Significance
The twin-arginine transport system (Tat) has the remarkable ability of transporting folded proteins across membranes while avoiding uncontrolled ion leakage. Tat is essential for plant photosynthesis and is required for bacterial pathogenesis. The mechanism by which folded proteins are translocated is poorly understood. We have determined the structure of the TatA oligomer, which is responsible for the translocation step, and evaluated its impact on lipid bilayers. The results suggest a mechanism of protein translocation involving thinning and perturbing the membrane bilayer. The approach used here will be useful for structural analysis of other oligomeric proteins that weakly assemble in the membrane.
Abstract
The twin-arginine translocase (Tat) carries out the remarkable process of translocating fully folded proteins across the cytoplasmic membrane of prokaryotes and the thylakoid membrane of plant chloroplasts. Tat is required for bacterial pathogenesis and for photosynthesis in plants. TatA, the protein-translocating element of the Tat system, is a small transmembrane protein that assembles into ring-like oligomers of variable size. We have determined a structural model of the Escherichia coli TatA complex in detergent solution by NMR. TatA assembly is mediated entirely by the transmembrane helix. The amphipathic helix extends outwards from the ring of transmembrane helices, permitting assembly of complexes with variable subunit numbers. Transmembrane residue Gln8 points inward, resulting in a short hydrophobic pore in the center of the complex. Simulations of the TatA complex in lipid bilayers indicate that the short transmembrane domain distorts the membrane. This finding suggests that TatA facilitates protein transport by sensitizing the membrane to transient rupture.
The twin-arginine translocase (Tat) pathway is one of two fundamentally different systems for translocating proteins out of the bacterial cytoplasm. In the Sec system, proteins are “threaded” across the membrane in an unfolded form (1). In the Tat system, which is structurally and mechanistically unrelated to the Sec translocase, proteins are translocated in a fully folded form (2, 3). The Tat pathway is required for important cellular processes, including energy metabolism (Tat can export cofactor-containing proteins), cell division, cell motility, quorum sensing, heavy metal resistance, iron acquisition, and biofilm formation (3). The Tat pathway is found in most bacterial pathogens and is normally required for virulence (4). The Tat system is also found in the thylakoid membrane of plant chloroplasts, where it is essential for the biogenesis of the photosynthetic apparatus (5, 6).
In Escherichia coli the Tat system consists of the three integral membrane proteins TatA, TatB, and TatC (7, 8). TatB and TatC form a tight complex (TatBC) that binds substrate proteins (9–12). TatA is then recruited to the TatBC/substrate complex, where it polymerizes to form the substrate translocation pathway (9, 13–17).
The physical mechanism by which folded substrates are translocated across the membrane is the most poorly understood aspect of the Tat pathway. It has been suggested that TatA polymerization produces a bespoke channel corresponding to the size of the transported substrate protein (18), or that it weakens or disorders the membrane bilayer to form a transport-permissive patch (19, 20).
E. coli TatA is an 89-residue monotopic integral membrane protein with the N terminus at the periplasmic face of the membrane (21). The monomer structure of the TatA family protein TatAd from Bacillus subtilis has been determined by NMR (22). As anticipated (23, 24), this determination shows that TatA comprises an N-terminal transmembrane helix (TMH; corresponding to residues 5–20 in E. coli TatA), followed by an amphipathic helix (APH; corresponding to residues 22–45 in E. coli TatA) and an unstructured and hydrophilic cytoplasmic tail (Fig. S1). The TMH and APH are oriented at approximately right angles to each other, forming an “L” shape. The junction between the two helices is centered on invariant Gly21 (E. coli TatA numbering) and is termed the “hinge.” The interhelix angle is maintained by packing interactions (the “hinge brace”).
A molecular-level description of the way TatA protomers assemble is an essential step in understanding Tat transport and will provide insight into likely modes of protein translocation. In native membranes TatA assembly is catalyzed by the substrate-loaded TatBC complex (14, 15) and requires a transmembrane proton motive force (9, 13). These conditions make structural studies in membranes extremely difficult. However, solubilization of TatA using mild detergents, such as dodecyl nonaoxyethylene ether (C12E9), digitonin, or dodecylmaltoside, favors TatA oligomerization and this manipulation has allowed determination of low-resolution electron microscopy structures of TatA and of TatE, a secondary TatA copy found in E. coli (12, 18, 25). The electron microscopy structures show ring-like complexes but provide no details of the arrangement of the oligomers within the electron density or knowledge about how TatA is able to mediate transport of folded proteins.
We report an atomic-level model for the TatA oligomer determined by solution NMR. We exploited the fact that oligomerization of TatA can be controlled by varying the concentration of the solubilizing detergent. The structure of the oligomeric complex was then assembled by combining a high-resolution structure of the monomer with experimentally determined intermolecular distance restraints in the oligomer. The oligomeric TatA structure reveals an assembly mediated entirely by the transmembrane helices. The transmembrane helices form a short hydrophobic pore, which modeling indicates would distort the membrane bilayer. The APH extend away from the pore, and would be accessible for interactions with the TatBC/substrate complex.
Results
Detergent-Dependent Oligomerization of TatA.
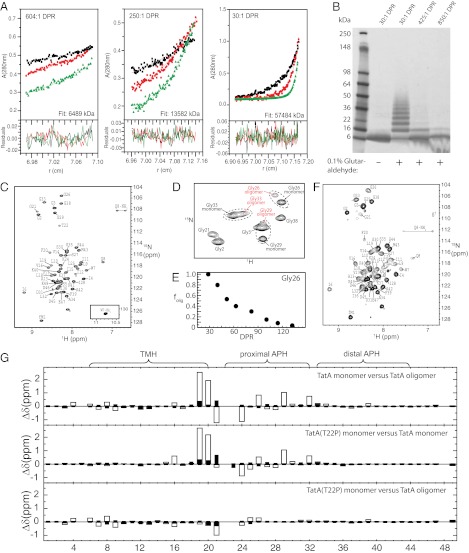
Understanding the molecular mechanism of the Tat translocation system requires a structural description of the complexes formed by TatA. TatA oligomers have been best characterized in the detergent C12E9 (18, 23, 26). In this detergent, TatA oligomers have variable subunit stoichiometry, with most complexes having apparent molecular masses between 130 and 390 kDa (18). The heterogeneity of these TatA complexes, as well as the observed flexibility of the TatA monomer (22), precludes structure determination by crystallization. We explored solution NMR as an alternative approach to elucidate the TatA complex structure. Solution NMR tolerates conformational flexibility and some heterogeneity. Nevertheless, the size and heterogeneity of the TatA complexes observed in C12E9 had to be significantly reduced to allow application of NMR methods. To achieve this result, we first used a truncated version of TatA that lacks the 40 unconserved and nonessential C-terminal residues of the cytoplasmic tail (27). These residues are disordered in the TatAd monomer NMR structure (22) and in the C12E9-solubilized E. coli TatA complex, as judged by circular dichroism measurements (23) and preliminary NMR experiments. The resultant truncated construct (henceforth referred to as TatA) is 49-aa long and retains the highly conserved TMH and APH domains (Fig. S1). Second, we screened for detergents in which TatA adopts a smaller, more homogeneous oligomeric state. We found that the oligomeric state of TatA in the detergent dodecylphosphocholine (DPC) can be manipulated by varying the detergent-to-protein ratio (DPR). This result is illustrated by equilibrium sedimentation analytical ultracentrifugation (Fig. 1A) and chemical cross-linking experiments (Fig. 1B), where the TatA concentration was kept constant but the detergent concentration varied. The average molecular mass of the TatA complexes and the number of TatA-TatA cross-links decreased with increasing DPR. At high DPRs TatA was predominantly monomeric as assessed by analytical ultracentrifugation and showed little cross-linking to other TatA molecules. Consistent with this analysis, NMR spectra of TatA at a DPR of 250 showed a homogeneous species with spectral line-widths indicative of a small, monodisperse protein (Fig. 1C). At lower DPRs an additional set of broader resonances appeared, which were most clearly seen for the well-resolved APH glycines (Fig. 1 D and E). By a DPR of 30, these new resonances had replaced the resonances arising from monomeric TatA, suggesting complete conversion of TatA to a previously undescribed form (Fig. 1F). Oligomers at least as large as an octamer can be detected at this DPR by cross-linking (Fig. 1B) and analytical ultracentrifugation indicates an average protein mass equivalent to an oligomeric state of approximately 9 (Fig. 1A). Thus, this unique spectral state is associated with an oligomeric form of TatA. The homogeneity of the oligomeric TatA spectra indicated a regular structure in which subunits adopt similar conformations and intermolecular packing interfaces. Although the oligomer state may represent a range of oligomers rather than a single oligomer, the spectral similarities indicate that the TatA protomers adopt highly similar structures and intersubunit relationships. Negative-stain electron microscopy at this DPR shows a ring-like morphology similar to that of TatA or TatE in other detergents (12, 18, 25) (Fig. S2A).
