifically, caspase-1 has been recently implicated in the control of body weight and glucose homeostasis (13, 17–19). These effects have largely been attributed to the elaboration of cytokines such as IL-1β, consistent with the findings that the caspase-1–dependent cytokines IL-1 and IL-18 seem to affect adipogenesis, adiposity, and lipid metabolism (13, 19–22). In addition, some glycolytic enzymes are proteolytic targets of caspase-1 (23), and several recent reports have suggested that mice deficient in inflammasome signaling have improved glucose homeostasis (18, 19).
In addition to alterations in glycolysis, caspase-1 may also affect cellular lipid metabolism. In CHO cells, caspase-1 activation by bacterial aerolysin leads to processing and nuclear translocation of sterol regulatory element-binding protein-1 and -2 to support membrane repair and perhaps other lipid-requiring processes (22). One group has also observed caspase-1–dependent proteolysis of peroxisome proliferator-activated receptor (PPAR)-γ in 3T3-L1 adipocytes (24), although this finding has proven difficult to repeat by independent investigators (25). In addition to direct effects of caspase-1 on lipogenic transcription factors, secretion of IL-1β also affects lipid metabolism. IL-1β is reported to induce hypertriglyceridemia through inhibition of lipoprotein lipase activity and to inhibit adipogenesis (19, 21, 26, 27). Mice deficient in IL-1 signaling become more obese than WT mice (28), whereas mice deficient in the natural antagonist of IL-1, IL-1ra (IL1rn) are protected from obesity (28–31). Oddly, although mice deficient in IL-18 are also reported to be obese, this is attributable to hyperphagia, rather than to altered adipogenesis or changes in energy expenditure (20, 32). Thus, different caspase-1–dependent cytokines have complementary roles in regulating whole-body metabolism.
Given the multitude of effects of IL-18 and IL-1 on metabolism, as well as reports of direct effects of caspase-1 on lipogenic transcription factors in vitro, we further investigated the role of caspase-1 in lipid metabolism. Here we find that caspase-1 plays a profound role in regulation of lipid metabolism in vivo by orchestrating clearance of circulating triglycerides (TGs) in young, lean mice. Surprisingly, this role is independent of signaling through the IL-1R1, IL-18R, or myeloid differentiation primary response gene 88 (MyD88), suggesting that caspase-1 can control lipid metabolism in vivo through multiple mechanisms, not limited to IL-1 family cytokines. Moreover, these data suggest that caspase-1 plays a vital role in metabolic control in the lean, healthy state, just as it does during pathophysiology. Caspase-1 blockade could improve upon beneficial clinical effects reported for IL-1β blockade by reducing dyslipidemia.
Results
Caspase-1−/− Mice Have Altered Steady State Lipid Homeostasis.
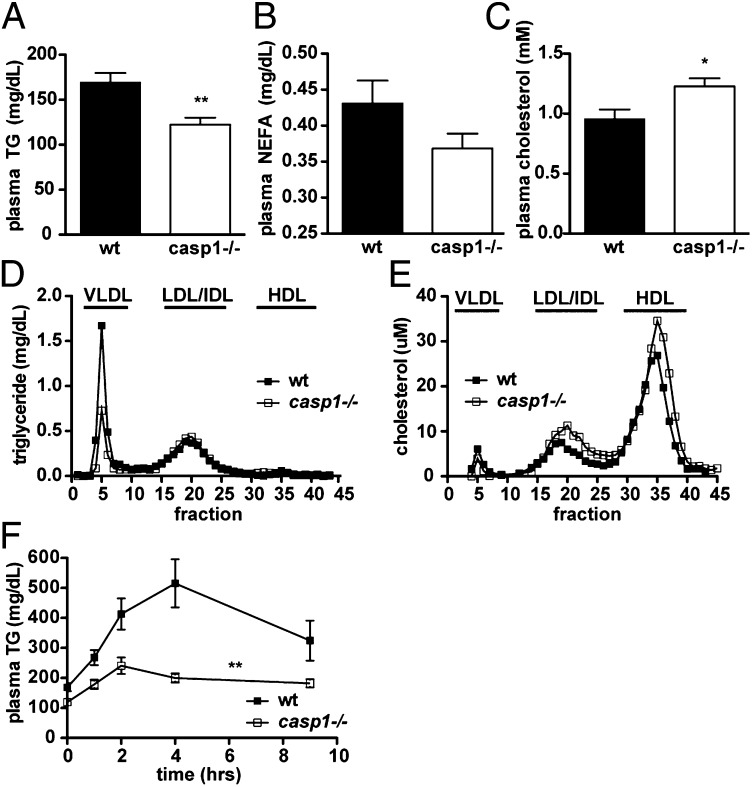
Given the previously reported roles of caspase-1 in body weight and glucose metabolism (13, 18, 19) and its observed influence on lipid metabolism in vitro (22), we investigated whether caspase-1 regulates lipid metabolism in vivo under physiologic conditions. When we examined young, lean, chow-fed, caspase-1–deficient (casp1−/−) mice, we found that they had significantly lower fasting plasma TG levels and tended to have lower fasting plasma free fatty acids (nonesterified fatty acids, NEFA) (Fig. 1 A and B) compared with WT mice, despite similar body weight and composition (Table 1) and similar indirect calorimetric measurements (Table S1). A substantial portion of free fatty acids in plasma in the fasted state are derived from hydrolysis of stored TG (33). However, we did not observe any difference in fasting plasma glycerol concentration (8.3 ± 0.7 mg/dL in WT vs. 8.5 ± 0.7 mg/dL in casp1−/−, n = 6 to 7 per group), suggesting that adipose tissue lipolysis was not responsible for the different levels of NEFA. Interestingly, whereas TG concentration was decreased, total plasma cholesterol was significantly increased in casp1−/− mice (Fig. 1C). The cholesterol content was increased in both the HDL and LDL fractions, whereas it was slightly decreased in very (V)LDL (Fig. 1E). TG content, as expected, was selectively decreased in VLDL (Fig. 1D).
