Abstract
Basal ganglia-thalamocortical circuits are critical for motor control and motor learning. Classically, basal ganglia nuclei are thought to regulate motor behavior by increasing or decreasing cortical firing rates, and basal ganglia diseases are assumed to reflect abnormal overall activity levels. More recent studies suggest instead that motor disorders derive from abnormal firing patterns, and have led to the hypothesis that surgical treatments, such as pallidotomy, act primarily by eliminating pathological firing patterns. Surprisingly little is known, however, about how the basal ganglia normally influence task-related cortical activity to regulate motor behavior, and how lesions of the basal ganglia influence cortical firing properties. Here, we investigated these questions in a songbird circuit that has striking homologies to mammalian basal ganglia-thalamocortical circuits but is specialized for singing. The “cortical” outflow nucleus of this circuit is required for song plasticity and normally exhibits increased firing during singing and song-locked burst firing. We found that lesions of the striato-pallidal nucleus in this circuit prevented hearing-dependent song changes. These basal ganglia lesions also stripped the cortical outflow neurons of their patterned burst firing during singing, without changing their spontaneous or singing-related firing rates. Taken together, these results suggest that the basal ganglia are essential not for normal cortical firing rates but for driving task-specific cortical firing patterns, including bursts. Moreover, such patterned bursting appears critical for motor plasticity. Our findings thus provide support for therapies that aim to treat basal ganglia movement disorders by normalizing firing patterns.
Keywords: auditory feedback, skill learning, anterior forebrain pathway, prefrontal cortex, Parkinson disease
Basal ganglia-thalamocortical circuits play a critical role in learning and producing complex motor behaviors, and are the locus of numerous movement disorders. Despite intense study of these circuits in both normal and disease states, how they process movement-related information and modulate cortical activity to regulate motor output remain poorly understood. Classic models of basal ganglia function emphasize the concept that changes in overall firing rates of basal ganglia nuclei affect the excitability of cortical neurons, and thereby facilitate or suppress particular motor programs (1, 2). For example, hyperkinetic disorders are thought to derive from insufficient pallidal inhibition of the thalamus, resulting in increased cortical activity that abnormally facilitates movement. Recent optogenetic manipulations that increased or decreased locomotion through activation of striatal pathways are consistent with such “firing-rate” models (3).
Although rate models provide a simple framework for understanding the pathophysiology of movement disorders, they do not explain many experimental and pathological findings. A well-known example is the paradoxical effect of surgical lesions of the globus pallidus internus (pallidotomy), a procedure used to ameliorate parkinsonian motor symptoms. According to firing-rate models, pallidotomy should chronically disinhibit thalamic neurons and increase excitatory drive to the cortex. This theory explains how pallidotomy can enable movements in hypokinetic subjects, but also predicts abnormal or increased movements. In practice, however, involuntary or excessive movements are not observed in patients following pallidotomy (4, 5). Classic firing-rate models also fail to explain the presence of abnormal firing patterns that have been observed throughout the basal ganglia and cortex, such as increased bursting, oscillations, and synchronized firing across neurons in both human patients and animal models of disease. In contrast to rate models, “firing-pattern” models hypothesize that such aberrant activity patterns disrupt the normal operation of cortical motor programs, and that pallidotomy acts by eliminating abnormal firing patterns in the downstream circuit, thus blocking the spread of pathologic signals to the cortex (4, 6, 7). The role of normal, task-related cortical firing patterns and their dependence on basal ganglia input, however, still remain unclear, and few electrophysiological studies have addressed these questions specifically (6, 8).
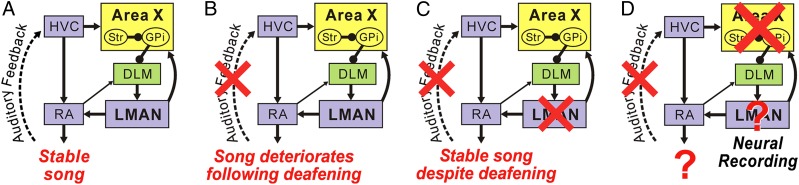
To examine basal ganglia contributions to task-related cortical activity and motor behavior, and to determine how damage to the circuit alters cortical firing properties, we investigated the songbird anterior forebrain pathway (AFP) (Fig. 1A), a discrete circuit that shares many similarities with basal ganglia-thalamocortical circuits in mammals (9, 10). Unlike its mammalian counterparts, however, the AFP is specialized for a single, well-defined behavior—singing—greatly facilitating investigation of information processing in basal ganglia-thalamocortical circuits and the link between neural activity and behavior. The AFP indirectly connects the song premotor cortical analog HVC (HVC is used as its proper name) and the song motor cortical analog RA (robust nucleus of the arcopallium) via the basal ganglia (striato-pallidal) nucleus Area X, the thalamus, and the prefrontal cortical analog LMAN (lateral magnocellular nucleus of the nidopallium) [see Fig. 1A for specific parallels to mammals; although these parallels are not complete (11, 12), for simplicity we hereafter refer to LMAN as cortical]. As in mammals, the AFP is not required for the production of well-learned behaviors, but it is critically important for motor plasticity (13–18). For example, in adult zebra finches, manipulations that distort or eliminate auditory feedback normally drive gradual changes in song structure (Figs. 1B and 2A, second group) (19–21), but lesions or inactivation of the AFP output nucleus LMAN prevent hearing-dependent changes to song (Fig. 1C) (16, 17). Although previous studies have shown that LMAN neurons exhibit robust singing-related activity, little is known of how the basal ganglia contribute to LMAN activity nor what features of LMAN activity are required for song plasticity. To study these questions, we made bilateral lesions of the basal ganglia nucleus Area X in adult birds, eliminating pallidal projections to the thalamus (akin to pallidotomy), and examined their effects on neural activity in LMAN, as well as on the song plasticity that normally follows adult deafening (Fig. 1D).
