Abstract
The animal and human intestinal mucosa secretes an assortment of compounds to establish a physical barrier between the host tissue and intestinal contents, a separation that is vital for health. Some pathogenic microorganisms as well as members of the commensal intestinal microbiota have been shown to be able to break down these secreted compounds. Our understanding of host-compound degradation by the commensal microbiota has been limited to knowledge about simplified model systems because of the difficulty in studying the complex intestinal ecosystem in vivo. In this study, we introduce an approach that overcomes previous technical limitations and allows us to observe which microbial cells in the intestine use host-derived compounds. We added stable isotope-labeled threonine i.v. to mice and combined fluorescence in situ hybridization with high-resolution secondary ion mass spectrometry imaging to characterize utilization of host proteins by individual bacterial cells. We show that two bacterial species, Bacteroides acidifaciens and Akkermansia muciniphila, are important host-protein foragers in vivo. Using gnotobiotic mice we show that microbiota composition determines the magnitude and pattern of foraging by these organisms, demonstrating that a complex microbiota is necessary in order for this niche to be fully exploited. These results underscore the importance of in vivo studies of intestinal microbiota, and the approach presented in this study will be a powerful tool to address many other key questions in animal and human microbiome research.
Keywords: nanoSIMS, stable isotope probing
A fundamental interaction between the intestinal microbiota and their host is the foraging of host-derived compounds by mutualistic and pathogenic microorganisms. Utilization of host-derived substrates is known to confer a selective advantage to some enteric pathogens during inflammation (1), but little is known about host-compound foraging in the healthy mammalian intestines. The secreted mucus, a heterogeneous mixture of compounds that covers epithelial tissue in two layers (∼150 μm total in the mouse colon) (2), is regenerated rapidly, on the order of less than in a single day (1, 3). The high production and turnover of secreted compounds represents a steady influx of nutrients and therefore constitutes an ecological niche for microorganisms capable of, or even specialized at, foraging host-secreted compounds. Therefore, the secretion not only of antimicrobial substances (2, 4) but also of specific substrates is a means for the host to shape the structure of its symbiont community (5).
Despite the obvious importance of host-compound foraging, technical difficulties in teasing apart this close interaction have limited studies to pure culture and simplified in vitro systems or gnotobiotic mouse models (6, 7). Results produced from simplified model systems and metaomics studies are valuable for generating hypotheses, but techniques to directly probe important intestinal processes such as host-compound foraging are urgently needed to test these hypotheses (8). Here, we explored host-compound foraging using a stable isotope tracer-based approach for in vivo labeling and quantitative imaging of individual gut bacteria with high-resolution secondary ion mass spectrometry (NanoSIMS). We used the amino acid threonine as a marker as, after injection, it is used by the intestines for biosynthesis of the threonine-rich mucin glycoproteins (6, 7) but will also be incorporated into several other secreted protein compounds.
Results and Discussion
To establish the tracer approach and to define the optimal sampling time for the NanoSIMS measurements, a physiologically relevant concentration of stable isotope labeled threonine (both 13C and 15N labeled) was given i.v. to mice and the transport of the stable isotopes was tracked in the blood serum, cecum tissue, and gut lumen using elemental analysis isotope ratio mass spectrometry (EA–IRMS). Enrichment of 13C and 15N was not observed in the lumen contents until after 4 h postinjection (h.p.i.) and δ13C and δ15N values peaked at about 8 h.p.i. (Fig. S1A, SI Results and Discussion). Concomitantly, acetate, propionate, and butyrate in the lumen, main products of microbial metabolism of secreted compounds, were also enriched in 13C (Fig. S1A). Consistent with the hypothesis that the microbiota plays a role in the transfer of tracer to the gut, the lumen contents of germ-free mice and gnotobiotic mice colonized with a low complexity intestinal microbiota (Fig. S2) were significantly less enriched than mice with a normal complexity (NC) microbiota (Fig. S1C; Student’s t test, P < 0.05). This indicated that the microbiota richness and/or composition are important modifiers of secretion.