Fig. 1.
(A) Analytical ultracentrifugation of 167 µM TatA at DPR of 604:1, 250:1, and 30:1. Molecular weights of 6,489 kDa, 13,582 kDa, and 57,484 kDa, respectively, were determined from simultaneous fitting to data at rotor speeds of 20 K (black), 30 K (red), and 40 K (green). The monomeric TatA molecular mass was calculated to be 6.2 kDa under analytical ultracentrifugation conditions (50% 2H2O), indicating average oligomeric states of 1.0, 2.2, and 9.3, at DPR of 604:1, 250:1, and 30:1, respectively. (B) SDS/PAGE of TatA (500 µM) cross-linked with glutaraldehyde at DPR of 30:1, 425:1, and 850:1. (C) 1H-15N HSQC of monomeric TatA (500 µM) at a DPR of 250, with resonance assignments indicated. Resonances arising from the C-terminal 6-His tag are indicated with an asterisk. (D) 1H-15N HSQCs of the glycine region of TatA at an intermediate DPR of 60. TatA at DPR of 60 shows peak doubling for Gly26, Gly29, and Gly33 corresponding to monomeric and oligomeric TatA molecules. (E) The oligomeric fraction of the total peak volume from the two resolved Gly26 peaks: folig = [volumeoligomer/(volumeoligomer + volumemonomer)] as a function of DPR. (F) 1H-15N SOFAST-HMQC of oligomeric TatA (500 µM) (DPR = 30) with resonance assignments indicated. (G) Chemical shift differences in 1H (open bars) and 15N (filled bars) between the indicated TatA constructs.
Monomeric TatA(T22P) Is Structurally Similar to the TatA Oligomeric Subunit Conformation.
The large size of the micelle-bound TatA oligomer (total mass estimated to be approximately 90 kDa) prevented a conventional structure determination by solution NMR. However, a structural model of an oligomeric protein may be constructed using sparse intermolecular restraints and well-defined structures of the monomers as long as the monomer structures are maintained in the oligomer (28).
Upon oligomerization, TatA exhibits extensive chemical shift perturbations near the TMH/APH hinge (Fig. 1G). These perturbations could, in principle, have arisen from either structural or environmental changes. Because structural changes may interfere with oligomer contacts and prevent accurate model building, variants of the TMH/APH hinge region based on substitutions commonly found in other TatA proteins were expressed and characterized for their ability to preserve the oligomeric subunit conformation. Substituting the threonine residue at position 22 in E. coli TatA with the proline found in some other TatA molecules, including B. subtilis TatAd, produced a variant that oligomerized in a similar way to wild-type TatA (Fig. S2 B and C), but which in the monomer state retained close similarity in APH resonances to the oligomeric state (Fig. S3). The chemical shifts of residues 27–32 in TatA oligomer and TatA(T22P) monomer are highly similar, suggesting that oligomerization-induced chemical shift perturbations in this region of the APH do not arise from physical contact between subunits but rather from an alteration of the structure or dynamics upon oligomer formation. The discovery that TatA(T22P) is conformationally similar to that of the TatA oligomeric subunit was serendipitous, but proved useful for further characterization of the TatA oligomer.
Monomer Structure of TatA(T22P).
Because spectral overlays indicated that the TatA(T22P) monomer structure is a close structural homolog of the oligomeric TatA subunit structure, we determined the structure of the TatA(T22P) monomer. In the high-resolution structure of monomeric TatA(T22P) (Fig. 2A, Fig. S4, and Table S1), the APH makes an angle of ∼103° with respect to the TMH. NOEs were observed between the methyl protons of Val17 in the TMH and Leu25 in the APH, similar to the hinge brace observed in TatAd (22). In the TatA(T22P) structure Leu32 and Gly33 form a ∼44° kink in the APH, which can also be seen as a discontinuity in 1H-15N residual dipolar couplings (RDCs) (Fig. 2B). The APH kink is present also in the wild-type TatA monomer (Fig. 2B) and divides the helix into proximal and distal segments with respect to the TMH.
Fig. 2.
(A) Ribbon diagram of TatA(T22P) monomer structure, with selected side-chains drawn as sticks. The side-chains of Val17 and Leu25 are drawn as spheres to show the TMH-APH contact. (B) 1H-15N RDCs for monomeric TatA (○) and TatA(T22P) (△). The average RDC values for the TMH (residues 6–20) and the proximal APH (residues 24–32) are indicated for TatA (short-dashed line) and TatA(T22P) (long-dashed lines).
The 1H-15N RDCs for residues in the TMH and proximal APH in monomeric wild-type TatA were of lower magnitude than those of TatA(T22P) (Fig. 2B). To evaluate whether this arises from a change in structure or mobility at the TMH/APH hinge, the TatA RDCs were fit to the representative TatA(T22P) structure and the RDCs back-calculated (Fig. S5). The correlation between the measured and calculated RDCs was 0.99, similar to that for the TatA(T22P) RDCs, suggesting that TatA does not adopt a different structure but rather has increased mobility at the TMH/APH hinge. Consistent with this interpretation, less intense methyl-to-methyl NOE intensities between Val17 and Leu25 were observed in monomeric TatA relative to monomeric TatA(T22P). Comparison of backbone 13C′ and 13Cα chemical shifts indicate that the proximal APH of TatA(T22P) is more helical than that of monomeric TatA (Fig. S6), suggesting that helicity also increases upon TatA oligomerization.
Spectral Analysis of the TatA Oligomer.
The spectral similarity of oligomeric TatA to monomeric TatA(T22P) permitted direct transfer of the APH backbone amide chemical shift assignments from monomeric TatA(T22P) to oligomeric TatA. However, there were still chemical shift differences between TatA(T22P) monomer and TatA oligomer in the TMH. Assignment confirmation and extension to TMH residues required a combined approach, beginning with NOE strip comparisons between 3D 13C-separated NOESYs recorded on fully protonated monomer and oligomer to obtain 1H-13C methyl assignments (Fig. 1F). For a small number of residues, confirmation of backbone amide assignments required observation of chemical shift perturbations after site-directed mutagenesis (29).