Fig. 1.
Contributions of an avian basal ganglia-thalamocortical circuit to vocal motor plasticity. (A) The AFP of songbirds indirectly connects the motor nuclei HVC and RA, and consists of the basal ganglia (striato-pallidal) nucleus Area X (9, 10), the thalamic nucleus DLM, and the cortical nucleus LMAN. Although LMAN is not a layered structure with classic pyramidal cells, it shares many anatomic and physiologic similarities with the mammalian prefrontal cortex: LMAN lies anterior to motor and premotor areas, receives input from a dorsal thalamic nucleus that is not a primary sensory area, projects to the basal ganglia and to the primary song motor nucleus RA, and is more densely innervated by dopaminergic fibers than the region surrounding it (31, 60–62). Moreover, as with the prefrontal cortex, stimulation of LMAN does not evoke motor (song) output (24, 46, 63). Alternatively, or in addition, with respect to the hypothesis that avian nuclei are homologous to mammalian cortical layers (11, 12, 64), LMAN shares some but not all of the features of layer II/III intracortically connecting neurons. Yellow, green, and purple boxes indicate basal ganglia, thalamic, and cortical structures, respectively. Str, striatum; GPi, internal segment of the globus pallidus. (B) Removal of auditory feedback (via deafening) induces gradual degradation of song (20). (C) Lesions of LMAN prevent deafening-induced changes in song (17). (D) Experimental paradigm: To investigate basal ganglia contributions to cortical activity and motor plasticity, lesions of Area X were made before deafening and their effects on LMAN activity and song were examined.
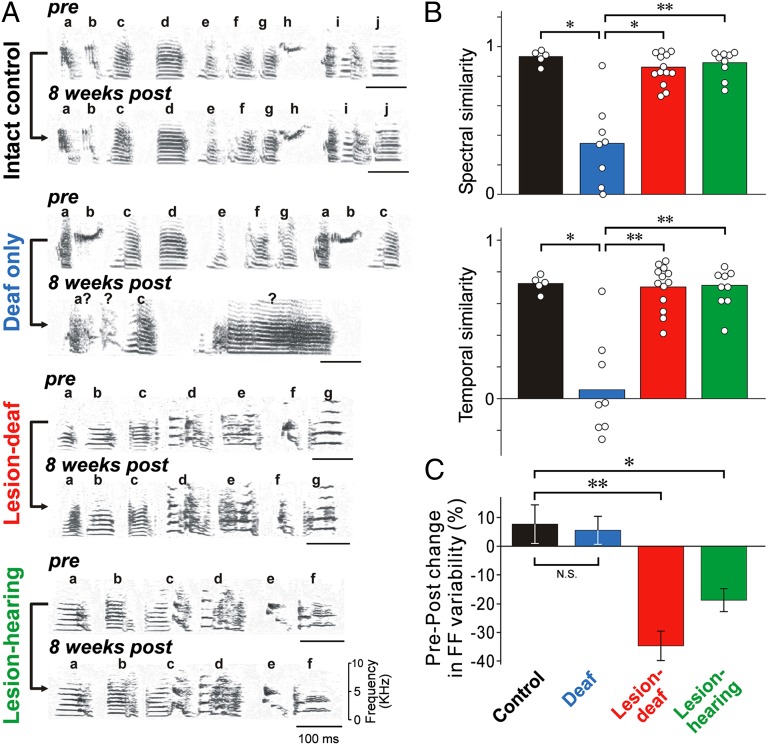
Fig. 2.
Bilateral lesions of Area X block vocal plasticity and acute song variability. (A) Spectrograms illustrate striking changes in the song of a deafened bird (second group) but not in those of an intact bird (first group), a bird that received lesions of Area X before deafening (third group), nor a hearing bird with lesions of Area X (fourth group). Pre- and 8-wk-postoperative songs are shown. Syllables are labeled with letters; question marks denote unidentifiable syllables. (B) Summary of spectral (Upper) and temporal (Lower) similarity between preoperative and 8-wk-postoperative song. Each point corresponds to one bird; *P < 0.005; **P < 0.001. (C) Percent differences in fundamental frequency (FF) variability between pre- and 1–3 d postoperative syllables (control: 16 syllables in four sham-lesion birds; deaf only: 23 syllables in five birds; lesion-deaf: 13 syllables in four birds; lesion-hearing: 28 syllables in nine birds). Error bars, SEM; *P < 0.02; **P < 10−4.