Fig. 1.
Chow-fed casp1−/− mice have altered lipid homeostasis. (A) Fasting plasma TG (data shown represent a combination of multiple independent experiments, with n > 26 per group), (B) NEFA (P = 0.1, n = 14 per group), and (C) fasting cholesterol (n = 6–7 per group). (D and E) Distribution of (D) cholesterol and (E) TG in fasting plasma samples separated by FPLC (pooled from 8 to 10 per group). (F) Plasma TG after an oral fat tolerance test (n = 17–19 per group).*P < 0.05; **P < 0.001. Error bars represent SEM.
Table 1.
WT and casp1−/− mice have similar body weight and composition when fed a chow diet
| Parameter | WT (n = 14) | casp1−/− (n = 14) | P value |
| Body weight (g) | 24.0 ± 0.5 | 24.9 ± 0.3 | 0.16 |
| Body fat (g) | 1.8 ± 0.2 | 1.6 ± 0.1 | 0.56 |
| % fat | 7.4 ± 1.0 | 6.4 ± 0.6 | 0.41 |
To better understand the altered TG metabolism in casp1−/− mice, we performed oral fat tolerance tests on overnight fasted mice. In addition to lower fasting TGs, casp1−/− mice showed a dramatically reduced TG excursion after an oral olive oil gavage (Fig. 1F). These reduced TG levels were paralleled by lower glycerol and free fatty acids (Fig. S1 A and B).
Dyslipidemia can cause insulin resistance through ectopic lipid distribution (34). Thus, we hypothesized that reduced TG levels could be the cause of the previously reported protection from insulin resistance in caspase-1–deficient animals (19). In our animal facility, however, casp1−/− mice showed similar insulin sensitivity to WT animals on both normal diet (Fig. S2A) and high-fat diet (Fig. S2 E and F). High fat-fed casp1−/− mice in our studies were prone to diet-induced obesity (Fig. S2 B–D), consistent with recent observations by Henao-Mejia et al. (17).
Caspase-1−/− Mice Show Accelerated TG Clearance.
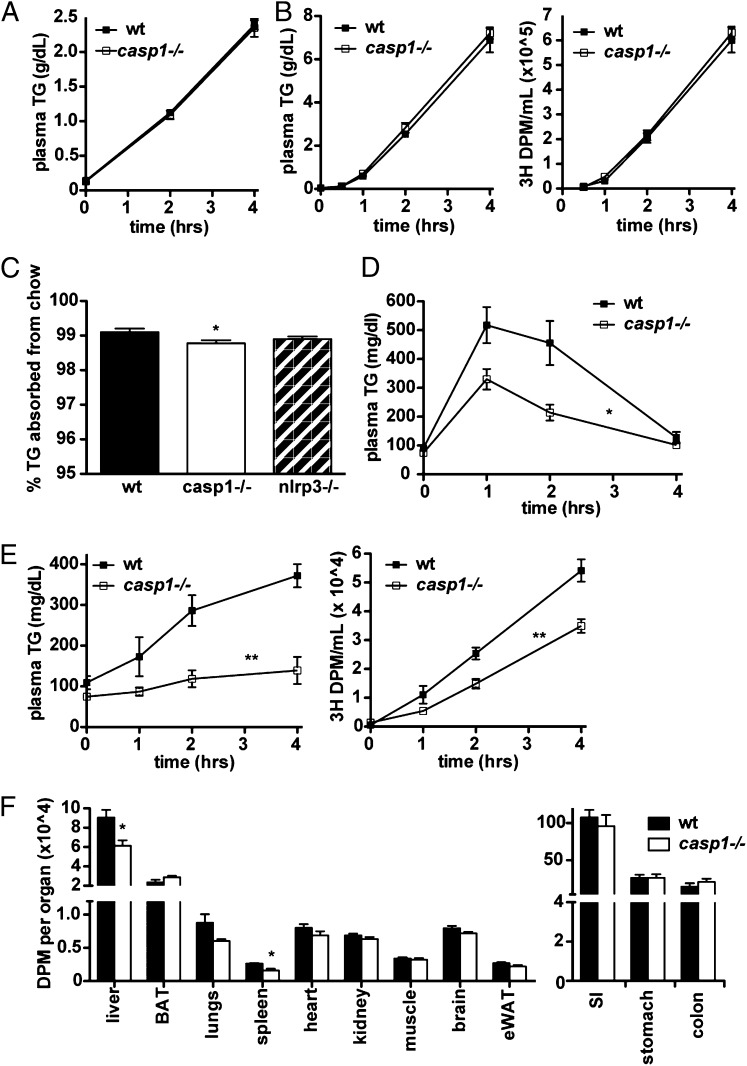
Decreased plasma TG could be caused by decreased entry of TG into the plasma as VLDL or chylomicrons or by accelerated clearance out of circulation. When we inhibited TG clearance from circulation in fasted mice by administering the nonionic surfactant poloxamer 407, we found that VLDL-TG accumulated similarly in casp1−/− and WT mice (Fig. 2A). Thus, TG export as VLDL did not seem to be quantitatively altered in casp1−/− mice. Similarly, when we inhibited TG clearance and gavaged mice with olive oil, we found that total TG (from chylomicrons and VLDL combined) accumulated similarly in casp1−/− and WT mice (Fig. 2B, Left), as did tracer counts from the labeled oral lipid load (Fig. 2B, Right). Notably, the amount of TG that accumulated in the plasma of gavaged mice was more than twice that of mice given only