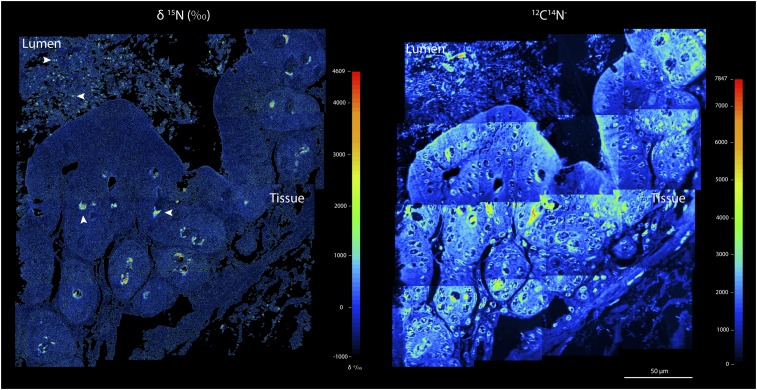
Using NanoSIMS we were able to visualize the transfer of label from the mouse cecum tissue to the gut lumen. Semithin sections of embedded samples showed distinctive hotspots of 15N in the tissue as well as in the lumen (Fig. 1). The 15N hotspots in the tissue were likely within secretory mucus cells (Fig. S3), which would be expected to have a higher demand for threonine to support the ongoing biosynthesis of mucins, although verification with Muc2 immunohistochemical staining was not possible without disturbing the isotope composition of the sample (SI Results and Discussion). By combining NanoSIMS imaging and fluorescence in situ hybridization (FISH) imaging of the same field of view, we were able to determine the isotope content of individual cells hybridized with specific phylogenetic probes (Table S1). Using gut lumen samples prepared with a FISH-compatible acrylic resin and probes targeting broad or highly abundant groups, we found widespread 15N enrichment. In addition, 15N hotspots were associated with cells targeted by the Bacteroidetes-specific (Bac303) and the Lachnospiraceae-specific (Erec482) probes, as well as with cells belonging to a highly abundant species-level phylotype in the Lachnospiraceae [Lachnospiraceae operational taxonomic unit (OTU)_11021, mean relative abundance in healthy mice of 9.5%] (8) (Table S2). These results were consistent with reports that the capacity to degrade mucin in vitro is phylogenetically widespread (9).
Fig. 1.
Imaging 15N enrichment (δ15N) in the cecum tissue and intestinal lumen 8 h after i.v. injection of 13C,15N threonine. A mosaic of 16 individual high-resolution NanoSIMS images is shown. 12C14N− secondary ion intensity distribution images of the same area are shown to illustrate the structure of the tissue and lumen biomass. 15N hotspots are indicated by white arrows. The patchy distribution of 15N hotspots in the tissue is likely attributable to the heterogeneity in mucus release activity both within and between mucus cells (24, 25).
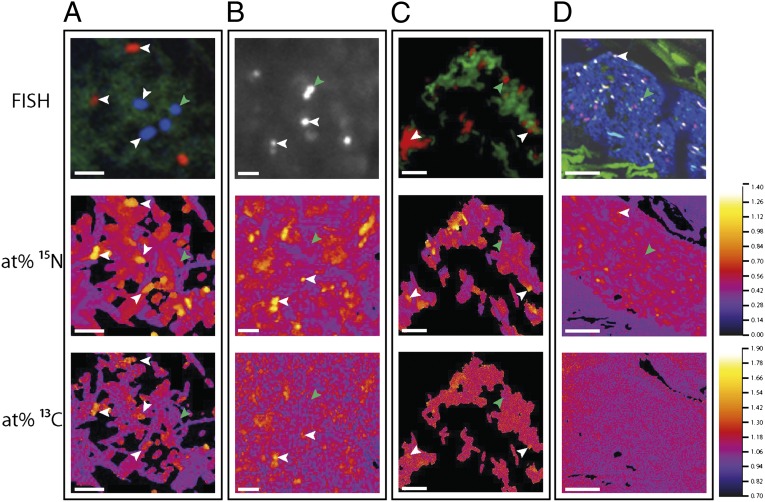
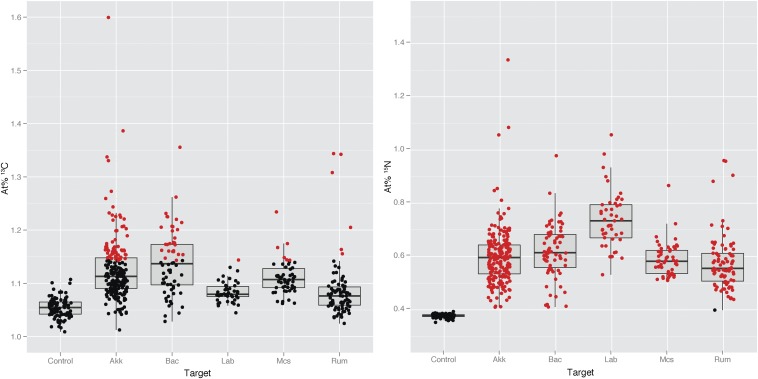
To quantify per-cell C and N isotope composition without carbon contributions from the resin (Fig. 2C, SI Results and Discussion), we analyzed nonembedded lumen contents. We applied a suite of probes based on abundant bacterial groups in a previous study of dextran sodium sulfate (DSS)–induced acute colitis in the same mice (8). These probes were specific for Akkermansia spp., Bacteroides acidifaciens, Mucispirillum spp., Lactobacillaceae/Enterococcaceae spp., and a Ruminococcaceae OTU_5807 species-level phylotype. All targeted microbial groups were enriched in 13C compared with unlabeled controls (Fig. 3), indicating phylogenetically widespread incorporation of 13C-labeled substrates either via direct utilization of host-derived compounds or cross-feeding. Importantly, however, 13C enrichment was not uniform between the groups, and significant 13C labeling was detected in a large subset of the cell population of B. acidifaciens (42%) as well as Akkermansia spp. (29%), and only to a much lesser extent in Ruminococcaceae OTU_5807 (7%), Mucispirillum spp. (12%), and Lactobacillaceae/Enterococcaceae spp. (2%) (Figs. 2 and 3). Past findings lend additional weight to this differential foraging pattern as Akkermansia muciniphila in pure culture degrades mucin and is an abundant member of the mouse and human intestinal microbiota (10). B. acidifaciens has not previously been shown to degrade mucin, but another member of the same genus, the human intestinal bacterium Bacteroides thetaiotaomicron, uses host compounds in a monoassociated mouse model (11). This