Results
Consistent with previous studies, we found that the songs of birds that were deafened as adults gradually deteriorated over a period of weeks, in comparison with the stable songs of normal adults (e.g., Fig. 2A, compare two top groups; all songs were recorded from birds singing alone; i.e., “undirected” song) (20). Deafening induced changes in both the structure of individual song elements (syllables) and the temporal pattern of song. In contrast, we found that the songs of birds that received bilateral lesions of Area X before deafening (hereafter “lesion-deaf” birds) (see Fig. S1 for lesions) remained largely unchanged (Fig. 2A, third group). Just like intact birds and hearing birds with lesions of Area X (e.g., Fig. 2A, fourth group) (13, 15), lesion-deaf birds continued to sing the same stereotyped sequence of syllables (motif), even eight wks after deafening. Effects of experimental manipulations on song were quantified using two measures that represent separately how well the spectral and temporal structure were maintained in postoperative songs (analyzed eight wks after deafening) in comparison with preoperative songs (Fig. 2B and Materials and Methods). Both measures of song similarity were significantly higher for lesion-deaf birds (n = 13) than for deafened birds (n = 6 deaf and n = 2 deaf and sham-lesion). Although there were subtle changes in the fine structure of songs of birds with lesions of Area X, such as increased spectral entropy, maintenance of the spectral and temporal structure of the songs of both hearing (n = 8) and deaf birds with lesions of Area X was comparable to that of intact birds (n = 5) over the same period (Fig. 2B) (P > 0.05). These findings demonstrate that the basal ganglia are required for normal, hearing-dependent vocal motor plasticity but not for the performance of well-learned song, and they strikingly mirror clinical findings that pallidotomy does not markedly affect the execution of well-practiced skills but impairs motor plasticity (22, 23).
Lesions of Area X also acutely reduced trial-by-trial variability in song, which has been hypothesized to be critical for song plasticity (24–27). Compared with control (sham-lesion) and deafened birds, variability in the pitch, or fundamental frequency, of individual syllables across repeated renditions was significantly lower in birds with lesions of Area X (both hearing and deaf; fundamental frequency variability was measured 1–3 d after surgery) (Fig. 2C).
Our finding that lesions of Area X block auditory feedback-dependent changes to song mimics the behavioral effects of lesions/inactivation of the cortical nucleus LMAN (16, 17), and suggests that basal ganglia lesions abolish cortical firing properties that are critical for motor plasticity. What might these critical properties be? As in mammals, pallidal neurons in Area X send strong inhibitory input to the thalamus, which in turn sends excitatory input to LMAN (Fig. 1A) (28–33). Previous work has shown that acute inactivation of Area X in anesthetized adult zebra finches tonically disinhibits thalamic neurons, inducing higher spontaneous firing rates in LMAN (34), consistent with findings in mammals (8, 35). According to firing-rate models of basal ganglia function, lesions of Area X should increase spontaneous firing rates in LMAN, which, in turn, could mask the normal singing-related rate increases observed in intact birds (36–38). Alternatively, lesions of Area X could change the firing statistics of LMAN neurons, as predicted by firing-pattern models, with or without affecting their overall firing rates. To examine these possibilities directly, we made extracellular recordings of LMAN activity in awake, singing birds following lesions of Area X (both hearing and deaf) and compared these with recordings in intact birds.
In birds with lesions of Area X (n = 2 hearing and n = 6 deaf), we found that the spontaneous firing rates of LMAN neurons (i.e., when birds were not singing) were similar to those in intact birds (Fig. 3 A, B Bottom, D and E; data from birds with lesions of Area X are plotted in two different colors, denoting hearing vs. deaf birds, but hereafter are described together as data from lesion birds, as results were similar between groups). This finding indicates that although pharmacological inactivation of Area X transiently disinhibits the thalamus and increases spontaneous firing rates in LMAN (34), excitatory drive to LMAN does not remain chronically elevated following lesions of Area X. Rather, spontaneous firing rates of LMAN neurons recover to normal levels, perhaps because of homeostatic mechanisms.
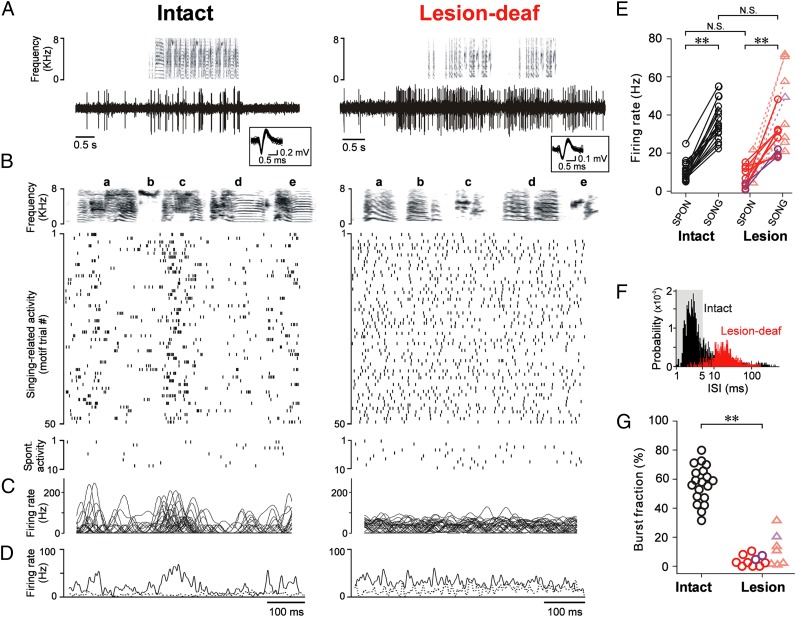
Fig. 3.