poloxamer, indicating that the olive oil was well absorbed in both strains of mice. Although the percentage of dietary TG extracted from the diet by casp1−/− mice was statistically less than that of WT mice (Fig. 2C), no significant difference was observed in nlrp3−/− mice, and we thought that it was unlikely that such a small difference could account for such a large observed effect on plasma TG. Moreover, when we injected mice i.p. with a TG emulsion to bypass gut absorption, we still observed a decreased TG excursion in casp1−/− mice relative to controls (Fig. 2D). Thus, neither intestinal absorption and chylomicron TG secretion nor hepatic VLDL-TG secretion were responsible for the reduced plasma TG levels observed in casp1−/− mice. This strongly suggested that accelerated clearance of TG was responsible for the phenotype of casp1−/− mice.
Fig. 2.
Chow-fed casp1−/− mice have accelerated TG clearance from circulation. (A) Plasma TG measured after administration of poloxamer into overnight fasted animals (n = 7–8 per group). (B) Plasma TG and 3H counts after injection of poloxamer combined with an oral lipid gavage containing 3H[9,10]triolein (n = 7 per group). (C) Fecal TG levels (n = 8–10 per group). (D) Plasma TG measured after an i.p. injection of lipid. (E) Plasma TG and 3H counts after administration of a labeled oral lipid gavage. (F) Tissue (3H) counts derived from the same experiment (n = 6 per group). *P < 0.05, **P < 0.005. Error bars represent SEM.
We wondered which tissue or organ was responsible for the rapid clearance of TG during a fat tolerance test. To examine this, we gavaged WT and casp1−/− mice with olive oil containing 3H[9,10] triolein as a tracer. Similar to our other studies, TG levels and tracer counts were substantially lower in casp1−/− mice compared with WT mice (Fig. 2 E and F), suggesting that the radioactive tracer, like other TG, was more rapidly cleared from circulation. Surprisingly, no tissue sampled showed greater tracer uptake, whereas liver and kidney showed lesser tracer uptake (Fig. 2F, Left). Brown adipose tissue (BAT) has been shown to be an important consumer of plasma TG (35). Although the BAT of casp1−/− mice accumulated slightly more tracer than that of WT mice, we found that LPL activity and expression in BAT were actually lower, rather than increased, in casp1−/− mice (Fig. S3). Importantly, there was no significant increase in tracer counts remaining in the gut of casp1−/− mice, further confirming that intestinal absorption was not altered (Fig. 2F, Right).
Accelerated TG Clearance in casp1−/− Mice May Be Attributable to Lipoprotein Composition.