is, however, proof that these organisms forage on host-derived compounds in a complex gut ecosystem, which is important because substrate utilization can be affected by ecological complexity (12). The variation in the percentage of cells enriched in each target population indicates physiological heterogeneity within phylogenetic probe-defined cells in the gut. This variation may either be due to the targeting of different genotypes or ecotypes with the same probe-stained population, or to heterogeneity in the location or activity of genetically similar or identical populations. It is, for example, possible that resource partitioning of mucosal products between related organisms leads to divergent or complementary ecological niches, a phenomenon that has been observed for Lactobacillus species in the forestomach of mice (13).
Fig. 2.
Representative NanoSIMS (at% 13C and 15N) and FISH images 8 h after i.v. injection of 13C,15N threonine. (A) B. acidifaciens (blue) and Ruminococcaceae OTU_5807 (red) cells (bar: 5 μm; prepared without embedding; other Bacteria shown in green), (B) Akkermansia spp. (white) cells (bar: 2 μm; prepared without embedding), and (C) Lactobacillaceae/Enterococcaceae spp. (red) cells (bar: 5 μm; prepared without embedding; other Bacteria shown in green). (D) Semithin sections of lumen contents with Lachnospiraceae OTU_11021 (yellow/white, overlap of green and red signals), all other Bacteria (blue), and autofluorescent dietary fibers (green) (bar: 10 μm). Exemplary cells with or without significant enrichment (white or green arrows) are indicated. No enrichment in carbon is visible in D because of dilution by unlabeled carbon in the resin.
Fig. 3.
Single-cell stable isotope labeling of selected bacterial groups in the mouse intestine 8 h after i.v. injection of 13C,15N threonine. At% 13C and 15N was calculated for each cell. FISH probes targeted Akkermansia spp. (Akk), Mucispirillum spp. (Mcs), B acidifaciens (Bac), Ruminococcaceae OTU_5807 (Rum), and Lactobacillaceae/Enterococcaceae spp. (Lab) (probe details in Table S1). Eub338-targeted unlabeled cells (control) from the gut were used as controls. Each point represents a single cell, and box plots summarize the quartiles of the target population. Red points are significantly enriched cells (>95% confidence intervals).
In contrast to 13C, almost all cells analyzed were enriched in 15N, which is likely due to an enrichment of 15N in the luminal-free ammonium pool and subsequent incorporation of the labeled ammonium via amino acid synthesis into biomass of all growing cells. 15N-ammonium can originate from microbial disproportionation during fermentation of labeled proteins, but may also result from secretion of labeled urea into the lumen and subsequent hydrolysis if threonine in the host was deaminated (14). However, there was a significant correlation between cellular 13C and 15N content of all labeled cells, indicating a specific coassimilation of 13C and 15N in addition to the general enrichment of 15N (Pearson correlation coefficient 0.763, P < 0.001) (Fig. S4). The most parsimonious explanation for this observation is that bacteria also assimilated organic matter that was directly sourced from secreted threonine-derived proteins.
We estimated that ∼1.6% of the total cecum microbiota (targeted by the EUB338 probe mix) was enriched in 13C (Table S2). Given the abundance of B. acidifaciens and Akkermansia spp. in these communities (relative abundance of 1.0 and 2.1%, respectively; Table S3) and the high proportions of 13C-enriched cells in these populations (42 and 29%, respectively), these two groups are the numerically dominant foragers of host-derived proteins in this mouse cecum community.