Complete assignment of the TatA oligomer spectrum indicated that the T22P mutation closely mimics the chemical shift changes that occur in several residues in the APH upon oligomerization, consistent with these changes arising from oligomerization-induced allosteric effects (Fig. 1G). By contrast, chemical shift changes in the TMH are similar between the TatA oligomer and either the TatA(T22P) or TatA monomer, suggesting that these differences arise from intermolecular packing interactions. In addition, large changes are observed in the carboxamide side-chain resonances of functionally essential TMH residue Gln8 following oligomerization (Fig. 1 C and F, and Fig. S3). The downfield 15N shift and increase in 1H dispersion upon oligomerization are characteristic of transfer of this side-chain to a more polar environment in the oligomer.
Assembly of an Oligomeric TatA Structure.
NOEs were obtained from methyl-based 3D NOESY experiments on either fully protonated protein or “methyl-protonated” samples in monomeric and oligomeric form (Fig. S7). NOEs detected between methyl groups for which intramolecular distances within an α-helix were too large to be compatible with direct contact within a single molecule were tentatively identified as intermolecular in nature, and confirmed by a second NOESY spectrum collected after monomerization of the protein by addition of DPC. Intermolecular NOEs were identified between the methyls of Ile12 and Val14, and Val16 and Leu18. Additional NOEs between Trp7 and Leu9, Leu18 and Val16, and Leu18 and Phe20 were subsequently obtained from aromatic and aliphatic 13C-edited NOESY-HSQCs (heteronuclear single-quantum coherence) on fully protonated oligomeric protein at 37 °C.
A structural model of the TatA oligomer was determined by the protocol summarized in Fig. 3A and described in more detail in SI Text. Nine TatA subunits were included in the final model on the basis of the analytical ultracentrifugation-derived average molecular mass of TatA at the experimental DPR (Fig. 1A). All intermolecular NOEs detected were from residues in the TMH. Thus, the orientation of the APH toward (APH-in) or away (APH-out) from the center of the oligomer was not explicitly defined by the NMR data. However, as in monomeric TatA(T22P) and TatA, intramolecular NOEs were observed between the methyls of Val17 and Leu25, indicating that the relative orientation of the TMH and APH are similar in the monomer and oligomer, and that an oligomeric model can be assembled using a rigid-body approach. The ∼90° angle between the TMH and APH results in severe steric clashes for the APH-in arrangement of the oligomer, and all low-energy structural models had an APH-out arrangement. The resulting oligomer forms as a bundle of TMHs, with the APHs extending outward from a ring of TMH (Fig. 3B and Fig. S8). The hydrophobic residues of the proximal APH (Leu25, Ile28, Leu32) and distal APH (Ile36, Phe39, Met43) are oriented toward the presumed membrane bilayer (Fig. 3C). The APH curves slightly upward toward the transmembrane domain, which can be attributed to the curved micelle surface (see Molecular Dynamics Simulations of TatA in Lipid Bilayers, below). The TMH packing interactions are shown in Fig. 3D. A pore is formed from the bundle of TMHs, with the cytoplasmic end of the pore formed by the hydrophobic side-chains of Ile11, Ile15, and Leu19. The amide group of Gln8 forms the most N-terminal (periplasmic) group within the structured portion of the pore (Fig. 3E), resulting in a pore with a hydrophobic length of ∼15 Å.
Fig. 3.
Structure of oligomeric TatA. (A) Outline of the procedure used to generate the structure of oligomeric TatA. (B) Cartoon diagram of oligomeric TatA from the cytoplasmic side of the membrane (Left) and an edge-on view from within the membrane (Right). (C) Surface plot of oligomeric TatA as viewed from the cytoplasmic side (Left) and periplasmic side (Right) of the membrane. Atoms are colored according to residue type: Lys (blue), Asp or Glu (red), Phe or Trp (pink), Ser or Thr (cyan), Gly (light orange), Pro (green), Gln (magenta), and Leu, Ile, Val, Ala, or Met (light gray). The flexible residues 1–3 are omitted for clarity. (D) A detailed view of the TMH packing interface. Only residues 4–21 of two subunits are shown. (E) Surface plot of the pore interior. The view into the pore was created by removal of three of the nine subunits. Atoms are colored according to type: nitrogen (blue), oxygen (red), sulfur (yellow), and carbon (light gray). The carboxamide of Gln8, and the aliphatic side-chains of the pore-lining Ile11, Ile15, and Leu19 are indicated.
Electron Spin Resonance Distance Measurements Are Consistent with an APH-Out Assembly.
Because NMR failed to detect intermolecular NOEs between the APH of TatA, we used electron paramagnetic resonance (EPR) measurements on spin-labeled TatA oligomers to test whether the modeled APH-out configuration is correct. Although NMR distance constraints determined through NOEs are restricted to short distances (< ∼5 Å), site-directed spin labeling allows the determination of longer distances (30). Distances between ∼10 and 17 Å can be determined by exploiting the dipolar broadening in continuous-wave (cw) EPR spectra of the spin label; distances above ∼17 Å can be determined using double electron-electron resonance (DEER) (31).
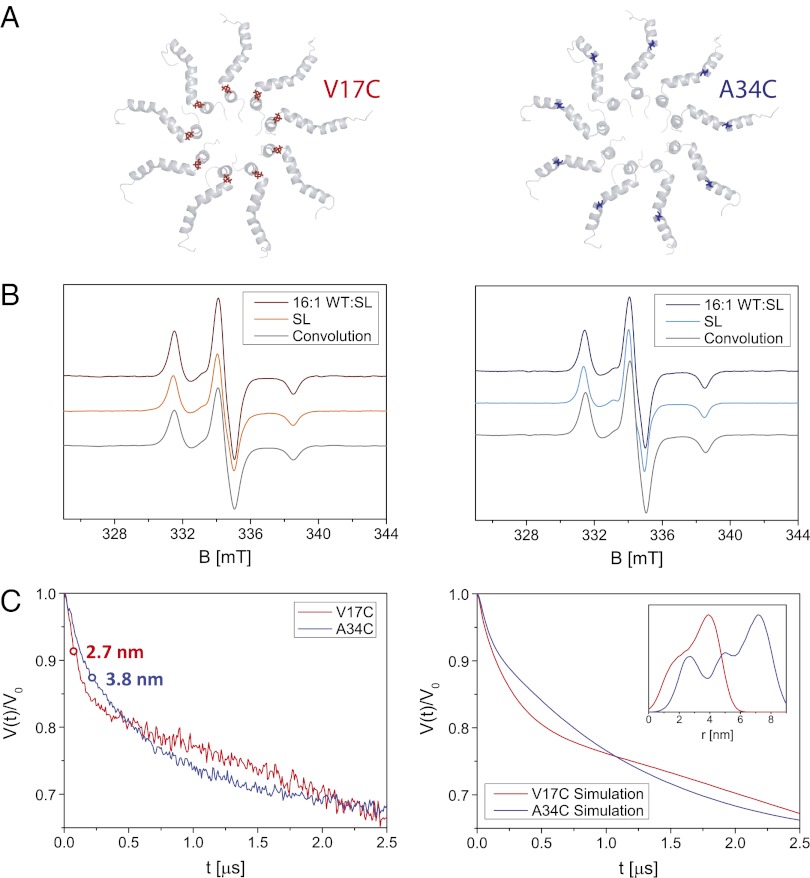
Single cysteine TatA variants were spin-labeled with MTSL [(1-Oxyl-2,2,5,5-tetramethyl-Δ3-pyrroline-3-methyl) methanethiosulfonate] (Fig. 4A). Cw EPR spectra recorded on oligomeric TatA samples with different ratios of spin-labeled subunits show a broadening of the nitroxide spectrum for a V17C variant (located in the TMH) at higher spin-labeling stoichiometry, but spectra for an A34C variant (located in the APH) are almost completely independent of spin-label stoichiometry (Fig. 4B). Large spin–spin interactions therefore broaden the EPR spectra of the V17C variant at higher spin concentrations but no such spin–spin interaction broadening can be discerned from the spectra of the A34C sample. These data suggest a closer average proximity between TMHs than between APHs (Fig. 4A). Further evidence for the presence of a shorter average distance between V17C residues relative to A34C residues can be qualitatively inferred from the half-time of the modulation decay in DEER experiments (Fig. 4C, Left). A quantitative analysis of both the cw EPR and DEER data was undertaken by first computing a spin-spin distance distribution using the NMR-derived 9-mer structure (Fig. 4C, Inset, Right); the MTSL positions on each APH spin-label site of the oligomer were defined by a mean position and broadened by a Gaussian distribution to account for spin label and protein flexibilities [the mean spin-label position was determined by the program MMM (32)]. Simulations using this distance distribution are shown for the cw EPR data in gray in Fig. 4B and for the DEER data in Fig. 4C, Right (see SI Text for further details). The experimental data strongly support the assignment of an APH-out oligomeric conformation and the simulations show agreement with the structure of the NMR-derived model.