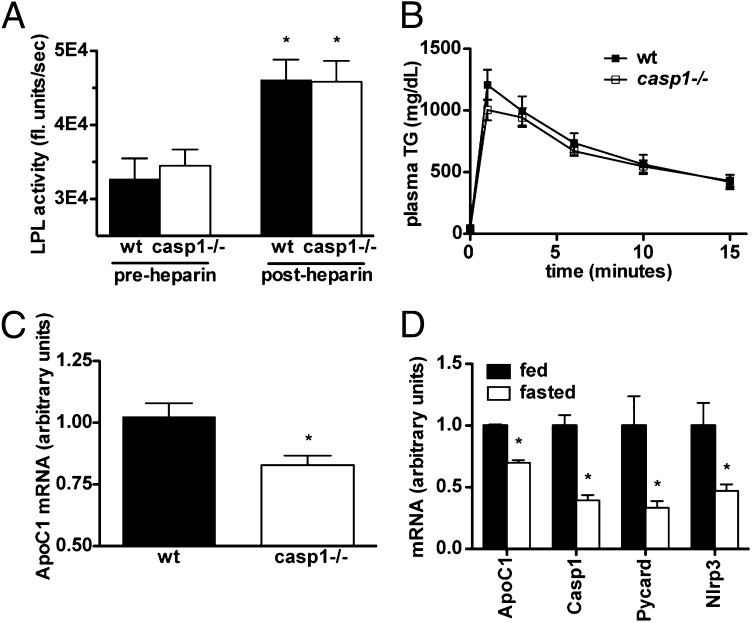
Lipoprotein lipase (LPL) is an enzyme produced by parenchymal cells that is active at the endothelial surface and hydrolyzes circulating TG to release NEFA for use in the relevant tissue. LPL is responsible for the bulk of TG clearance from circulation and has previously been shown to be inhibited by IL-1 (21). Thus, we hypothesized that altered expression of LPL could contribute to the accelerated clearance seen in casp1−/− mice. However, postheparin plasma LPL activity on an artificial substrate did not differ between genotypes (Fig. 3A). Interestingly, when we injected TG emulsion (lacking apolipoproteins) i.v. into casp1−/− and WT mice, we found that it was cleared at a similar rate (Fig. 3B). Having already established that VLDL production and gut absorption did not differ between genotypes, this left us with possibility that although LPL quantity may not be different, its activity may differ owing to the composition of lipoproteins such as ApoC1-3 or other lipoproteins that act as inhibitors or cofactors of LPL; such effect may not be seen when using an artificial TG emulsion.
Fig. 3.
Clearance may depend on lipoprotein composition, but not LPL activity. (A) LPL activity measured in postheparin plasma on an artificial TG emulsion (n = 5–6 per group) (*vs. preheparin plasma). (B) Plasma TG measured during an i.v. fat tolerance test (n = 6–9 per group). (C) Hepatic ApoC1 mRNA expression (n = 15–16 per group). (D) mRNA expression of ApoC1, Casp1, Pycard (ASC), and Nlrp3 in overnight-fasted vs. ad libitum fed WT mice (n = 4 per group). *P < 0.01. Error bars represent SEM.
Unfortunately, very few reagents exist to examine mouse apolipoproteins. To get around this, we performed FPLC on mouse plasma to separate lipoproteins and then examined the proteins coeluting with each lipoprotein fraction by Coomassie staining. One such protein, which was reduced in casp1−/− mice, was tentatively identified by mass mapping as ApoC1. Because ApoC1 is expressed almost exclusively in the liver, we performed quantitative PCR analysis on livers of WT and casp1−/− mice and found that its expression was indeed reduced in casp1−/− mice (Fig. 3C). Interestingly, we found that ApoC1 mRNA was coordinately regulated with inflammasome expression in the liver during the fasted-to-fed transition (Fig. 3D). Further quantitative PCR analysis also identified a subtle array of genes suggestive of increased PPAR-α activation (Fig. S4), although it is unclear whether these differences in gene expression are biologically significant.
Lipid Clearing Effect Seen in casp1−/− Mice Depends on the NLRP3 Inflammasome but Is Independent of IL-1 Family Cytokines.