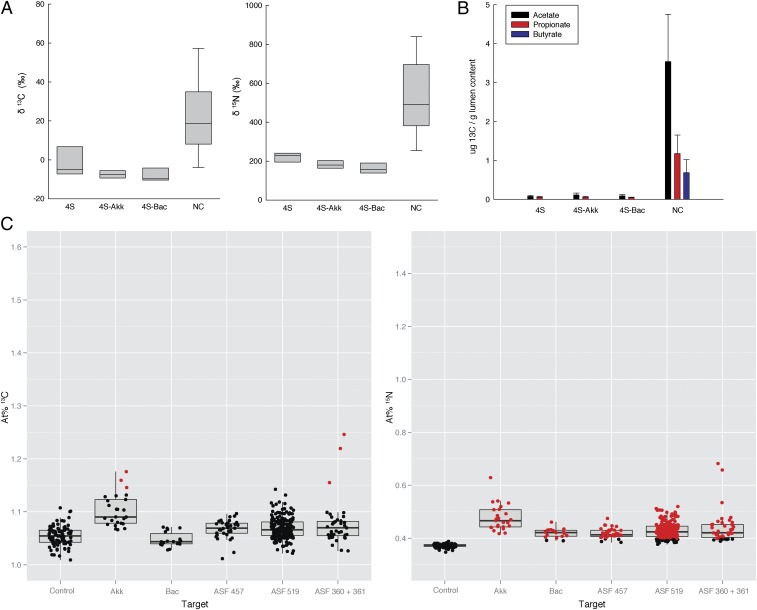
We used a gnotobiotic mouse model to test the foraging performance and system-wide impacts of the important host-compound foragers B. acidifaciens and A. muciniphila in a more controlled ecosystem. The type strains from these species were allowed to colonize mice with a four-member gut microbiota separately and together for 10 d and reached high abundances (the “4S” community; SI Results and Discussion). Surprisingly, however, colonization with A. muciniphila or B. acidifaciens was unable to increase 13C and 15N in the lumen or 13C enrichment in the fermentation product pools, and NanoSIMS analysis verified a low level of enrichment in single cells of the two added strains as well as the 4S microbiota (Fig. 4). It is notable that A. muciniphila, which is generally considered to be a dedicated mucin degrader, is able to colonize effectively and reach a significantly higher relative abundance in 4S than in NC microbiota mice (Table S3) without assimilating much host-derived compounds.
Fig. 4.
Mice that host a four-member intestinal microbiota (4S microbiota) consisting of Lactobacillus acidophilus [Altered Schaedler Flora (ASF) 360], Lactobacillus murinus (ASF 361), Mucispirillum schaedleri (ASF 457), and a Parabacteroides sp. (ASF 519) were colonized with either A. muciniphila (4S-Akk) or B. acidifaciens (4S-Bac) or both together (4S-Akk/Bac). Isotope ratios (δ) are given in units of permil (‰) and are relative to Vienna PeeDee Belemnite (V-PDB) for δ13C and to atmospheric air (atm. air) for δ15N. Colonization with A. muciniphila or B. acidifaciens or both strains together had no significant effect in enrichment of 13C or 15N in the lumen. (A) EA–IRMS data of carbon and nitrogen in lumen contents 8 h after i.v. injection of 13C15N threonine in mice that had been colonized for 10 d. (B) Amount of total 13C in lumen acetate, propionate, and butyrate, measured with LC–IRMS. Values from NC microbiota mice used in (Fig. 1 B and C) are shown in A and B for comparison. (C) Per-cell at% 13C and 15N of selected bacterial groups. Measurements of ASF 360/361, 457, and 519 cells from 4S, 4S-Akk, and 4S-Bac mice were pooled because there were no significant differences between mouse types and Eub338-targeted cells that were not labeled (control) are shown for comparison. Each point represents the at% of a single cell, and box plots summarize the quartiles of the target population. Red points are significantly enriched cells (>95% confidence interval).
Our results suggest that although some organisms benefit differentially from components of host-secreted compounds, the activity of host foragers depends on other community members. Specialized degraders may require the presence of other organisms to efficiently harvest host-derived compounds due to the structural heterogeneity of the glycoprotein substrates and the associated enzymatic diversity required for complete degradation (15, 16). Alternatively, host foragers may spread into other niches such as degradation of dietary compounds in the absence of competition. The identification of these abundant host-protein foragers and the ecological dependence of their activity raises intriguing research questions for future studies to address, including how the availability of diet-derived compounds affects foraging behavior and the mechanisms by which the commensal microbiota is able to stimulate host-protein secretion. As we have demonstrated here, single-cell stable-isotope probing complements popular metaomics approaches (17, 18) by offering an unprecedented ability to explore the eco-physiology of uncultivated gut microorganisms and directly interrogate their metabolic interactions. In vivo labeling in combination with NanoSIMS imaging will therefore be a powerful approach to address other key questions in animal and human microbiome research, ranging from host selection of specific microbes (5) to the biological basis of enterotypes (17).