Lesions of Area X alter firing patterns in LMAN without affecting firing rate increases during singing. (A) Song spectrograms (Upper) and singing-related activity of single LMAN neurons (Lower) in an intact (Left) and a lesion-deaf bird (Right). Note the increase in activity both before and during singing in both cases. (Inset) Fifty randomly selected spike waveforms sorted from single LMAN neurons are superimposed. (B) Raster plots of activity during 50 motif renditions (Upper) and when the bird was quiet (spontaneous activity, Bottom) for the neurons in A. (C) Smoothed instantaneous firing rates computed from the spikes of the same LMAN neurons (30 motifs overlaid). (D) Corresponding firing rate histograms for motif-aligned (solid line) and spontaneous activity (dotted line). (E) LMAN neurons in intact [n = 19 single units (circles) in nine birds] and lesion birds [n = 10 single units (circles) and 8 small clusters of units (triangles) in eight birds] increased their firing rates during singing. Red, data from lesion-deaf birds; purple, data from lesion-hearing birds. Statistical comparisons across experimental groups were performed on single units. (F) ISI histograms of LMAN neurons during singing in intact (black) and lesion-deaf birds (red) shown in A–D; shading highlights ISIs ≤ 5 ms (bursts). (G) Mean fraction of spikes that occurred as part of bursts (burst fraction). LMAN neurons exhibited burst firing in intact birds but not in lesion birds. Conventions as in E. **P < 0.0005.
Lesions of Area X also had little effect on singing-related increases in the firing rates of LMAN neurons. In both intact and deaf birds, LMAN neurons increase their discharge before and during singing, consistent with premotor signaling (36–38). In the absence of Area X, we found that LMAN neurons continued to exhibit robust increases in firing rate before song initiation and during singing (Fig. 3 A–E, Right, and Fig. S2). Singing-related firing rates did not differ significantly between lesion and intact birds (Fig. 3E). Our results show that the maintained singing-related increases in LMAN firing in birds with lesions of Area X are not sufficient to drive deafening-induced song plasticity, and challenge models of basal ganglia function that focus solely on firing rates.
We therefore analyzed the firing patterns of LMAN neurons after lesions of Area X, and found that they were dramatically altered in several ways. Previous studies have shown that LMAN neurons in intact birds typically fire brief, high-frequency bursts of action potentials when they sing alone (Fig. 3B, Left) (36, 37). This result is characterized by a prominent peak at short intervals (∼2–3 ms) in the distribution of interspike intervals (ISI) (Fig. 3F, black). On average, in intact birds, more than half of all LMAN spikes occur as part of bursts [ISIs ≤ 5 ms; mean burst fraction = 57 ± 13% (SD)] (Fig. 3G, Left). In contrast, in lesion birds, LMAN neurons rarely fired bursts of action potentials (e.g., Fig. 3B, Right, and Figs. S3 and S4). There were no peaks at short intervals in the ISI distributions (e.g., Fig. 3F, red), and the mean burst fraction was significantly smaller than in control birds (Fig. 3G, Right) (mean burst fraction = 4 ± 3.7%). This loss of burst firing in LMAN was not a result of the absence of auditory feedback, as LMAN neurons in deafened birds with intact Area X exhibited prominent burst firing similar to that in intact birds (mean burst fraction = 54 ± 18%; n = 10 neurons in three deaf birds; P > 0.5 vs. control birds). These results indicate that LMAN burst firing in adult birds depends strongly on afferent input from the basal ganglia (via the thalamus), and is not purely an intrinsic property of this cortical nucleus.
Lesions of Area X also eliminated the song-locked firing patterns characteristic of LMAN neurons in adult birds. Previous work has shown that in both intact and deaf birds, LMAN neurons typically fire phasically at particular times in song (Fig. 3 B–D, Left) (36–38). In contrast, in lesion birds, such motif-locked spike patterns were not apparent, despite the increased firing during singing (Fig. 3 B–D, Right, and Figs. S3 and S4). Across all neurons, modulation of the average, song-aligned rate histograms was significantly smaller in lesion birds than in intact birds (Figs. 3D and 4A).
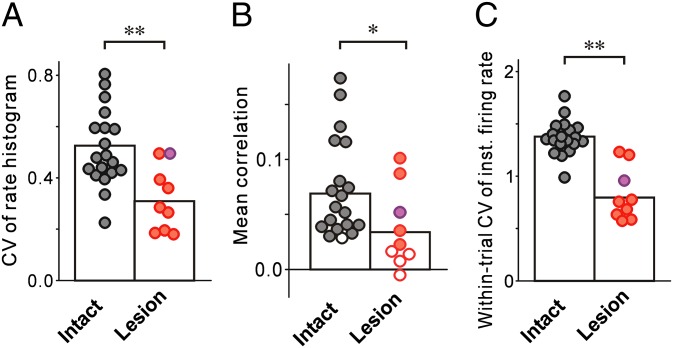
Fig. 4.
