Gibberellins (GA) represent a key class of hormone signals that promote plant growth and development (1). A key part of the Green Revolution, which saw crop yields more than double, was the development of new dwarf varieties, many of which were later found to have mutations in the GA pathway (2–4). Thus, understanding GA’s growth regulation represents a prime target for further increasing crop production. Considerable progress has recently been made dissecting the molecular basis of GA action (reviewed in ref. 1); however, despite these advances, it remains unclear how this key hormone promotes growth at either the cellular, tissue, or organ levels of organization. In PNAS, Shani et al. (5) describe how GA is distributed within root tissues of the model plant Arabidopsis thaliana. By developing a fluorescent-labeled GA, the authors were able to demonstrate that this key growth-promoting hormone signal accumulates within a specific root tissue and developmental zone.
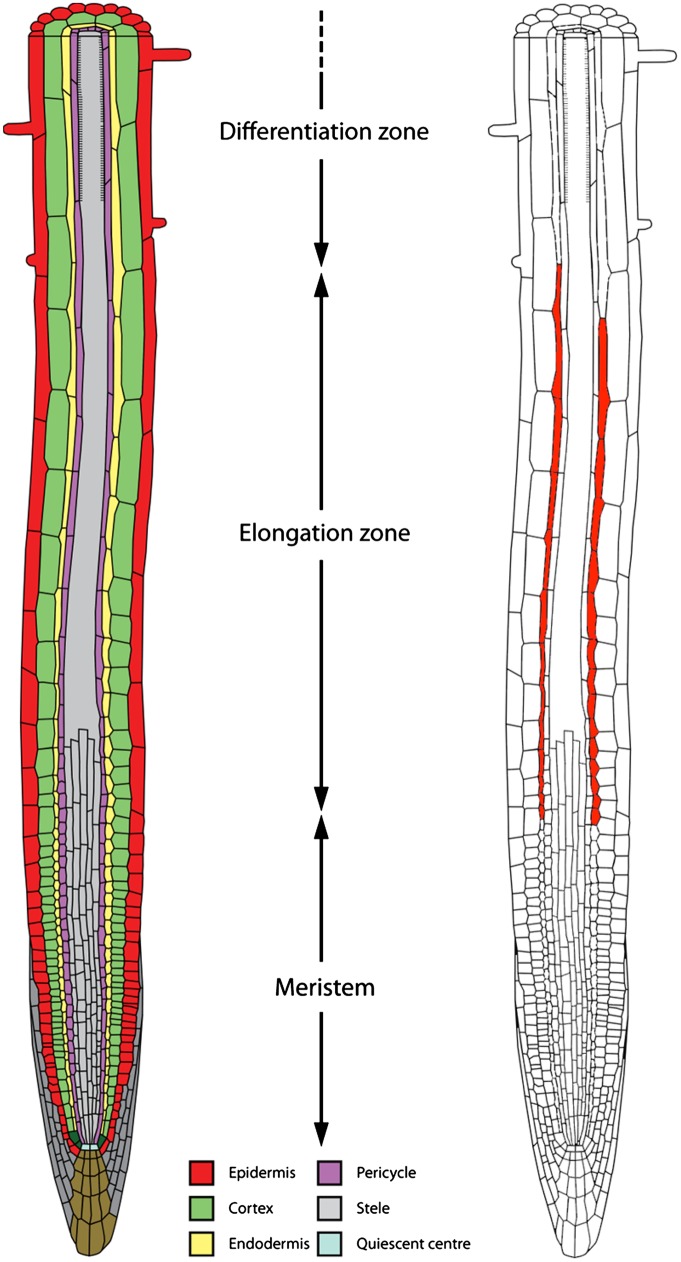
The Arabidopsis primary root has a simple structure composed, along the radial axis, of concentric layers of epidermal, cortical, endodermal, pericycle, and stele (vascular) tissues; and along the apical-basal axis of spatially distinct meristem, elongation, and differentiation zones (see schematic in Fig. 1). Cells divide close to the root tip in the meristematic zone; then, after stopping dividing and entering the elongation zone, cells undergo rapid expansion; cells then eventually cease growth upon entering the differentiation zone. Studies have found that mutating components of the GA biosynthesis or signaling pathways results in a significantly shorter root length (6, 7) because of GA promoting cell division in the root meristem (8, 9) and cell expansion in the elongation zone (7, 10).