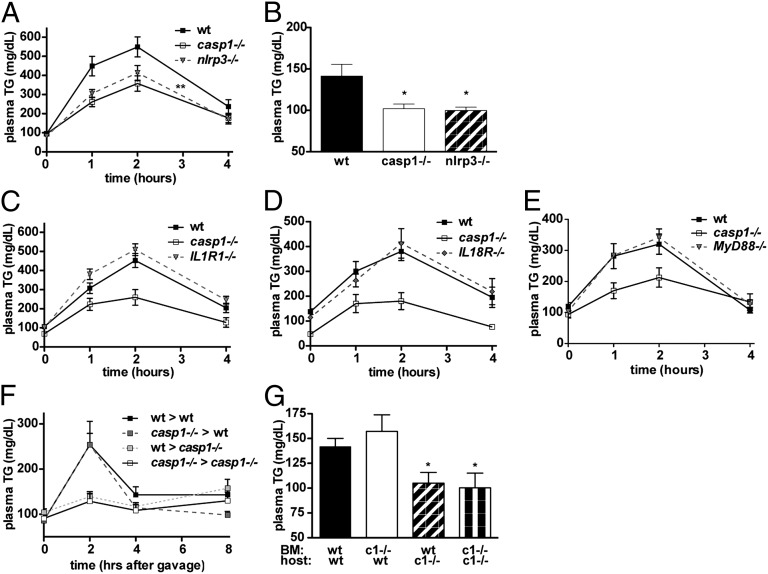
Caspase-1 can be activated by several members of the NLR family (10). NLRP3 in particular is known to be activated by multiple signals associated with tissue stress or damage. We therefore tested whether NLRP3 deficiency phenocopies the accelerated lipid clearance seen in casp1−/− mice. Indeed, we found that nlrp3−/− mice, like casp1−/− mice, had lower fasting TGs and accelerated lipid clearance compared with WT mice (Fig. 4 A and B), although the lipid clearing effect was slightly less than that observed in casp1−/−. Surprisingly, this phenotype was not observed in IL1R1−/− mice, which are deficient in IL-1α and IL-1β signaling (Fig. 4C), IL18R−/− mice, which are deficient in IL-18 signaling (Fig. 4D), or in MyD88−/− mice (Fig. 4E), which have defective signaling from all known IL-1 family receptors, as well as toll-like receptors. This is interesting because IL-1–deficient mice also have altered lipid handling, and the in vivo metabolic phenotypes previously described for caspase-1 have been primarily attributed to the metabolic effects of IL-1β (18, 19, 29, 30, 36).
Fig. 4.
Lipid clearing effect is mediated by the NLRP3 inflammasome in nonhematopoietic cells. (A) Intraperitoneal fat tolerance test (P < 0.07 vs. WT by ANOVA, P < 0.03 vs. WT t test of area under the curve, n = 13–14 per group) and (B) fasting plasma lipids in nlrp3−/− mice (n = 6–8 per group). (C) Intraperitoneal fat tolerance tests in IL1R1−/− mice (n = 12 per group), (D) IL18R−/− mice (n = 12 per group), and (E) MyD88−/− mice (n = 6–7 per group). (F) Oral fat tolerance test and (G) fasting plasma TG in bone marrow chimeras (n = 8–10 per group; c1−/− indicates casp1−/−). *P < 0.05. Error bars represent SEM.
Lipid Clearing Effect Is Due to Caspase-1 Activity in Nonhematopoietic Cells.
Although the vast majority of work on caspase-1 has focused on its role in macrophages, several investigators have now reported caspase-1 activity in distinct cell types, including keratinocytes (16) and pancreatic β cells (14, 15). To determine whether the lipid clearing effect of caspase-1 is due to caspase-1 activity in macrophages or another cell type, we generated bone marrow chimeras in which we transplanted casp1−/− or WT bone marrow into lethally irradiated casp1−/− or WT hosts. WT hosts transplanted with either type of bone marrow exhibited slower lipid clearance than casp1−/− mice with either type of bone marrow (Fig. 4F). Similarly, the reduced fasting plasma TG was observed in casp1−/− hosts with either type of transplant, but not in WT hosts (Fig. 4G). This suggests that the lipid clearing effect seen in casp1−/− mice is due to caspase-1 activity in a nonhematopoietic cell type, although we cannot formally rule out the potential role of a radio-resistant hematopoietic cell type.
Discussion
It has become abundantly clear over the last decade that caspase-1 has important metabolic effects in rodents and humans that are induced by IL-1β. Although the inflammasome remains incompletely understood, it seems likely that its various functions constitute a carefully orchestrated response, of which IL-1β production is just one aspect.
We found that caspase-1–deficient (casp1−/−) mice have profoundly altered lipid metabolism. Under fasting conditions, the mice demonstrated a consistent decrease in plasma TG with a concomitant increase in total plasma cholesterol. When challenged with a bolus of lipid, as might occur after a high-fat meal, caspase-1–deficient mice demonstrated dramatically accelerated clearance of lipid from plasma with no quantitative alteration of lipid production from the gut or liver. Surprisingly, these data contrast with the recently published work by van Diepen et al. (37), in which the authors reported reduced TG absorption and reduced hepatic VLDL production. Of note, these authors used Triton, rather than poloxamer, to inhibit LPL and demonstrate altered VLDL production. Although neither of these inhibitors is specific to LPL, we chose poloxamer to avoid some of the potential confounding effects of Triton (38). Moreover, we opted to combine poloxamer with lipid gavage to more clearly distinguish between absorption and clearance, as well as to bypass the gut entirely by delivering lipid i.p. Like van Deipen et al., however, we could not identify a tissue that was acting as the sink for TG clearance. It is possible that the lipid was rapidly absorbed into a widely distributed tissue that could not be effectively sampled using this methodology. Although postheparin LPL did not differ between caspase-1–deficient animals and WT, we believe that the clearance effect could still be due to an LPL activity difference imparted by altered apolipoprotein expression. We found that ApoC1 expression was suppressed in livers of casp1−/− mice, which would be expected to disinhibit LPL-mediated hydrolysis and TG clearance. Such differences in apolipoprotein expression could theoretically be imparted by the altered flora reported in inflammasome-deficient mice (17), or through caspase-1–dependent effects of transcription factors (24) or transcriptional machinery.