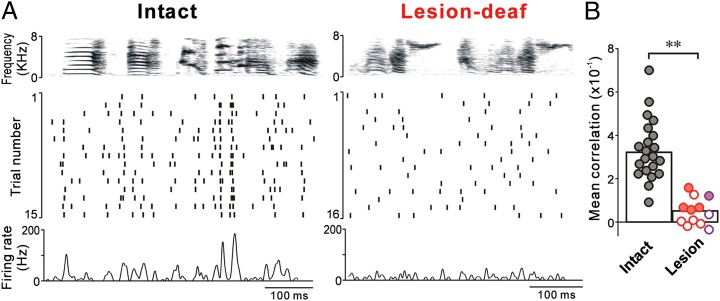
Song-locked rate modulations of LMAN neurons are reduced in lesion birds. CVs of the motif-aligned rate histogram (A), mean cross-trial correlations in spike trains (B), and within-trial CVs of instantaneous firing rates (C) of LMAN neurons were lower in lesion birds (red, lesion-deaf; purple, lesion-hearing) than in intact birds (black). In B, filled circles indicate a significant difference from correlations computed from time-shuffled spike-trains (Materials and Methods). *P < 0.05; **P < 0.01.
Reduced modulation of the motif-aligned rate histograms in lesion birds stems from two factors. First, LMAN activity in lesion birds was no longer aligned to songs across repeated trials. Correlations in the spike trains across different renditions of the motif were significantly smaller in lesion birds compared with intact birds (Figs. 3C and 4B, and Materials and Methods). For 44% of the neurons in lesion birds, correlation distributions did not differ significantly from those calculated after random time shifts were added to the spike trains (versus 5% in intact birds) (Fig. 4B and Materials and Methods). Second, firing rates of LMAN neurons in lesion birds were not strongly modulated even during individual motifs, but were instead relatively tonic (Fig. 3 B and C, Right). The average coefficient of variation (CV) of the instantaneous firing rate (computed within each trial and averaged across trials) was significantly smaller in lesion birds than in intact birds (Fig. 4C).
The disruption of song-locked firing patterns following lesions of Area X was especially apparent during courtship singing to females. Normally, in intact birds, the precision and reliability of song-locked firing of LMAN neurons are greatest when males sing to females, and cross-trial correlations in spike trains are high (Fig. 5, Left) (36, 39). In lesion birds, however, we found that cross-trial correlations were small even during courtship song (Fig. 5, Right, and Fig. S5), and in 58% of the neurons, LMAN activity was random with respect to song (versus 0% in intact birds). Thus, in addition to eliminating burst firing, lesions of Area X also stripped LMAN neurons of much of their song-locked firing pattern without affecting overall activity levels. Together with our behavior data, these results demonstrate a critical contribution of Area X both to song-locked firing patterns of LMAN neurons and to song plasticity.
Fig. 5.
LMAN neurons in lesion birds do not exhibit song-locked firing, even during courtship singing. (A) Motif-aligned raster plots and corresponding rate histograms of LMAN neurons recorded during courtship singing in an intact (Left) and a lesion-deaf bird (Right). (B) Mean cross-trial correlations in spike trains were lower in lesion birds than in intact birds. Conventions as in Fig. 4B. **P < 0.001.
Discussion
Our experiments in the songbird basal ganglia-thalamocortical circuit address outstanding questions about how the basal ganglia modulate task-related cortical activity to regulate motor behavior. We found that lesions of the striato-pallidal nucleus Area X eliminated the normal phasic, song-locked rate modulations and burst firing of LMAN neurons without altering their overall firing rates (neither spontaneous nor during singing). Concomitant with the loss of temporally patterned activity in the AFP, vocal plasticity in response to deafening was largely eliminated. Taken together, these results indicate that singing-related increases in LMAN firing rates, which do not depend on Area X, are not sufficient for driving changes in adult song. Rather, it is the temporally patterned burst firing in LMAN—which depends on input from Area X—that appears to be critical for driving gradual, hearing-dependent changes in song. Given the high degree of homology between avian and mammalian basal ganglia-thalamocortical circuits (9, 10), our findings imply that a key function of the intact basal ganglia is to modulate cortical activity to generate task-related firing patterns critical for motor plasticity.
Our study also demonstrates that removal of basal ganglia input to the thalamus does not induce chronic increases in cortical activity, as had been previously thought based on synaptic connectivity and acute inactivation studies (8, 34, 35). The normal levels of cortical activity that we observed in birds with pallidal lesions are consistent with the observation that patients with Parkinson disease do not exhibit involuntary or excessive movements after pallidotomy. Moreover, the loss of behavior-locked cortical firing patterns in our birds supports the idea that pallidotomy ameliorates Parkinsonian motor deficits by blocking the spread of abnormal firing patterns from the basal ganglia to the cortex (4, 6, 7). Finally, the elimination of auditory feedback-driven motor plasticity in our birds by pallidal lesions parallels the impaired ability of postpallidotomy patients to learn new motor sequences (22, 23).