Fig. 4.
(A) Cartoon of the 9-mer structure of oligomeric TatA from the cytoplasmic side of the membrane with highlighted amino acid positions used for spin labeling, Val17 in red (Left) and Ala34 in blue (Right). (B) Cw EPR spectra recorded at 170 K on oligomeric TatA samples (DPR of 30) for different relative concentrations of wild-type (WT) and spin-labeled (SL) protein for TatA labeled in position 17 (Left) and 34 (Right). Experimental conditions: 9.40 GHz microwave frequency, 0.1 mT modulation amplitude. The convolution of the 16:1 wild-type to spin-labeled EPR spectrum with the distance distribution obtained from the model of the 9-mer is shown in gray. (C) Four- and three-pulse DEER data recorded for oligomeric TatA labeled at position 17 (red line) or 34 (blue line) for a sample with 4:1 wild-type to spin-labeled protein and combined using the DEER-Stitch procedure (50) (Left). The individual four- and three-pulse DEER traces are shown in Fig. S9. As a qualitative guide, the half-time (the time the reduced form factor takes to decay to half its maximum value) is indicated by a circle and related to the mean distance according to ref. 51 using DeerAnalysis (52). Simulations of the DEER data for distance distributions calculated for the model structure of the 9-mer labeled in position 17 (red line) or 34 (blue line) are shown (Right). The distance distributions are shown (Inset). Details on the calculation of the distance distribution and the simulations can be found in the SI Text.
Water Accessibility of the TatA Oligomer in Detergent Micelles.
The water accessibilities of TatA monomer and oligomer were probed using the paramagnetic metal chelate Mn+2EDDA−2, which results in paramagnetic relaxation enhancements for residues exposed to water (33, 34) (Fig. 5). In both states of TatA, the backbone amides of TMH residues 9–20 were mostly sequestered from water, as expected because of embedding within the detergent micelle, and suggest that detergent molecules are present in the central pore of the oligomer. Water exposure of the side-chain amide of the glutamine at position eight, which is a conserved polar residue within the TMH, was greatly increased upon oligomerization, consistent with the increased deshielding of the side-chain amide chemical shifts upon oligomerization (Fig. 1 C and F). The proximal and distal regions of the APH in both states were partially protected from water exposure in both states, likely through interactions with the detergent micelle, although greater water exposure was detected in the region of the APH kink.
Fig. 5.
Water accessibility of monomeric (○) and oligomeric (■) TatA amides as a function of residue number as determined by resonance broadening because of addition of water-soluble Mn+2EDDA−2. A decrease in the ratio (I/Io) of the peak intensity after (I) and before (Io) addition of Mn+2EDDA−2 indicates increased water accessibility. Average values for the two side-chain amide resonances of Gln8 are indicated.
TatA Mode of Assembly Is Consistent with Oligomeric Sizes Observed in Membranes.
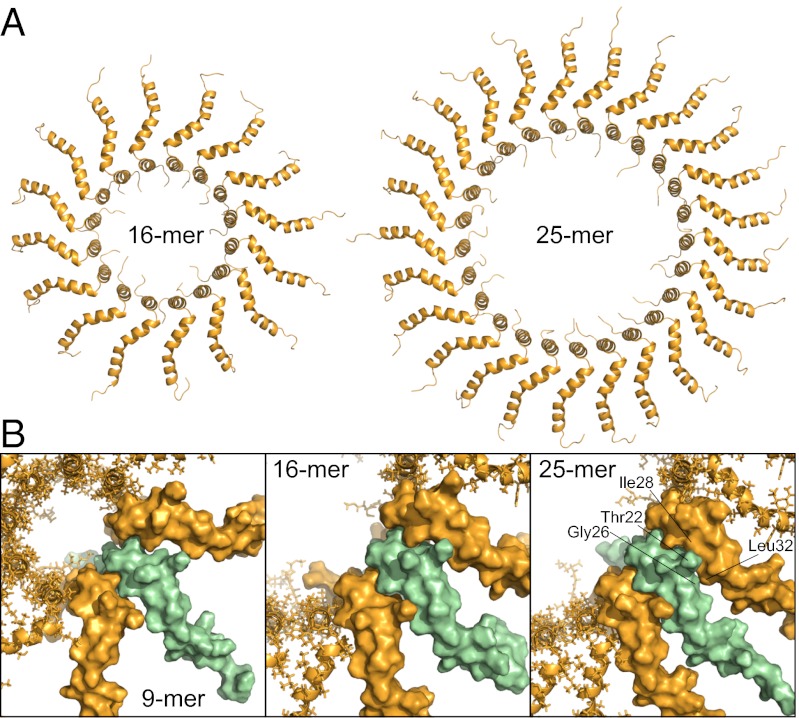
Fluorescence measurements in E. coli membranes indicate formation of complexes containing an average of ∼25 TatA molecules (16), and kinetic analysis of transport through the Tat pathway in thylakoids indicates a requirement of ∼26 molecules of Tha4, the TatA homolog, for maximal transport efficiency (35). To test whether the mode of assembly determined for TatA in DPC is consistent with larger membrane complexes, a model-building procedure similar to that for the 9-mer was applied to representative complexes consisting of 16 and 25 subunits. Energies for restrained simulations of 16- and 25-mer structures were comparable to those of the 9-mer and no violations of the intermolecular distance restraints were observed. Thus, the experimentally determined mode of assembly can account for the larger complexes observed in membranes (Fig. 6A). In contrast to the smaller oligomers, intersubunit packing interactions were formed in the proximal APH of the 25-mer between Thr22 and Ile28, and Gly26 and Leu32 (Fig. 6B). Attempts to build a 26-mer resulted in nonplanar arrangements of the subunits to accommodate APH packing. Thus, although interactions between APH do not appear to be required for stabilizing the TatA oligomeric assembly, APH packing interactions may dictate an upper limit on complex size.
Fig. 6.
(A) Structural models of TatA oligomers containing 16 and 25 subunits. (B) APH subunit packing in the 9-mer, 16-mer, and 25-mer. Surface models of three adjacent subunits are shown, with the central subunit colored in green to highlight packing interactions. Potential clashes in higher-order oligomers between Thr22 and Gly26 in one subunit and Ile28 and Leu32 in an adjacent subunit are indicated for the 25-mer model.