IL-1β has also been reported to influence TG metabolism through its effects on LPL expression and/or activity. Surprisingly, the lipid clearing phenotype of casp1−/− animals was not dependent on signaling through IL-1R1. Moreover, as discussed, LPL availability was not affected. This suggests that caspase-1 coordinates lipid metabolism through multiple mechanisms. Although independent of IL-1 or IL-1 family signaling, the effect we observed was dependent on NLRP3. Recent work has demonstrated activation of the NLRP3 inflammasome in response to fatty acids (13). Thus, it is possible that sensing of lipids could stimulate inflammasome activation to regulate lipid metabolism. Given that activity of the double-stranded RNA-dependent protein kinase (PKR) is increased during overnutrition (39), and also controls inflammasome activation in response to a wide variety of stimuli (40), it is interesting to consider whether this kinase may also play an important role in our observed phenotype. Also of interest is the question of whether an identical metabolic response occurs in response to other means of NLRP3 activation, such as by alum or uric acid crystals, or whether different NLRP3 ligands can lead to distinct biological outcomes.
Ours is one of a minority of reports currently in the literature of caspase-1 activity within nonhematopoietic cells. Although inflammasome components are expressed at very low levels in most cell types other than macrophages, our data suggest that even low expression of inflammasome components can have important functional consequences. Moreover, caspase-1 activity seems to be important under healthy, uninfected conditions, as well as conditions of profound injury, infection, or stress such as have been previously described. It is possible that the effects of caspase-1 on metabolism are distinct, depending on the overall physiological status of the organism. At low levels, for example, caspase-1 may modulate lipids through a cytokine-independent effect, whereas at higher levels of activation, such as during infection, caspase-1–dependent elaboration of IL-1β or IL-18 may drive metabolic shifts. This could explain why no IL-1–dependent effect was observed under the conditions we tested.
In summary, this work is consistent with a model wherein caspase-1 inhibits TG clearance. Inhibition of caspase-1 activity may have the potential to prevent the progression of dyslipidemia and its pathologic consequences in human patients.
Materials and Methods
Animal Care and Maintenance.
Caspase-1−/− (casp1−/−) (41), nlrp3−/− (42), MyD88−/−, and IL1R1−/− (Jackson Laboratory) mice on a B6 background (backcrossed ≥nine times) or age-matched C57BL/6J (WT) mice (NCI or littermates), were maintained on a constant 12-h light:12-h dark cycle with free access to water and ad libitum access to standard chow diet (2018s, Harlan Teklad) and studied at 7–12 wk of age. Because our experiments demonstrated similar results whether using littermates or nonlittermate age- and sex-matched controls, a mixture of these controls was used. For high-fat diet studies, mice were maintained on standard chow until 6–8 wk of age before switching to high-fat diet (60% kCal from fat; D12492, Research Diets) for 12 wk. For bone marrow chimeras, casp1−/− mice were age-matched with CD45.1 congenically marked C57BL/6 mice (NCI Frederick). At ∼8 wk of age, mice were lethally irradiated with a dose of 10 Gy of X-ray irradiation and then i.v. injected with 15E6 red blood cell-depleted bone marrow cells from the appropriate donor. Mice were maintained on sulfatrim/baytril for 4 wk after irradiation and studied at least 2 wk thereafter. Effective engraftment was determined by fluorescence-activated cell sorting of peripheral mononuclear cells using antibodies against CD45.1 and CD45.2 (BD Bioscience). All animal studies were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All procedures were approved by the Institutional Animal Care and Use Committee of Yale University (Protocol 2008-08006).
Metabolic Parameters and Indirect Calorimetry.
Metabolic rate, food intake, and activity were measured using the Comprehensive Laboratory Animal Monitoring System (Columbus Instruments) over 48 h, and body composition by in vivo 1H magnetic resonance spectroscopy (MiniSpec, Bruker).
Lipid was extracted from equal masses of dried fecal pellets by 2:1 chloroform:methanol according to the Folsch method (43) from equal masses of tissue and then assayed using enzymatic kits (Triglyceride SL and Cholesterol Assay Kit, Cayman Chemical).
For glucose tolerance tests, overnight fasted mice were injected i.p. with 2.0 g/kg of glucose (for chow-fed mice) or 1.0 g/kg (for high-fat diet mice). Blood was sampled from the retroorbital plexus before injection and at the indicated times, and glucose was measured using a OneTouch Ultra glucometer (LifeScan). For insulin tolerance test, 4 h-fasted animals were injected i.p. with 1.0 U/kg of human recombinant insulin (Novolin, Novo Nordisk).