Our finding that basal ganglia input contributes critically to song-locked cortical firing patterns in adult birds contrasts strongly with a recent study in juvenile birds that examined the basal ganglia-recipient thalamic nucleus that projects to LMAN [medial portion of the dorsolateral thalamus (DLM)] (Fig. 1A) (40). That study suggested that song-locked rate modulations in DLM are driven primarily by excitatory input from the motor cortical nucleus RA, because they were not eliminated by lesions of Area X. The discrepancy between the two studies raises some intriguing possibilities. First, because the previous study recorded thalamic activity after lesions of Area X but did not examine LMAN activity, it is possible that coordinated inputs from both RA and Area X are required to endow thalamic neurons with the ability to drive patterned activity in their cortical target LMAN. Alternatively, or in addition, there may be a developmental change in the relative contributions of basal ganglia inputs (Area X) and descending cortical inputs (RA) to patterned activity in thalamocortical circuits, such that inputs from RA decline with age or contributions from Area X increase (41–43). Such a developmental shift would explain the contradictory findings that lesions of Area X acutely reduce song variability in adult birds (Fig. 2C) but not in juvenile birds (13, 44). Regardless of the reasons for the discrepancy between the two studies, our findings indicate that although RA-to-thalamus projections are a likely source of the maintained singing-related increases in LMAN activity that we observed, pallidal inputs are the primary drivers of song-locked firing patterns in LMAN in adult birds.
In contrast to previous studies that eliminated all activity in LMAN, here, by removing basal ganglia input, we altered LMAN firing patterns without changing overall activity levels and found that song-locked bursting appears essential for song plasticity. How might basal ganglia-dependent, song-locked bursting in LMAN influence activity in the motor pathway and contribute to song plasticity? First, LMAN bursting may be especially effective at modulating activity in the song motor nucleus RA, driving fluctuations in song that could function as motor exploration (24–27, 36, 45–47). Second, given recent evidence that LMAN can adaptively bias song output (16, 48, 49), it is also possible that song-locked bursting contributes to song plasticity directly, by instructing specific changes in the synaptic connections between the premotor nucleus HVC and RA via spike-timing–dependent mechanisms (24, 27, 36, 47, 50, 51). Third, burst firing might also increase the synchrony of activity across multiple neurons in LMAN, and thus enhance their effectiveness in influencing RA and subsequent song output. Although the mechanisms by which bursts contribute to motor plasticity may differ across systems, our findings suggest that task-related bursting is an important feature of normal basal ganglia-thalamocortical circuits. Moreover, our results raise the possibility that when such activity is altered and is no longer tightly locked to movement, as in the case of the exaggerated bursting and synchronized firing observed in Parkinson disease (4, 6, 7), it can disturb information processing in these circuits and contribute to motor deficits.
In summary, our results shed light on how the basal ganglia regulate motor behavior by modulating cortical firing patterns. Numerous studies in mammals have shown that basal ganglia neurons change their task-related firing during learning (52, 53), and that such changes can precede changes in cortical activity and behavior (54–56). Here, we extend these findings by directly demonstrating that the basal ganglia are required for specific, task-related cortical firing patterns that are critical for song motor plasticity. Although our findings do not rule out rate models of basal ganglia function in mammals, by dissociating firing patterns from changes in firing rates in the song circuit, our data suggest that therapeutic strategies that aim to normalize discharge patterns may be especially useful for ameliorating motor deficits of basal ganglia disorders (57).
Materials and Methods
Subjects and Surgery.
Subjects were adult male zebra finches (Taeniopygia guttata). All procedures were approved by the University of California at San Francisco Institutional Animal Care and Use Committee. Young adult birds (∼110 d posthatch) were deafened as described previously (58). In a subset of these birds, bilateral lesions of Area X were made 0–2 d before deafening by local injection of 1% ibotenic acid. Lesions of Area X were also made in a third group of birds with intact hearing. All lesions were evaluated in tissue labeled with an antibody to Substance P (lesion sizes: 75–100%).
Song Analysis.
To quantify song changes, we randomly chose 5 preoperative and 10 postoperative (8 wk after) songs, and measured the spectral and temporal similarity between all possible pairs of pre- and postoperative songs using Sound Analysis Pro (v 1.04) (59) and custom-written software. These analyses were also performed on the songs of intact, age-matched birds.
Neural Recording.
Recordings and analyses of singing-related activity in LMAN were performed as previously described (36, 37). Briefly, extracellular neural signals were recorded in awake, behaving birds using a microdrive carrying tungsten electrodes. Spikes were sorted offline, and spike trains during quiet (baseline) periods and during singing were extracted. Neural activity during repeated motifs was aligned and linearly time-warped using a reference motif. Mean firing rates and the fraction of spikes that occurred as part of bursts (ISI ≤ 5 ms) were calculated. To analyze firing rate modulations, spike trains were smoothed with a Gaussian filter (SD = 5 ms). Trial-by-trial differences in activity patterns were quantified by computing the average correlation coefficient between instantaneous firing rates for all pairs of trials (36). Neural data from intact birds were collected previously (36) and are replotted here for comparison. More detailed methods of surgery, song analyses, neural recording, and statistical tests are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank M. Stryker, M. Brainard, M. Joshua, and W. H. Mehaffey for comments on this manuscript, and A. Arteseros for technical assistance. This work was supported by Grants MH55987 and MH078824 from the National Institutes of Health and by the National Alliance for Research on Schizophrenia and Depression (A.J.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216308110/-/DCSupplemental.