Mutations in the APH Lead to Long-Range Perturbations.
Mutagenesis studies of highly conserved residues in the APH (Fig. S1) were carried out to better understand the role of the APH in TatA structure. Alanine substitution of Asp31 in the proximal APH, Gly33 in the APH kink, or Phe39 in the distal APH, resulted in extensive backbone amide chemical shift changes throughout the APH in monomer form (Fig. S10). Because both the proximal and distal APH interact with the detergent micelle (Fig. 5), we propose that the observed chemical shift perturbations arise from a combination of altered helical stability and altered contacts with the detergent micelle either through rotation along the helical axis or changes in the APH kink.
Chemical shift perturbations in the distal APH residues 42–44 were observed for all substitutions, with the largest changes in 42–44 occurring for the F39A substitution (Fig. S10). Phe39 is invariant and a F39A substitution inhibits translocation (36). The TatA structure indicates that Met43 forms a hydrophobic, membrane-facing patch with Phe39 (Fig. 3C). Taken together, these results suggest that Phe39 anchors the distal APH to the membrane surface and that this anchoring may be important for translocation activity.
Molecular Dynamics Simulations of TatA in Lipid Bilayers.
The behavior of TatA monomer and oligomer structures in a membrane environment was investigated through multiscale molecular dynamic simulations using a simple model of an E. coli inner-membrane lipid bilayer (37). Table S2 contains details of the simulations performed. In all simulations of oligomeric TatA the TMH inserted parallel to the bilayer normal. The APH adopted an altered conformation in which both the proximal and distal segments lie in the plane of the bilayer, interacting with the lipid headgroup region (Fig. 7). This finding suggests that the more acute angle between the TMH and distal APH in the NMR structure reflects adaptation of the APH to a curved micelle surface (Fig. 3B). Simulations of oligomeric TatA in a DPC micelle environment support this. The lipid bilayer surrounding the transmembrane domain is severely distorted on the periplasmic side of the oligomer because of the unusually short TMHs (Fig. 7). The periplasmic N terminus of the transmembrane domain is water-accessible, and the Gln8 side-chains interact with water molecules (Fig. S11A), consistent with the paramagnetic relaxation enhancement measurements (Fig. 5) and the chemical shifts (Fig. 1F) of the Gln8 side-chain amides in oligomeric TatA in DPC.
Fig. 7.
Coarse-grained molecular dynamics simulations of monomeric (1-mer), tetrameric (4-mer), and nonameric (9-mer) TatA structures in an E. coli lipid membrane. (Top) Backbone traces of TatA. The protein is displayed as a cartoon colored cyan, with the phosphate particles of the lipid headgroups shown as red spheres. Gln8 (blue) and Phe39 (yellow) residues are shown in stick representation. (Middle) Protein displayed as a surface with backbone particles colored pink and side-chains yellow. Lipid headgroup particles are shown in red and the rest of the lipid is shown in gray. Water and ions are omitted for clarity. (Bottom) Water particles shown in green. Lipids and ions are omitted for clarity. In the representations, one and four subunits have been removed from the 4- and 9-mer oligomers, respectively, to reveal the central pore.
In contrast to the bilayer distortions observed for the oligomer, simulations of the TatA monomer indicated that the TMH inserts at an angle to the membrane normal, in an orientation similar to that reported for B. subtilis TatAd in lipid bilayers (38). The tilted conformation draws the N terminus of the proximal APH deeper into the membrane and the hydrophobic depth of the membrane is more closely matched by that of the protein (Fig. 7). As a result, the extent of bilayer distortion in the presence of monomeric TatA is greatly decreased compared with that in the presence of the oligomer.
In monomer and oligomer simulations in lipid bilayers the APH region was observed to adopt two conformations in the plane of the phospholipid headgroups, namely straight or kinked at Gly33. The distal APH remains anchored in the membrane in both conformations via burial of the Phe39 side-chain, a residue that is essential for Tat function (36). In coarse-grained molecular dynamic simulations of the monomer, dynamic switching between the two conformations occurred on an ∼100-ns timescale (Fig. S11B). The degree of kinking in the APH at Gly33 was found to decrease as a function of oligomer size, with the most populated kink angle (between the proximal and distal regions of the APH) of the monomer approximately 90°, decreasing to ∼20° in the higher-order oligomers, likely because of spatial constraints. The Gly33 kink angle may play a role in stabilizing and regulating oligomer formation. Only the kinked conformation was observed in simulations in the DPC micelle. Some unfolding of the APH was observed in all simulations of oligomers, possibly as a result of the large proportion of helix-destabilizing Gly and Ser residues.
Comparison of simulations with oligomerization states ranging from n = 1–4 to 9 suggest that oligomers from n = 4–9 and above are all likely to result in significant bilayer distortion. Although interactions between TMH are sufficient to maintain a stable helix bundle for n = 4; for n = 9 the bilayer distortion and movements of the APH seem to destabilize the TMH intermolecular contacts. Thus, additional factors (e.g., the transbilayer proton motive force, substrate, or other Tat proteins) are likely needed to stabilize higher-order TatA oligomers in a lipid bilayer environment. The majority of simulations were performed with lipids present within the pore. In AT simulations with no lipid density in the pore at the start of the simulation, collapse of the TMH bundle occurred rapidly, such that water was completely excluded from the hydrophobic pore within 40 ns.
Discussion
TatA Assembly.
TatA and TatE (a TatA paralog) form ring-like oligomers in detergent (12, 18, 25) (Fig. S2A), consistent with diffusion measurements of fluorescent TatA complexes in cell membranes (16). The precise oligomeric state of TatA is variable in cell membranes (16) and detergent micelles (12, 18, 25). At low concentrations of DPC, TatA adopts an average oligomer size of approximately 9, which is smaller than that of TatA in C12E9 (18) and digitonin (12) but comparable to that of TatE in dodecylmaltoside (25). The interfacial contacts between TatA TMHs in DPC micelles determined here by NMR are consistent with previous EPR studies of full-length TatA in C12E9 micelles (26).
In the TatA 9-mer the average intersubunit crossing angle for the TMH is 4.2°. This angle provides a narrow contact surface over which large changes in subunit numbers result in only small changes in intersubunit contacts. In contrast, the APH extends outward from the pore axis, thereby avoiding APH contacts that would vary with subunit number. The APH may, however, provide an upper limit to oligomer size as inter-APH distances gradually decrease with the addition of subunits.
TatA Oligomerization Changes APH Interactions with the Micelle.
Substitution of Thr22 with a proline provided a surrogate structure for studying the subunit conformation of oligomeric TatA. Comparison of the structural properties of monomeric TatA and TatA(T22P) suggests that the predominant structural changes upon oligomerization are a change in the TMH/APH dynamics and an increase in helicity in the proximal APH. Helix stabilization in the APH of the TatA(T22P) variant may be a result of proline N-capping or to changes in interactions with the phospholipid headgroups of the detergent micelle. However, the similar changes in helicity that accompany oligomerization of the wild-type protein must be driven primarily by altered contacts with the detergent micelle, likely through a change in the angle at the TMH/APH hinge.