Methods
Ethics Statement.
All animal experiments were approved by the University of Veterinary Medicine, Vienna, institutional ethics committee and the Austrian Ministry of Science and Research (BMWF) and were conducted in accordance with protocols approved by the Austrian laws (BMWF-66.006/0002-II/10b/2010) and German authorities (Regierung von Oberbayern).
Animal Experiments.
Specific pathogen-free (SPF) C57BL/6 mice that had NC microbiota were used for all experiments not involving direct manipulation of the microbiota and were housed in a SPF facility according to recommendations of the Federation of European Laboratory Animal Science Association. Germfree Naval Medical Research Institute (NMRI) mice were purchased from the University of Bern and transported to the Max von Pettenkofer Institute (Munich) in germfree shippers. NMRI mice colonized in a low-complexity microbiota (Fig. S2) were obtained from the Rodent Center HCI Eidgenössiche Technische Hochschule Zürich. Gnotobiotic female C57BL/6 mice colonized with four strains of the altered Schaedler flora (19) (ASF 360, 361, 457, and 519; 4S microbiota, 4S) were bred at the Ludwig-Maximilians-University of Munich in flexible film isolators with Hepa-filtered air under germfree conditions and fed with autoclaved chow and water. 4S mice were associated by gavage with cultures of A. muciniphila (ATCC BAA-835) or B. acidifaciens (DSM 15896) that had been grown overnight at 37 °C in anaerobic brain-heart infusion (BHI) broth [37 g/L BHI (OXOID), 0.25 g/L Cysteine-HCl⋅H2O, 0.25 g/L Na2S⋅9H2O]. Gavaging of the mice with sterile BHI was used as control. Mice were kept in individually ventilated cages for the duration of association. Upon harvest, blood, cecum epithelial tissue, scraped mucus, and lumen contents were collected, and combined tissue and lumen were embedded in either epoxy (low-viscosity epoxy resin, LVR; Agar 100, Agar Scientific) or acrylic (LR White, Ted Pella) resin as described in detail below.
DSS Treatment.
For DSS challenge, mice were provided with 2% (mass/vol) DSS (molecular weight, 36–50 kDa; MP Biomedicals) in autoclaved drinking water ad libitum for 72 h. No weight loss was observed, and intact gut barrier was confirmed with a fecal occult blood assay (Hemoccult SENSA, Beckman Coulter).
Collection of Blood Serum.
Mice were anesthetized and blood was collected from the retro-orbital sinuses with a microhematocrit blood tube. Blood was allowed to clot for 20 min at room temperature, then centrifuged in a tabletop centrifuge (14,000 × g) for 10 min. Serum was transferred to a new tube, snap-frozen in liquid nitrogen, and stored at –80 °C.
Cecum Contents.
Immediately after collection of blood serum, mice were killed. The intestines were opened, and cecum contents were removed to a microcentrifuge tube. High-purity water (200 µL) was added to the contents, the mixture was homogenized by vortexing, and the homogenate was centrifuged (14,000 × g) for 2 min. The supernatant was transferred to a new microcentrifuge tube and snap-frozen in liquid nitrogen for later analysis of short chain fatty acids. The solid contents were divided and either flash-frozen for subsequent DNA extraction, dried in a vacuum concentrator (Eppendorf Concentrator 5301) for elemental analysis, or fixed for FISH. Fixation was performed by resuspending the contents in 2% paraformaldehyde and incubating overnight at 4 °C. This fixation was appropriate for both Gram-positive and –negative cells, and >99% of SYBR green-stained cells also had a positive signal with the EUB338 probe mix. The contents were then pelleted and washed with PBS two times and then resuspended in a solution of 60% ethanol/40% PBS and stored at –20 °C.
Secreted Mucus Collection.
Mucus was collected by opening the cecum, carefully removing the lumen contents, and gently washing the tissue with sterile PBS. The insoluble mucus was then collected by lightly scraping the inner wall of the tissue with bent forceps and transferring mucus either to a microcentrifuge tube, where it was flash-frozen in liquid nitrogen for further purification, or to a tin cup, where it was dried in a vacuum concentrator (Eppendorf Concentrator 5301) for elemental analysis.