References
- 1.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12(10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 2.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13(7):281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 3.Kravitz AV, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown P, Eusebio A. Paradoxes of functional neurosurgery: Clues from basal ganglia recordings. Mov Disord. 2008;23(1):12–20, quiz 158. doi: 10.1002/mds.21796. [DOI] [PubMed] [Google Scholar]
- 5.Marsden CD, Obeso JA. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson’s disease. Brain. 1994;117(Pt 4):877–897. doi: 10.1093/brain/117.4.877. [DOI] [PubMed] [Google Scholar]
- 6.Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119(7):1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64(1):20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 8.Inase M, Buford JA, Anderson ME. Changes in the control of arm position, movement, and thalamic discharge during local inactivation in the globus pallidus of the monkey. J Neurophysiol. 1996;75(3):1087–1104. doi: 10.1152/jn.1996.75.3.1087. [DOI] [PubMed] [Google Scholar]
- 9.Gale SD, Perkel DJ. Anatomy of a songbird basal ganglia circuit essential for vocal learning and plasticity. J Chem Neuroanat. 2010;39(2):124–131. doi: 10.1016/j.jchemneu.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doupe AJ, Perkel DJ, Reiner A, Stern EA. Birdbrains could teach basal ganglia research a new song. Trends Neurosci. 2005;28(7):353–363. doi: 10.1016/j.tins.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Dugas-Ford J, Rowell JJ, Ragsdale CW. Cell-type homologies and the origins of the neocortex. Proc Natl Acad Sci USA. 2012;109(42):16974–16979. doi: 10.1073/pnas.1204773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Brzozowska-Prechtl A, Karten HJ. Laminar and columnar auditory cortex in avian brain. Proc Natl Acad Sci USA. 2010;107(28):12676–12681. doi: 10.1073/pnas.1006645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: Implications for vocal learning. J Neurosci. 1991;11(9):2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224(4651):901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- 15.Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990;53(1):51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- 16.Andalman AS, Fee MS. A basal ganglia-forebrain circuit in the songbird biases motor output to avoid vocal errors. Proc Natl Acad Sci USA. 2009;106(30):12518–12523. doi: 10.1073/pnas.0903214106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000;404(6779):762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- 18.Williams H, Mehta N. Changes in adult zebra finch song require a forebrain nucleus that is not necessary for song production. J Neurobiol. 1999;39(1):14–28. [PubMed] [Google Scholar]
- 19.Leonardo A, Konishi M. Decrystallization of adult birdsong by perturbation of auditory feedback. Nature. 1999;399(6735):466–470. doi: 10.1038/20933. [DOI] [PubMed] [Google Scholar]
- 20.Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behav Neural Biol. 1992;57(1):58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- 21.Tumer EC, Brainard MS. Performance variability enables adaptive plasticity of ‘crystallized’ adult birdsong. Nature. 2007;450(7173):1240–1244. doi: 10.1038/nature06390. [DOI] [PubMed] [Google Scholar]
- 22.Obeso JA, et al. What can man do without basal ganglia motor output? The effect of combined unilateral subthalamotomy and pallidotomy in a patient with Parkinson’s disease. Exp Neurol. 2009;220(2):283–292. doi: 10.1016/j.expneurol.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 23.Brown RG, et al. Pallidotomy and incidental sequence learning in Parkinson’s disease. Neuroreport. 2003;14(1):21–24. doi: 10.1097/00001756-200301200-00004. [DOI] [PubMed] [Google Scholar]
- 24.Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433(7026):638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- 25.Ölveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol. 2005;3(5):e153. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doya K, Sejnowski TJ. Advances in Neural Information Processing Systems. Cambridge, MA: MIT Press; 1995. pp. 101–108. [Google Scholar]
- 27.Fiete IR, Fee MS, Seung HS. Model of birdsong learning based on gradient estimation by dynamic perturbation of neural conductances. J Neurophysiol. 2007;98(4):2038–2057. doi: 10.1152/jn.01311.2006. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg JH, Adler A, Bergman H, Fee MS. Singing-related neural activity distinguishes two putative pallidal cell types in the songbird basal ganglia: Comparison to the primate internal and external pallidal segments. J Neurosci. 2010;30(20):7088–7098. doi: 10.1523/JNEUROSCI.0168-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo M, Perkel DJ. A GABAergic, strongly inhibitory projection to a thalamic nucleus in the zebra finch song system. J Neurosci. 1999;19(15):6700–6711. doi: 10.1523/JNEUROSCI.19-15-06700.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livingston FS, Mooney R. Development of intrinsic and synaptic properties in a forebrain nucleus essential to avian song learning. J Neurosci. 1997;17(23):8997–9009. doi: 10.1523/JNEUROSCI.17-23-08997.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bottjer SW, Halsema KA, Brown SA, Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol. 1989;279(2):312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- 32.Luo M, Perkel DJ. Long-range GABAergic projection in a circuit essential for vocal learning. J Comp Neurol. 1999;403(1):68–84. [PubMed] [Google Scholar]