Plasma Analytes.
Plasma harvested from overnight-fasted mice was assayed for TG, NEFA, glycerol, and cholesterol using enzymatic kits (Triglyceride SL, Genzyme Diagnostics; NEFA-HR, Wako; glycerol and cholesterol assay kits, Cayman Chemical) according to the manufacturer’s instructions. Plasma was collected from overnight-fasted mice 10 min after i.v. injection of 100 U/kg heparin and assayed for LPL activity using a commercially available kit (Roar Biomedical).
For FPLC, plasma collected from overnight-fasted mice was pooled (n = 8–10 per group) and run on Superose 6 columns at a flow rate of 0.25 mL/min. A portion of each 0.5-mL fraction was assayed for TG and cholesterol content using commercially available kits (L-type Triglyceride, Wako; and cholesterol assay kit, Cayman Chemical). Fractions corresponding to lipoprotein peaks were pooled and Coomassie stained after SDS/PAGE. Bands of differing intensity between WT and casp1−/− mice were cut and sent for mass mapping at the Stanford proteomics core.
Lipid Tolerance Tests.
For oral fat tolerance tests, mice were fasted overnight 14–16 h in standard cages with ad libitum access to water. Mice were gavaged with 0.5 mL of olive oil, and blood was collected for measurement of TG content at the indicated time points by Triglyceride-SL (Genzyme). Concurrent analyses of glycerol and NEFA were measured using commercially available kits (glycerol assay, Cayman Chemical; NEFA-HR, Wako). Intraperitoneal fat tolerance tests were performed by injecting overnight-fasted mice with 200 μL of Intralipid 20% (vol/vol) fat emulsion (Sigma or Fresenius Kabi), harvesting blood from the retroorbital plexus at the indicated time points, and immediately separating it from plasma. Jugular venous catheters were implanted 6–7 d before i.v. fat tolerance tests. Intravenous fat tolerance tests were performed by injecting overnight-fasted mice with 100 μL of Intralipid 20% fat emulsion. To measure hepatic lipid export, overnight-fasted mice were injected with 1 g/kg poloxamer 407 (Pluronic F-127, Sigma), and plasma was collected at the indicated time points for analysis by Triglyceride-SL. To measure intestinal TG production, overnight-fasted mice were injected with 1 g/kg poloxamer. After 1 min the mice were gavaged with 0.5 mL olive oil, and plasma was collected at the indicated time-points and assayed with Triglyceride-SL. For studies involving radioactive tracer, 1 μCi of 3H[9,10]-triolein (Perkin-Elmer) was included in the olive oil gavage. Unfractionated plasma was subjected to scintillation counting in Ultima Gold mixture (Perkin-Elmer) on a TriCarb scintillation counter (Perkin-Elmer). For tissue radioactivity, individual tissues were flash frozen immediately after harvest, weighed, and then dissolved in Solvable (Perkin-Elmer) tissue solubilization buffer according to the manufacturer’s instructions. Solubilized samples were bleached with hydrogen peroxide before scintillation counting.
Quantitative RT-PCR.
For analysis of gene expression, total RNA was isolated from tissue by phenol/chloroform extraction followed by cleanup with RNeasy (Qiagen) according to the manufacturer’s instructions. Poly(A) mRNA was reverse transcribed, and PCR was performed using intron-spanning gene-specific primers and SYBR green master mix (Qiagen or Quanta) on a Stratagene machine (Agilent Technologies). A list of examined genes is included in Table S2. Fold change in mRNA expression was determined using the ΔΔcT method normalize to HPRT.
Statistical Analysis.
Data are expressed as mean ± SEM. Statistical significance was determined by t test or two-way ANOVA (for longitudinal studies), as appropriate. Statistically significant results are reported for P < 0.05.
Supplementary Material
Acknowledgments
We thank Jelena Bezbradica, Derek Erion, Patrick Tso, and Fred Gorelick for advice and technical assistance, and Blas A. Guigni, João-Paulo Camporez, Debbie Jiang, Julie Serr, and Sophie Cronin for technical assistance. This work was supported by National Institutes of Health Grants AI R01 046688 and AI R01 055502 (to R.M.), DK R01 40936 and U24 DK 059635 (to G.I.S.), MSTP TG 2T32GM07205 (to M.E.K.), and Yale Diabetes Endocrine Research Center Grant 2 P30 DK 045735 (to R.M.). R.M. and G.I.S. are investigators of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1301996110/-/DCSupplemental.