Fig. 1.
Schematic illustrations of (Left) the tissue organization and zones within the Arabidopsis primary root, and (Right) the cellular distribution of the hormone signal GA. The concentric layers of epidermal, cortical, endodermal, pericycle, and stele (vascular) tissues are color coded (see key); the GA distribution is denoted in red.
At the molecular scale, GA promotes growth by triggering the degradation of the growth-repressing DELLA proteins. GA could promote Arabidopsis root growth by coordinating the simultaneous degradation of DELLA proteins in every tissue, or alternatively, DELLA degradation may only be required in one or more tissues. Several pieces of evidence suggest that GA is distributed unequally between tissues and growth zones. For example, the root endodermis has been shown to be particularly important in GA regulation of root growth: Ubeda Tomás et al. (8, 10) reported that by tissue specifically expressing a nondegradable form of DELLA, root growth was blocked when GA signaling was prevented within endodermal cells. Recently, direct measurements of GA metabolites using a mass spectrometry (MS)-based approach in the maize leaf (which has equivalent developmental zones to the Arabidopsis root) have revealed that bioactive GA levels are high in the so-called transition zone where cells cease to divide and start to expand (11). Nevertheless, directly determining exactly where the GA signal accumulated at a cellular level of resolution to control organ growth remained to be resolved until now.
Shani et al. (5) are able to address exactly where GA accumulated in root tissues by adopting a state-of-the-art imaging (rather than MS-based) solution. By attaching a fluorescent tag using variable lengths of an acyl amide linker to the C6 position of the tetracyclic di-terpenoid structures of GA3 and GA4, the authors are able to recover several fluorescent GA-surrogates (termed GA3-Fl and GA4-Fl) that retained bioactivity. GA3-Fl and GA4-Fl were also shown to promote the interaction between the GA receptor GID1 and its DELLA target using in vitro coimmunoprecipitation and yeast two-hybrid assays. Importantly, no breakdown products of GA3-Fl and GA4-Fl were detected after incubation with roots, indicating that these unique GA variants were stable in planta. Hence, wherever GA3-Fl and GA4-Fl accumulated was likely to represent the tissue site of GA.
Using these fluorescent compounds, Shani et al. (5) showed that GA accumulates within a specific primary root tissue and developmental zone. After short incubations (∼15 min) with GA3-Fl and GA4-Fl, Arabidopsis roots were observed to accumulate these GA variants specifically in endodermal cells within the elongation zone (see schematic summary in Fig. 1). In contrast, the pattern of GA3-Fl and GA4-Fl accumulation was not observed in the shr-2 radial patterning mutant that lacked an endodermal layer. The striking endodermal accumulation of GA3-Fl and GA4-Fl in wild-type roots is in complete agreement with earlier functional studies (7, 8) and the tissue distribution of the GA response reporter, RGApro::RGA-GFP (5). Hence, root endodermal cells in the transition/elongation zones (that accumulated GA3-Fl and GA4-Fl) appear to represent the cellular/tissue site of GA response. Unlike the root meristem, cells within the transition zone can either continue to divide or expand. This decision influences the rate at which cells enter the elongation zone and therefore profoundly impacts root growth rate, hence explaining its importance as a target for hormone regulation.