The APH of TatA does not participate directly in TatA oligomerization, but residues within the APH are highly intolerant to substitution (39–41). The TatA APH has been shown previously to interact with liposomes (23, 42), and here it is shown that single mutations in the APH produce chemical shift changes in the membrane-interacting face of the distal APH that likely reflect alterations in protein/phosphoplipid headgroup contacts. Because of greater malleability detergent micelles are expected to only weakly maintain amphipathic directionality, and thus the perturbations observed here are expected to be small relative to those that might be expected in phospholipid bilayers. Phospholipid composition has been shown to regulate Tat activity (43, 44, 45), and alteration of TatA interactions with phospholipid headgroups may therefore contribute to the sensitivity of the Tat system to TatA APH mutations.
TatA Pore.
TatA proteins contain a conserved polar residue near the N-terminal end of the transmembrane domain. In E. coli it is Gln8, which is the only residue within the transmembrane domain that is essential for translocase function (39). In the oligomeric structure of E. coli TatA, Gln8 points toward the center of the TatA pore and is water-accessible. The position of Gln8 within the TMH, combined with the short length of the helix, results in a pore that is only hydrophobic for three turns of an α-helix. This is approximately half the length of the hydrophobic phase of a typical membrane bilayer. Molecular dynamics simulations indicate that the pore can accommodate lipids, but that they are distorted compared with lipids in bulk membrane. The thinned and distorted membrane within the TatA oligomer pore provides an obvious pathway for translocation of substrate protein.
Lipids in the pore may provide an energetic barrier to the loss of protons and other small molecules across the membrane while the Tat translocase is assembling. The lipids would, however, need to be removed or pushed aside during the translocation step, and it is unlikely that leaking of small molecules through the pore is completely prevented during protein translocation. Measurements for the chloroplast Tat pathway indicate that ∼80,000 protons are lost in each transport event (46). As expected for a system that permeabilizes active membranes, TatA polymerization is highly regulated, requiring interaction with a substrate-bound TatBC complex. Our simulations in membranes indicate that the TatA pore complex may disassemble rapidly during or after protein transport.
Model for Tat Translocation.
Formation of the active Tat translocation site requires substrate-induced assembly of a TatA oligomer from a pool of TatA in the membrane. Our experimental TatA oligomer model in conjunction with molecular dynamics simulations suggests how this may enable TatA to mediate transmembrane transport (Fig. 8).
Fig. 8.

Model of the regulation of TatA pore formation by TatBC and substrate. (A) The inactive monomeric form of TatA accommodates the depth of the membrane by tilting the short TMH, increasing the angle between the TMH and the APH, and deeply inserting the APH into the membrane. (B) Interactions with TatBC/substrate complex force an orientation of the TatA TMH roughly parallel to the membrane normally favoring TatA polymerization. The N terminus is pulled into the membrane thinning and distorting the bilayer in the pore and sensitizing the membrane to disruption. The TatA APH serves as a platform for the substrate that is tethered to the TatBC complex by a signal peptide. In the drawing the TatA oligomeric ring is represented by two opposing subunits. (C) Pulling of substrate protein into the membrane forces TatA subunits apart and ruptures the membrane.
Our molecular dynamics analysis indicates that the TatA monomer can adopt an orientation within the membrane in which the TMH is tilted relative to the membrane normal, the angle between the TMH and APH is increased, and the proximal APH is deeply inserted into the membrane (Figs. 7 and 8A, and Fig. S11). This tilted orientation is required to accommodate the unusually short TMH of TatA and is consistent with solid-state NMR measurements of B. subtilis TatAd in bicelles (38), as well as with labeling accessibility studies of the chloroplast Tha4 protein (47). Flexibility at the APH kink enables the distal APH to remain parallel to the membrane surface with the functionally critical residue Phe39 (36) inserted into the bilayer interior.
Upon oligomerization, TatA must reorient in the membrane to allow the shallow crossing angles required for TMH packing. This reorientation causes the whole of the APH to now lie along the surface of the membrane, which is in agreement with the changes in APH accessibility observed upon Tha4 activation (47). Relocalization of the APH in turn results in the TMH ring being pulled into the cytoplasmic leaflet of the membrane. The effective length of the TMH is further reduced on the pore face of the oligomer because the conserved polar amino acid at position 8 in each protomer points into the TMH pore. The resulting hydrophobic mismatch with the bilayer causes membrane thinning and lipid disordering in the pore, thereby sensitizing the membrane to disruption (Fig. 8B).
This model for regulating oligomerization may explain why the TatA state is shifted toward the oligomer in detergent. In the absence of the long-range amphipathic order and hydrophobic depth mismatch, TMH “tilting” becomes irrelevant and permits intermolecular packing to dominate. Although the isolated TatA oligomer is unstable in a simulated bilayer environment, in the cell it will be rendered metastable by interactions with the TatBC/substrate complex, which controls TatA polymerization.
The TatA oligomer structure suggests that the APHs form a platform upon which the substrate lies, an arrangement that is consistent with cross-linking studies (17, 47, 48). Nonspecific substrate-APH interactions would then provide cross-bridging contacts between APHs that could assist in stabilizing the TatA oligomer. Additional stabilization would be provided by the highly charged C-tails of TatA if these wrap around the substrate (47).
Our model suggests that at this stage the substrate protein is located over a thinned and disordered patch of membrane that is susceptible to rupture. A possible scenario for the final step of translocation is that substrate is pulled into the TatA pore, forcing the TatA subunits apart and transiently diluting pore lipids (Fig. 8C). In this way the membrane is ruptured permitting substrate translocation and at the same time disassembling the TatA oligomer.
Materials and Methods
Plasmids.
Plasmid pET24a-TatA and pET24a-TatA(Δ40) was used to express full-length and residues 1–49 of E. coli TatA, respectively, with a carboxyl-terminal hexahistidine tag. The plasmids were obtained by PCR amplification of tatA from E. coli genomic DNA (primers ccgcgccatatgGGTGGTATCAGTATTTGGCAG, cgcgtgaagcttagtgatggtgatggtgatgCACCTGCTCTTTATCG and cgcgtgaagcttagtgatggtgatggtgatgCTTTGGTTCATCATCGCTC), cut with NdeI/HindIII and ligated into pET24a (Novagen). Site-specific mutations were introduced into the tatA gene in pET24-TatA(Δ40) using the QuikChange method (Stratagene).
Protein Expression and Purification.
E. coli strain BL21 pLysS (Invitrogen) transformed with the appropriate expression plasmid was cultured aerobically at 37 °C in M9 medium supplemented with the appropriate isotopes. A methyl-protonated sample labeled with 1H and 13C at valine γ-methyls and leucine and isoleucine δ-methyls, and deuterated at all other nonexchangeable proton positions, was produced by expression in M9 medium containing 70 mg/L 2-Ketobutyric acid-4-13C,3,3-d2 and 120 mg/L 2-Keto-3-methyl-13C-butyric-4-13C, 3-d (Sigma-Aldrich) (49). When the cultures reached an A600 nm of 0.6, expression of the tatA allele was induced with 1-mM final concentration of isopropyl β-D-thiogalactoside, and the growth continued for 7 h at 30 °C before harvesting. Cells were resuspended in 20 mM Tris, pH 8.0 and 200 mM NaCl (buffer A) supplemented with DNase I and protease inhibitors (Roche Applied Science; complete EDTA-free protease inhibitor mixture). Cells were disrupted by three passages through a French pressure cell at 8,000 psi, and cell debris was removed by centrifugation at 10,000 × g and 4 °C for 15 min. Membranes were isolated by ultracentrifugation at 150,000 × g and 4 °C for 60 min and solubilized with C12E9 (Sigma). The protein was bound to Ni-NTA agarose (Qiagen), washed with buffer A containing 0.1% C12E9 and 50 mM imidazole, then with buffer A containing 0.1% (wt/vol) DPC and 50 mM imidazole and eluted with buffer A containing 0.1% (wt/vol) DPC and 800 mM imidazole. TatA-containing fractions were pooled and concentrated using a 30-kDa molecular mass cutoff Microcon centrifugal concentrator (Millipore). The concentrated sample was subjected to size-exclusion chromatography on a Superdex 200 10/300 GL column (GE Healthcare) in 50 mM sodium phosphate pH 7.0 and 3 mM DPC. TatA-containing fractions were identified by SDS/PAGE, pooled and stored at –80 °C until use. Sample DPC concentrations following size-exclusion chromatography were ∼15 mM, as determined by comparison of signal intensities in a 1D 1H NMR spectrum against those from samples of known concentration.