References
- 1.Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol. 2008;8(12):923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 3.Weissman C. The metabolic response to stress: An overview and update. Anesthesiology. 1990;73(2):308–327. doi: 10.1097/00000542-199008000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95(5):2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 9.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389(6651):610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 10.Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol. 2011;11(3):213–220. doi: 10.1038/nri2936. [DOI] [PubMed] [Google Scholar]
- 11.Martinon F, Mayor A, Tschopp J. The inflammasomes: Guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 12.Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev. 2011;243(1):152–162. doi: 10.1111/j.1600-065X.2011.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen H, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12(5):408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: A sensor for metabolic danger? Science. 2010;327(5963):296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 15.Youm YH, et al. Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage. Endocrinology. 2011;152(11):4039–4045. doi: 10.1210/en.2011-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller M, Rüegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132(5):818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 17.Henao-Mejia J, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482(7384):179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stienstra R, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12(6):593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Netea MG, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12(6):650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- 21.Beutler BA, Cerami A. Recombinant interleukin 1 suppresses lipoprotein lipase activity in 3T3-L1 cells. J Immunol. 1985;135(6):3969–3971. [PubMed] [Google Scholar]
- 22.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126(6):1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 23.Shao W, Yeretssian G, Doiron K, Hussain SN, Saleh M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem. 2007;282(50):36321–36329. doi: 10.1074/jbc.M708182200. [DOI] [PubMed] [Google Scholar]
- 24.He F, Doucet JA, Stephens JM. Caspase-mediated degradation of PPARgamma proteins in adipocytes. Obesity (Silver Spring) 2008;16(8):1735–1741. doi: 10.1038/oby.2008.269. [DOI] [PubMed] [Google Scholar]
- 25.Guilherme A, Tesz GJ, Guntur KV, Czech MP. Tumor necrosis factor-alpha induces caspase-mediated cleavage of peroxisome proliferator-activated receptor gamma in adipocytes. J Biol Chem. 2009;284(25):17082–17091. doi: 10.1074/jbc.M809042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price SR, Mizel SB, Pekala PH. Regulation of lipoprotein lipase synthesis and 3T3-L1 adipocyte metabolism by recombinant interleukin 1. Biochim Biophys Acta. 1986;889(3):374–381. doi: 10.1016/0167-4889(86)90201-6. [DOI] [PubMed] [Google Scholar]
- 27.Grégoire F, et al. Interferon-gamma and interleukin-1 beta inhibit adipoconversion in cultured rodent preadipocytes. J Cell Physiol. 1992;151(2):300–309. doi: 10.1002/jcp.1041510211. [DOI] [PubMed] [Google Scholar]
- 28.García MC, et al. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes. 2006;55(5):1205–1213. doi: 10.2337/db05-1304. [DOI] [PubMed] [Google Scholar]
- 29.Matsuki T, Horai R, Sudo K, Iwakura Y. IL-1 plays an important role in lipid metabolism by regulating insulin levels under physiological conditions. J Exp Med. 2003;198(6):877–888. doi: 10.1084/jem.20030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Somm E, et al. Decreased fat mass in interleukin-1 receptor antagonist-deficient mice: impact on adipogenesis, food intake, and energy expenditure. Diabetes. 2005;54(12):3503–3509. doi: 10.2337/diabetes.54.12.3503. [DOI] [PubMed] [Google Scholar]
- 31.Chida D, et al. Increased fat:carbohydrate oxidation ratio in Il1ra (-/-) mice on a high-fat diet is associated with increased sympathetic tone. Diabetologia. 2008;51(9):1698–1706. doi: 10.1007/s00125-008-1075-z. [DOI] [PubMed] [Google Scholar]
- 32.Zorrilla EP, et al. Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci USA. 2007;104(26):11097–11102. doi: 10.1073/pnas.0611523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehnholm C. Cellular Lipid Metabolism. Berlin: Springer; 2009. [Google Scholar]
- 34.Samuel VT, Shulman GI. Mechanisms for insulin resistance: Common threads and missing links. Cell. 2012;148(5):852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartelt A, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17(2):200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 36.de Roos B, et al. Attenuation of inflammation and cellular stress-related pathways maintains insulin sensitivity in obese type I interleukin-1 receptor knockout mice on a high-fat diet. Proteomics. 2009;9(12):3244–3256. doi: 10.1002/pmic.200800761. [DOI] [PubMed] [Google Scholar]
- 37.Van Diepen JA, et al. Caspase-1 deficiency in mice reduces triglyceride absorption and hepatic triglyceride secretion. J Lipid Res. 2013;54(2):448–456. doi: 10.1194/jlr.M031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millar JS, Cromley DA, McCoy MG, Rader DJ, Billheimer JT. Determining hepatic triglyceride production in mice: Comparison of poloxamer 407 with Triton WR-1339. J Lipid Res. 2005;46(9):2023–2028. doi: 10.1194/jlr.D500019-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura T, et al. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140(3):338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu B, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488(7413):670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267(5206):2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 42.Sutterwala FS, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24(3):317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.