How is this striking root cell-specific pattern of GA accumulation regulated? It is well established that the hormone auxin is actively transported between neighboring cells, so that the cell-scale regulation of membrane proteins creates organ-scale auxin fluxes that control plant development (12). Despite a recent report that a member of the NRT1/PTR carrier family can transport the hormone signals ABA and GA in yeast (13), and earlier studies suggesting active transport does occur (14–16), GA transporters functioning in planta remain to be identified. Shani et al. (5) are able to demonstrate that GA3-Fl and GA4-Fl are transported actively (rather than via diffusion) and could be blocked with metabolic inhibitors or by lower incubation temperatures. Hence, a saturable, energy-dependent GA transport pathway appears to exist that controls GA accumulation in root tissues. GA3-Fl and GA4-Fl promise to provide invaluable tools with which to perform genetic screens and identify and characterize these unique classes of GA carriers.
GA distribution also appears to be controlled at the subcellular scale. Shani et al. (5) observed that GA3-Fl and GA4-Fl accumulated in elongating endodermal cell nuclei and vacuoles, raising the fascinating question: Do distinct GA transporters exist to fine-tune the hormone’s subcellular distribution? We recently hypothesized that the subcellular redistribution of GA into vacuoles plays a crucial role in diluting this growth-promoting signal as root cells expand, resulting in the cessation of cell growth (10). Identifying and manipulating the GA carriers that mediate this subcellular transport pathway could provide a novel means to control the cytoplasmic/nuclear GA concentration, and thus control organ growth.
How can GA regulate root growth by accumulating in expanding endodermal cells when it represents just one of several root tissues? Other studies have also reported that hormones, like auxin (12) and brassinosteroids (17), control root growth by targeting expanding epidermal cells (reviewed in ref. 18). Because expansion of inner and outer root tissues is regulated by distinct hormones, communication between tissues may
The present study by Shani et al. elegantly illustrates the benefits of developing fluorescently labeled plant hormones.
be required to coordinate organ growth. Identification of the signals controlling tissue-to-tissue communication will be crucial to unravel the mechanisms coordinating growth. A clue may come from another observation by Shani et al. (5) that the gaseous hormone ethylene blocks the accumulation of GA3-Fl and GA4-Fl in elongating endodermal cells. Hence, ethylene may control root growth by modulating GA response, in part through down-regulation of GA transport. Ethylene inhibition of root growth can be blocked by disrupting the auxin response in every elongation zone tissue (19). It is therefore possible that ethylene coordinates root growth, in part, by controlling auxin and GA response and transport pathways.
In summary, the present study by Shani et al. (5) elegantly illustrates the benefits of developing fluorescently labeled plant hormones. By developing such reporters, one can obtain quantitative cell-scale data on dynamic hormone levels that was not feasible, or had to be determined indirectly using previously available methods (11, 20). Combining such quantitative data with mechanistic models will accelerate our understanding of the regulation of organ growth (as has recently been demonstrated in refs. 10 and 21). In addition to regulating organ growth, GA has been shown to affect many developmental processes, including seed germination, floral development, and leaf shape (1). Although Shani et al. (5) focused their studies on the Arabidopsis primary root, in the longer term these fluorescent-tagged GA variants will prove invaluable tools to understand the diverse roles of GA in many different plant organs and species.
Acknowledgments
This work was supported by the Leverhulme Trust (L.R.B.), the Biological and Biotechnological Science Research Council (M.J.B.), and the European Research Council (M.J.B.).
Footnotes
The authors declare no conflict of interest.
See companion article on page 4834.