Cecum Epithelial Tissue Preparation.
Cecum tissue was washed in sterile PBS and small sections (∼100 mg) were homogenized in 1 M perchloric acid solution to release soluble components in a carbon-free solvent (16,000 rpm, 30 s, 4 °C; Polytron PT-DA 07/2EC-B101). The proteins were separated from acid-soluble amino acids by centrifugation (13,000 × g, 25 min, 4 °C). The pellet was resuspended in 1 M perchloric acid and centrifuged, and decanted two times more to ensure removal of free threonine. Tissue material was then resuspended in high-purity water (Milli-Q), added to a tin cup, and dried in a vacuum concentrator for elemental analysis.
Tissue and Lumen Harvesting for Embedding.
Cecum tissue and contents were harvested together by carefully tying the edges of the selected area closed with thread, excising the area, and fixing in Carnoy’s solution (6:3:1 mixture of ethanol–acetic acid–chloroform) at 4 °C for 2 h. Fixed samples were then transferred to a solution of 70% ethanol/30% PBS and stored at –20 °C until embedding.
Resin Embedding and Sectioning.
Samples were dehydrated using an increasing ethanol series (six steps: 80%, 80%, 90%, 90%, 96%, and 96%) for 10 min at each step using molecular-grade ethanol. Samples were then further dehydrated by three successive incubations in 100% ethanol for 20 min each. For epoxy resin embedding, which is ideal for preservation of tissue structure but is incompatible with FISH, the samples were placed in 100% acetone for 20 min three times. LVR (Agar 100, Agar Scientific) was infiltrated in a series of steps: a 1:3 mixture of LVR–acetone for 3 h, a 1:1 mixture for 8–12 h, a 3:1 mixture for 8–12 h, pure LVR for 3 h, and pure LVR overnight. Samples were then placed in a mold and into a 60 °C oven for 24 h to polymerize the resin. For embedding in FISH-compatible acrylic resin (LR White, Ted Pella), following the ethanol dehydration, the sample was infiltrated in a series of steps: 3:1 mixture of ethanol–LR White for 3 h, a 1:1 mixture for 8–12 h, a 3:1 mixture for 8–12 h, and pure LR White for 8–16 h three times. Samples in LR White were enclosed in gelatin capsules and incubated at 48 °C for 24 h to polymerize the resin. Semithin sections (300–500 nm) were cut with an ultramicrotome (Leica EM UC6).
Pyrosequencing.
DNA was extracted using a standard phenol-chloroform protocol (8). DNA was subjected to a two-step barcoded PCR targeting the 16S rRNA gene as described previously (20). Sequencing was performed on a GS FLX instrument (454/Roche) at the Norwegian High-Throughput Sequencing Centre and data were analyzed using QIIME (21) as described previously (8).
Isotope-Labeled Compounds and Pulse-Chase Labeling.
For all labeling experiments, stable isotope-labeled L-threonine [98 atom (at) % U-13C4, 98 at% 15N, Sigma-Aldrich] or D-glucose (99 at% U-13C6, Cambridge Isotope Laboratories Inc.) was used. Nonlabeled L-threonine (Sigma-Aldrich) was used as a 12C-control. Compounds were dissolved in sterile saline solution ([0.90% (wt/vol) of NaCl] and delivered to mice via a 50 µL lateral tail vein injection. Each mouse received either threonine (1.8 µmol or 18 µmol) or glucose (12 µmol). Mice were then killed at selected time points (pre-injection, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 16, or 24 h.p.i.). (For further details, see SI Results and Discussion and Figs. S1 and S4–S7.)
EA–IRMS.
To measure the isotopic composition (at% 15N and at% 13C) of blood serum, cecum epithelial tissue, and intestinal lumen contents, 0.5–1.2 mg (dry weight) of each sample was placed in a tin capsule and dried at 60 °C overnight. Samples were analyzed with an elemental analyzer (EA 1110, CE Instruments) coupled via a ConFlo III device to the IRMS (DeltaPLUS, Thermo Fisher). The precision of measurements in standards was better than ±0.00005 at% 15N and better than ±0.00011 at% 13C.
Liquid Chromatography IRMS.
For compound-specific carbon isotope analysis of acetate, propionate, and butyrate, an HPLC system (Dionex Corporation) coupled to a Delta V Advantage Mass Spectrometer by a Liquid Chromatography (LC) IsoLink Interface (Thermo Fisher Scientific) was used (22). Samples were acidified with 1 M phosphoric acid to remove bicarbonate, and subsequently separated on a Macherey-Nagel Nucleogel Sugar 810H column at 75 °C with 0.5 mL⋅min−1 20 mM phosphoric acid as eluent. Peaks were converted to CO2 at 99 °C with 50 µL⋅min−1 of each 0.5 M sodium persulfate and 1.7 M phosphoric acid. Concentrations and isotopic compositions of individual compounds were calibrated against external standards as described elsewhere (22).