Chemical Cross-Linking.
Glutaraldehyde at the indicated concentrations were added to protein samples and incubated at 25 °C. The reaction was stopped after 5 min by adding 0.2 M Tris buffer (pH 8.0) followed by SDS/PAGE and immunoblotting.
Supplementary Material
Acknowledgments
We thank Nick Soffe, Jonathan Boyd, and Jolyon Claridge for assistance with NMR data acquisition. This study was supported in part by a European Molecular Biology Organization Long-Term fellowship (to F.R.); the E. P. Abraham Cephalosporin Trust (F.R.); Dr. Catherine Venien-Bryan (A.D.R.); ScalaLife (European Union) and the Engineering and Physical Sciences Research Council (Collaborative Computational Project for Biomolecular Simulation) (S.L.R.); electron paramagnetic resonance studies were supported by the Engineering and Physical Sciences Research Grant EP/D044855D/1, supporting the Oxford Centre for Advanced Electron Spin Resonance; and all other work was funded by the Biotechnology and Biological Sciences Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 2LZR and 2LZS).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219486110/-/DCSupplemental.
References
- 1.Park E, Rapoport TA. Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu Rev Biophys. 2012;41:21–40. doi: 10.1146/annurev-biophys-050511-102312. [DOI] [PubMed] [Google Scholar]
- 2.Fröbel J, Rose P, Müller M. Twin-arginine-dependent translocation of folded proteins. Philos Trans R Soc Lond B Biol Sci. 2012;367(1592):1029–1046. doi: 10.1098/rstb.2011.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer T, Berks BC. The twin-arginine translocation (Tat) protein export pathway. Nat Rev Microbiol. 2012;10(7):483–496. doi: 10.1038/nrmicro2814. [DOI] [PubMed] [Google Scholar]
- 4.De Buck E, Lammertyn E, Anné J. The importance of the twin-arginine translocation pathway for bacterial virulence. Trends Microbiol. 2008;16(9):442–453. doi: 10.1016/j.tim.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Barkan A, Miles D, Taylor WC. Chloroplast gene expression in nuclear, photosynthetic mutants of maize. EMBO J. 1986;5(7):1421–1427. doi: 10.1002/j.1460-2075.1986.tb04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celedon JM, Cline K. Intra-plastid protein trafficking: How plant cells adapted prokaryotic mechanisms to the eukaryotic condition. Biochim Biophys Acta. 2013;1833(2):341–351. doi: 10.1016/j.bbamcr.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sargent F, et al. Overlapping functions of components of a bacterial Sec-independent protein export pathway. EMBO J. 1998;17(13):3640–3650. doi: 10.1093/emboj/17.13.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiner JH, et al. A novel and ubiquitous system for membrane targeting and secretion of cofactor-containing proteins. Cell. 1998;93(1):93–101. doi: 10.1016/s0092-8674(00)81149-6. [DOI] [PubMed] [Google Scholar]
- 9.Alami M, et al. Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol Cell. 2003;12(4):937–946. doi: 10.1016/s1097-2765(03)00398-8. [DOI] [PubMed] [Google Scholar]
- 10.Bolhuis A, Mathers JE, Thomas JD, Barrett CM, Robinson C. TatB and TatC form a functional and structural unit of the twin-arginine translocase from Escherichia coli. J Biol Chem. 2001;276(23):20213–20219. doi: 10.1074/jbc.M100682200. [DOI] [PubMed] [Google Scholar]
- 11.Cline K, Mori H. Thylakoid ΔpH-dependent precursor proteins bind to a cpTatC-Hcf106 complex before Tha4-dependent transport. J Cell Biol. 2001;154(4):719–729. doi: 10.1083/jcb.200105149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarry MJ, et al. Structural analysis of substrate binding by the TatBC component of the twin-arginine protein transport system. Proc Natl Acad Sci USA. 2009;106(32):13284–13289. doi: 10.1073/pnas.0901566106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mori H, Cline K. A twin arginine signal peptide and the pH gradient trigger reversible assembly of the thylakoid ΔpH/Tat translocase. J Cell Biol. 2002;157(2):205–210. doi: 10.1083/jcb.200202048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dabney-Smith C, Cline K. Clustering of C-terminal stromal domains of Tha4 homo-oligomers during translocation by the Tat protein transport system. Mol Biol Cell. 2009;20(7):2060–2069. doi: 10.1091/mbc.E08-12-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dabney-Smith C, Mori H, Cline K. Oligomers of Tha4 organize at the thylakoid Tat translocase during protein transport. J Biol Chem. 2006;281(9):5476–5483. doi: 10.1074/jbc.M512453200. [DOI] [PubMed] [Google Scholar]
- 16.Leake MC, et al. Variable stoichiometry of the TatA component of the twin-arginine protein transport system observed by in vivo single-molecule imaging. Proc Natl Acad Sci USA. 2008;105(40):15376–15381. doi: 10.1073/pnas.0806338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fröbel J, Rose P, Müller M. Early contacts between substrate proteins and TatA translocase component in twin-arginine translocation. J Biol Chem. 2011;286(51):43679–43689. doi: 10.1074/jbc.M111.292565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gohlke U, et al. The TatA component of the twin-arginine protein transport system forms channel complexes of variable diameter. Proc Natl Acad Sci USA. 2005;102(30):10482–10486. doi: 10.1073/pnas.0503558102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cline K, Theg SM. In: The Enzymes. Dabley RE, Koehler CM, Tamanoi F, editors. New York: Elsevier; 2007. [Google Scholar]
- 20.Brüser T, Sanders C. An alternative model of the twin arginine translocation system. Microbiol Res. 2003;158(1):7–17. doi: 10.1078/0944-5013-00176. [DOI] [PubMed] [Google Scholar]
- 21.Koch S, Fritsch MJ, Buchanan G, Palmer T. Escherichia coli TatA and TatB proteins have an N-out C-in topology in intact cells. J Biol Chem. 2012;287(18):14420–14431. doi: 10.1074/jbc.M112.354555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y, Zhao E, Li H, Xia B, Jin C. Solution NMR structure of the TatA component of the twin-arginine protein transport system from Gram-positive bacterium Bacillus subtilis. J Am Chem Soc. 2010;132(45):15942–15944. doi: 10.1021/ja1053785. [DOI] [PubMed] [Google Scholar]
- 23.Porcelli I, et al. Characterization and membrane assembly of the TatA component of the Escherichia coli twin-arginine protein transport system. Biochemistry. 2002;41(46):13690–13697. doi: 10.1021/bi026142i. [DOI] [PubMed] [Google Scholar]
- 24.Settles AM, et al. Sec-independent protein translocation by the maize Hcf106 protein. Science. 1997;278(5342):1467–1470. doi: 10.1126/science.278.5342.1467. [DOI] [PubMed] [Google Scholar]