References
- 1.Fleet CM, Sun TP. A DELLAcate balance: The role of gibberellin in plant morphogenesis. Curr Opin Plant Biol. 2005;8(1):77–85. doi: 10.1016/j.pbi.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Peng J, et al. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature. 1999;400(6741):256–261. doi: 10.1038/22307. [DOI] [PubMed] [Google Scholar]
- 3.Ueguchi-Tanaka M, et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature. 2005;437(7059):693–698. doi: 10.1038/nature04028. [DOI] [PubMed] [Google Scholar]
- 4.Fu X, et al. Gibberellin-mediated proteasome-dependent degradation of the barley DELLA protein SLN1 repressor. Plant Cell. 2002;14(12):3191–3200. doi: 10.1105/tpc.006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shani E, et al. Gibberellins accumulate in the elongating endodermal cells of Arabidopsis root. Proc Natl Acad Sci USA. 2013;110:4834–4839. doi: 10.1073/pnas.1300436110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu X, Harberd NP. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature. 2003;421(6924):740–743. doi: 10.1038/nature01387. [DOI] [PubMed] [Google Scholar]
- 7.Ubeda-Tomás S, et al. Root growth in Arabidopsis requires gibberellin/DELLA signalling in the endodermis. Nat Cell Biol. 2008;10(5):625–628. doi: 10.1038/ncb1726. [DOI] [PubMed] [Google Scholar]
- 8.Ubeda-Tomás S, et al. Gibberellin signaling in the endodermis controls Arabidopsis root meristem size. Curr Biol. 2009;19(14):1194–1199. doi: 10.1016/j.cub.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Achard P, et al. Gibberellin signaling controls cell proliferation rate in Arabidopsis. Curr Biol. 2009;19(14):1188–1193. doi: 10.1016/j.cub.2009.05.059. [DOI] [PubMed] [Google Scholar]
- 10.Band LR, et al. Growth-induced hormone dilution can explain the dynamics of plant root cell elongation. Proc Natl Acad Sci USA. 2012;109(19):7577–7582. doi: 10.1073/pnas.1113632109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelissen H, et al. A local maximum in gibberellin levels regulates maize leaf growth by spatial control of cell division. Curr Biol. 2012;22:1–5. doi: 10.1016/j.cub.2012.04.065. [DOI] [PubMed] [Google Scholar]
- 12.Swarup R, et al. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat Cell Biol. 2005;7(11):1057–1065. doi: 10.1038/ncb1316. [DOI] [PubMed] [Google Scholar]
- 13.Kanno Y, et al. Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proc Natl Acad Sci USA. 2012;109(24):9653–9658. doi: 10.1073/pnas.1203567109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drake GA, Carr DJ. Flux studies and compartmentation analysis of gibberellin A1 in oat coleoptiles. J Exp Bot. 1981;32(1):103–119. [Google Scholar]
- 15.Nour JM, Rubery PH. The uptake of gibberellin A1 by suspension-cultured Spinacia oleracea cells has a carrier-mediated component. Planta. 1984;160(5):436–443. doi: 10.1007/BF00429760. [DOI] [PubMed] [Google Scholar]
- 16.Ohlrogge JB, García-Martínez JL, Adams D, Rappaport L. Uptake and subcellular compartmentation of gibberellin a(1) applied to leaves of barley and cowpea. Plant Physiol. 1980;66(3):422–427. doi: 10.1104/pp.66.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hacham Y, et al. Brassinosteroid perception in the epidermis controls root meristem size. Development. 2011;138(5):839–848. doi: 10.1242/dev.061804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ubeda-Tomás S, Beemster GT, Bennett MJ. Hormonal regulation of root growth: Integrating local activities into global behaviour. Trends Plant Sci. 2012;17(6):326–331. doi: 10.1016/j.tplants.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Swarup R, et al. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell. 2007;19(7):2186–2196. doi: 10.1105/tpc.107.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells DM, Laplaze L, Bennett M, Vernoux T. Biosensors for phytohormone quantification: challenges, solutions, and opportunities. Trends Plant Sci. 2013 doi: 10.1016/j.tplants.2012.12.005. pii:S1360–S1385(12)00256-7. [DOI] [PubMed] [Google Scholar]
- 21.Band LR, et al. Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proc Natl Acad Sci USA. 2012;109(12):4668–4673. doi: 10.1073/pnas.1201498109. [DOI] [PMC free article] [PubMed] [Google Scholar]