FISH.
LR White semithin sections or gut lumen content were mounted on either boron-doped silicon or indium tin oxide coated glass slides (7 × 7 mm) for use in the NanoSIMS. FISH was performed on these samples with monolabeled 16S rRNA-targeted oligonucleotide probes using a standard protocol (23). Specificity and coverage of all applied probes with genus or higher coverage (Table S1) was confirmed using the Ribosomal Database Project II probe match tool with database release 10, Update 31, containing 2,639,157 bacterial and archaeal 16S rRNA sequences. The search for each probe was restricted to sequences of good quality with data in the target region. The specificity of species-level phylotype probes was confirmed in either a previous study (8) by checking them against 16S rRNA gene pyrosequencing data from the same mice or in this study by recovering near full-length 16S rRNA sequences from clone libraries of the same samples. The intersection of species-level phylotype probes used for a single target had a perfect match only to the target sequence and/or to similar sequences in the SILVA Small Subunit Non-Redundant Reference (SSURef NR) Release 111 database (>97% sequence similarity) (Table S1). For most species-level phylotype targets, more than a single probe was applied with fully consistent results between probes, which supported the specificity of the probes in these samples. Specificity for the probe targeting Akkermansia (Akk1437) was also experimentally tested by simultaneously hybridizing Akk1437 and the EUB338 III probe (which targets the phylum Verrucomicrobia), which confirmed that all Akk1437 signals were also EUB338 III positive. The optimal hybridization stringency of all genus-level and species-level phylotype probes was determined in either this study or a previous study (8) by hybridizing gut lumen samples enriched in the target group (as determined with sequencing data) under a range of formamide concentrations to produce formamide dissociation profiles for each probe. Hybridized samples were imaged on an epi-fluorescence laser microdissection microscope (LMD, Leica LMD 7000) using a 40× air objective. Markings were made on the sample using the LMD laser to properly orient the sample and to record the same field of view in the NanoSIMS.
NanoSIMS.
NanoSIMS measurements were performed on an NS50L (Cameca). Data were recorded as images by scanning with a finely focused Cs+ primary ion beam (2–4.5 pA) and detection of negative secondary ions and secondary electrons. Recorded images had a 512 × 512 pixel resolution and field-of-view ranging from 30 × 30 to 60 × 60 μm2. The mass spectrometer was tuned for a mass resolving power of ca. 10,000 at mass 26 to separate 12C14N from the isobaric species 13C2. Analysis areas were presputtered to establish a steady-state secondary ion formation before multicycle image acquisition. All images were recorded with a dwell time of 5–15 ms/pixel/cycle. Signal intensities were corrected for dead time effects and quasi-simultaneous arrival (QSA) effects using QSA sensitivity factors (i.e., “beta” values) of 1.10 for C− and 1.05 for CN− ions. (For further details, see SI Results and Discussion and Figs. S3 and S8.)
Image Processing.
FISH images were manually aligned to NanoSIMS images in Photoshop (CS5, Adobe). Cells were identified in aligned FISH images by automatic thresholding in the Fiji implementation of ImageJ, and resulting regions of interest were manually curated. NanoSIMS images were processed using the OpenMIMS plugin in Fiji (v2.5, National Resource for Imaging Mass Spectrometry). NanoSIMS images were autotracked and atomic percent 13C and 15N were calculated from 12C− and 13C− and 12C14N− and 12C15N− channels, respectively. Summary statistics from each region of interest were calculated for single-cell analysis. Individual cells were considered significantly enriched in 13C,15N if the mean cellular at% 13C,15N was above the 95th percent confidence interval of unlabeled control cells from the gut lumen and if the measurement error (3σ, Poisson) was smaller than the difference between the at% of the labeled cell and the mean at% of unlabeled control cells.
Isotope Notation.
Stable isotopes are given either in atom percent or δ notation. For an element X, with heavy isotope H, and light isotope L, atom percent is given by:
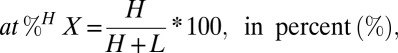 |
and δ is given by:
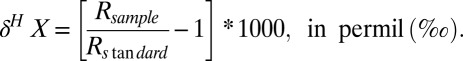 |
To interconvert isotope values between notations, the following equation can be used:
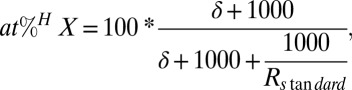 |
where Rstandard refers to Vienna PeeDee Belemnite for δ13C (0.011180) and to atmospheric air for δ15N (0.0036765).