- 25.Baglieri J, Beck D, Vasisht N, Smith CJ, Robinson C. Structure of TatA paralog, TatE, suggests a structurally homogeneous form of Tat protein translocase that transports folded proteins of differing diameter. J Biol Chem. 2012;287(10):7335–7344. doi: 10.1074/jbc.M111.326355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White GF, et al. Subunit organization in the TatA complex of the twin arginine protein translocase: A site-directed EPR spin labeling study. J Biol Chem. 2010;285(4):2294–2301. doi: 10.1074/jbc.M109.065458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee PA, Buchanan G, Stanley NR, Berks BC, Palmer T. Truncation analysis of TatA and TatB defines the minimal functional units required for protein translocation. J Bacteriol. 2002;184(21):5871–5879. doi: 10.1128/JB.184.21.5871-5879.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clore GM. Accurate and rapid docking of protein-protein complexes on the basis of intermolecular nuclear overhauser enhancement data and dipolar couplings by rigid body minimization. Proc Natl Acad Sci USA. 2000;97(16):9021–9025. doi: 10.1073/pnas.97.16.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sprangers R, Gribun A, Hwang PM, Houry WA, Kay LE. Quantitative NMR spectroscopy of supramolecular complexes: Dynamic side pores in ClpP are important for product release. Proc Natl Acad Sci USA. 2005;102(46):16678–16683. doi: 10.1073/pnas.0507370102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeschke G. Determination of the nanostructure of polymer materials by electron paramagnetic resonance spectroscopy. Macromol Rapid Commun. 2002;23(4):227–246. [Google Scholar]
- 31.Banham JE, et al. Distance measurements in the borderline region of applicability of CW EPR and DEER: A model study on a homologous series of spin-labelled peptides. J Magn Reson. 2008;191(2):202–218. doi: 10.1016/j.jmr.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 32.Polyhach Y, Bordignon E, Jeschke G. Rotamer libraries of spin labelled cysteines for protein studies. Phys Chem Chem Phys. 2011;13(6):2356–2366. doi: 10.1039/c0cp01865a. [DOI] [PubMed] [Google Scholar]
- 33.Altenbach C, Greenhalgh DA, Khorana HG, Hubbell WL. A collision gradient method to determine the immersion depth of nitroxides in lipid bilayers: Application to spin-labeled mutants of bacteriorhodopsin. Proc Natl Acad Sci USA. 1994;91(5):1667–1671. doi: 10.1073/pnas.91.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau TL, Partridge AW, Ginsberg MH, Ulmer TS. Structure of the integrin beta3 transmembrane segment in phospholipid bicelles and detergent micelles. Biochemistry. 2008;47(13):4008–4016. doi: 10.1021/bi800107a. [DOI] [PubMed] [Google Scholar]
- 35.Celedon JM, Cline K. Stoichiometry for binding and transport by the twin arginine translocation system. J Cell Biol. 2012;197(4):523–534. doi: 10.1083/jcb.201201096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hicks MG, et al. The Escherichia coli twin-arginine translocase: Conserved residues of TatA and TatB family components involved in protein transport. FEBS Lett. 2003;539(1–3):61–67. doi: 10.1016/s0014-5793(03)00198-4. [DOI] [PubMed] [Google Scholar]
- 37.Stansfeld PJ, Sansom MS. From coarse-grained to atomistic: A serial multi-scale approach to membrane protein simulations. J Chem Theory Comput. 2011;7(4):1157–1166. doi: 10.1021/ct100569y. [DOI] [PubMed] [Google Scholar]
- 38.Walther TH, Grage SL, Roth N, Ulrich AS. Membrane alignment of the pore-forming component TatAd of the twin-arginine translocase from Bacillus subtilis resolved by solid-state NMR spectroscopy. J Am Chem Soc. 2010;132(45):15945–15956. doi: 10.1021/ja106963s. [DOI] [PubMed] [Google Scholar]
- 39.Greene NP, et al. Cysteine scanning mutagenesis and disulfide mapping studies of the TatA component of the bacterial twin arginine translocase. J Biol Chem. 2007;282(33):23937–23945. doi: 10.1074/jbc.M702972200. [DOI] [PubMed] [Google Scholar]
- 40.Barrett CM, Mathers JE, Robinson C. Identification of key regions within the Escherichia coli TatAB subunits. FEBS Lett. 2003;537(1-3):42–46. doi: 10.1016/s0014-5793(03)00068-1. [DOI] [PubMed] [Google Scholar]
- 41.Hicks MG, Lee PA, Georgiou G, Berks BC, Palmer T. Positive selection for loss-of-function tat mutations identifies critical residues required for TatA activity. J Bacteriol. 2005;187(8):2920–2925. doi: 10.1128/JB.187.8.2920-2925.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan CS, Haney EF, Vogel HJ, Turner RJ. Towards understanding the Tat translocation mechanism through structural and biophysical studies of the amphipathic region of TatA from Escherichia coli. Biochim Biophys Acta. 2011;1808(9):2289–2296. doi: 10.1016/j.bbamem.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mikhaleva NI, Santini CL, Giordano G, Nesmeyanova MA, Wu LF. Requirement for phospholipids of the translocation of the trimethylamine N-oxide reductase through the Tat pathway in Escherichia coli. FEBS Lett. 1999;463(3):331–335. doi: 10.1016/s0014-5793(99)01661-0. [DOI] [PubMed] [Google Scholar]
- 44.Ma X, Browse J. Altered rates of protein transport in Arabidopsis mutants deficient in chloroplast membrane unsaturation. Phytochemistry. 2006;67(15):1629–1636. doi: 10.1016/j.phytochem.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Sikdar R, Doerrler WT. Inefficient Tat-dependent export of periplasmic amidases in an Escherichia coli strain with mutations in two DedA family genes. J Bacteriol. 2010;192(3):807–818. doi: 10.1128/JB.00716-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alder NN, Theg SM. Energetics of protein transport across biological membranes. A study of the thylakoid ΔpH-dependent/cpTat pathway. Cell. 2003;112(2):231–242. doi: 10.1016/s0092-8674(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 47.Aldridge C, Storm A, Cline K, Dabney-Smith C. The chloroplast Twin Arginine Transport (Tat) component, Tha4, undergoes conformational changes leading to Tat protein transport. J Biol Chem. 2012;287(41):34752–34763. doi: 10.1074/jbc.M112.385666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pal D, Fite K, Dabney-Smith C. Direct interaction between a precursor mature domain and transport component Tha4 during twin arginine transport of chloroplasts. Plant Physiol. 2013;161(2):990–1001. doi: 10.1104/pp.112.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tugarinov V, Kanelis V, Kay LE. Isotope labeling strategies for the study of high-molecular-weight proteins by solution NMR spectroscopy. Nat Protoc. 2006;1(2):749–754. doi: 10.1038/nprot.2006.101. [DOI] [PubMed] [Google Scholar]
- 50.Lovett JE, Lovett BW, Harmer J. DEER-Stitch: Combining three- and four-pulse DEER measurements for high sensitivity, deadtime free data. J Magn Reson. 2012;223:98–106. doi: 10.1016/j.jmr.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 51.Jeschke G. In: Structure and Bonding. Timmel CR, Harmer J, editors. Berlin, Heidelberg: Springer; 2012. pp. 1–38. [Google Scholar]
- 52.Banham JE, Timmel CR, Abbott RJ, Lea SM, Jeschke G. The characterization of weak protein-protein interactions: Evidence from DEER for the trimerization of a von Willebrand Factor A domain in solution. Angew Chem Int Ed Engl. 2006;45(7):1058–1061. doi: 10.1002/anie.200503720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.