Supplementary Material
Acknowledgments
We thank Margarete Watzka and Julia Ramesmayer for technical assistance and the Norwegian High Throughput Sequencing Centre for pyrosequencing. This work was financially supported by the Austrian Federal Ministry of Science and Research [Austrian Genome Research Program (GEN-AU) III InflammoBiota], the European Research Council [Advanced Grant Nitrification Reloaded (NITRICARE) 294343], and a German Federal Ministry of Education and Research Infektionsgenomik grant. NanoSIMS measurements were supported by large infrastructure grants from the University of Vienna and the City of Vienna.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219247110/-/DCSupplemental.
References
- 1.Winter SE, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467(7314):426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansson MEV, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105(39):15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faure M, et al. Development of a rapid and convenient method to purify mucins and determine their in vivo synthesis rate in rats. Anal Biochem. 2002;307(2):244–251. doi: 10.1016/s0003-2697(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 4.Vaishnava S, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334(6053):255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10(5):323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Schoor SR, Wattimena DL, Huijmans J, Vermes A, van Goudoever JB. The gut takes nearly all: Threonine kinetics in infants. Am J Clin Nutr. 2007;86(4):1132–1138. doi: 10.1093/ajcn/86.4.1132. [DOI] [PubMed] [Google Scholar]
- 7.Schaart MW, et al. Threonine utilization is high in the intestine of piglets. J Nutr. 2005;135(4):765–770. doi: 10.1093/jn/135.4.765. [DOI] [PubMed] [Google Scholar]
- 8.Berry D, et al. Phylotype-level 16S rRNA analysis reveals new bacterial indicators of health state in acute murine colitis. ISME J. 2012;6(11):2091–2106. doi: 10.1038/ismej.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGuckin MA, Lindén SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9(4):265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 10.Derrien M, Collado MC, Ben-Amor K, Salminen S, de Vos WM. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microbiol. 2008;74(5):1646–1648. doi: 10.1128/AEM.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonnenburg JL, et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307(5717):1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 12.Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012;6(8):1535–1543. doi: 10.1038/ismej.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tannock GW, et al. Resource partitioning in relation to cohabitation of Lactobacillus species in the mouse forestomach. ISME J. 2012;6(5):927–938. doi: 10.1038/ismej.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuller MF, Reeds PJ. Nitrogen cycling in the gut. Annu Rev Nutr. 1998;18:385–411. doi: 10.1146/annurev.nutr.18.1.385. [DOI] [PubMed] [Google Scholar]
- 15.Png CW, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010;105(11):2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 16.Hoskins LC, Boulding ET. Mucin degradation in human colon ecosystems. Evidence for the existence and role of bacterial subpopulations producing glycosidases as extracellular enzymes. J Clin Invest. 1981;67(1):163–172. doi: 10.1172/JCI110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arumugam M, et al. MetaHIT Consortium Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, et al. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci USA. 2008;105(6):2117–2122. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dewhirst FE, et al. Phylogeny of the defined murine microbiota: Altered Schaedler flora. Appl Environ Microbiol. 1999;65(8):3287–3292. doi: 10.1128/aem.65.8.3287-3292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry D, Ben Mahfoudh K, Wagner M, Loy A. Barcoded primers used in multiplex amplicon pyrosequencing bias amplification. Appl Environ Microbiol. 2011;77(21):7846–7849. doi: 10.1128/AEM.05220-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wild B, Wanek W, Postl W, Richter A. Contribution of carbon fixed by Rubisco and PEPC to phloem export in the Crassulacean acid metabolism plant Kalanchoe daigremontiana. J Exp Bot. 2010;61(5):1375–1383. doi: 10.1093/jxb/erq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daims H, Stoecker K, Wagner M. In: Advanced Methods in Molecular Microbial Ecology. Osborn A, Smith C, editors. Abingdon, UK: Bios-Garland; 2005. pp. 213–239. [Google Scholar]
- 24.Halm DR, Halm ST. Secretagogue response of goblet cells and columnar cells in human colonic crypts. Am J Physiol Cell Physiol. 2000;278(1):C212–C233. doi: 10.1152/ajpcell.2000.278.1.C212. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Vilar J, Mabolo R, McVaugh CT, Bertozzi CR, Boucher RC. Mucin granule intraluminal organization in living mucous/goblet cells. Roles of protein post-translational modifications and secretion. J Biol Chem. 2006;281(8):4844–4855. doi: 10.1074/jbc.M510520200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.