- 33.Farries MA, Perkel DJ. A telencephalic nucleus essential for song learning contains neurons with physiological characteristics of both striatum and globus pallidus. J Neurosci. 2002;22(9):3776–3787. doi: 10.1523/JNEUROSCI.22-09-03776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kojima S, Doupe AJ. Activity propagation in an avian basal ganglia-thalamocortical circuit essential for vocal learning. J Neurosci. 2009;29(15):4782–4793. doi: 10.1523/JNEUROSCI.4903-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deniau JM, Chevalier G. Disinhibition as a basic process in the expression of striatal functions. II. The striato-nigral influence on thalamocortical cells of the ventromedial thalamic nucleus. Brain Res. 1985;334(2):227–233. doi: 10.1016/0006-8993(85)90214-8. [DOI] [PubMed] [Google Scholar]
- 36.Kao MH, Wright BD, Doupe AJ. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J Neurosci. 2008;28(49):13232–13247. doi: 10.1523/JNEUROSCI.2250-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hessler NA, Doupe AJ. Singing-related neural activity in a dorsal forebrain-basal ganglia circuit of adult zebra finches. J Neurosci. 1999;19(23):10461–10481. doi: 10.1523/JNEUROSCI.19-23-10461.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leonardo A. Experimental test of the birdsong error-correction model. Proc Natl Acad Sci USA. 2004;101(48):16935–16940. doi: 10.1073/pnas.0407870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hessler NA, Doupe AJ. Social context modulates singing-related neural activity in the songbird forebrain. Nat Neurosci. 1999;2(3):209–211. doi: 10.1038/6306. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg JH, Fee MS. A cortical motor nucleus drives the basal ganglia-recipient thalamus in singing birds. Nat Neurosci. 2012;15(4):620–627. doi: 10.1038/nn.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vates GE, Vicario DS, Nottebohm F. Reafferent thalamo- “cortical” loops in the song system of oscine songbirds. J Comp Neurol. 1997;380(2):275–290. [PubMed] [Google Scholar]
- 42.Wild JM. Descending projections of the songbird nucleus robustus archistriatalis. J Comp Neurol. 1993;338(2):225–241. doi: 10.1002/cne.903380207. [DOI] [PubMed] [Google Scholar]
- 43.Luo M, Perkel DJ. Intrinsic and synaptic properties of neurons in an avian thalamic nucleus during song learning. J Neurophysiol. 2002;88(4):1903–1914. doi: 10.1152/jn.2002.88.4.1903. [DOI] [PubMed] [Google Scholar]
- 44.Goldberg JH, Fee MS. Vocal babbling in songbirds requires the basal ganglia-recipient motor thalamus but not the basal ganglia. J Neurophysiol. 2011;105(6):2729–2739. doi: 10.1152/jn.00823.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ölveczky BP, Otchy TM, Goldberg JH, Aronov D, Fee MS. Changes in the neural control of a complex motor sequence during learning. J Neurophysiol. 2011;106(1):386–397. doi: 10.1152/jn.00018.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamaguchi K, Mooney R. Recurrent interactions between the input and output of a songbird cortico-basal ganglia pathway are implicated in vocal sequence variability. J Neurosci. 2012;32(34):11671–11687. doi: 10.1523/JNEUROSCI.1666-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mooney R, Konishi M. Two distinct inputs to an avian song nucleus activate different glutamate receptor subtypes on individual neurons. Proc Natl Acad Sci USA. 1991;88(10):4075–4079. doi: 10.1073/pnas.88.10.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Charlesworth JD, Warren TL, Brainard MS. Covert skill learning in a cortical-basal ganglia circuit. Nature. 2012;486(7402):251–255. doi: 10.1038/nature11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warren TL, Tumer EC, Charlesworth JD, Brainard MS. Mechanisms and time course of vocal learning and consolidation in the adult songbird. J Neurophysiol. 2011;106(4):1806–1821. doi: 10.1152/jn.00311.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sizemore M, Perkel DJ. Premotor synaptic plasticity limited to the critical period for song learning. Proc Natl Acad Sci USA. 2011;108(42):17492–17497. doi: 10.1073/pnas.1104255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stark LL, Perkel DJ. Two-stage, input-specific synaptic maturation in a nucleus essential for vocal production in the zebra finch. J Neurosci. 1999;19(20):9107–9116. doi: 10.1523/JNEUROSCI.19-20-09107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leventhal DK, et al. Basal ganglia beta oscillations accompany cue utilization. Neuron. 2012;73(3):523–536. doi: 10.1016/j.neuron.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howe MW, Atallah HE, McCool A, Gibson DJ, Graybiel AM. Habit learning is associated with major shifts in frequencies of oscillatory activity and synchronized spike firing in striatum. Proc Natl Acad Sci USA. 2011;108(40):16801–16806. doi: 10.1073/pnas.1113158108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433(7028):873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- 55.Brasted PJ, Wise SP. Comparison of learning-related neuronal activity in the dorsal premotor cortex and striatum. Eur J Neurosci. 2004;19(3):721–740. doi: 10.1111/j.0953-816x.2003.03181.x. [DOI] [PubMed] [Google Scholar]
- 56.Williams ZM, Eskandar EN. Selective enhancement of associative learning by microstimulation of the anterior caudate. Nat Neurosci. 2006;9(4):562–568. doi: 10.1038/nn1662. [DOI] [PubMed] [Google Scholar]
- 57.Rosin B, et al. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron. 2011;72(2):370–384. doi: 10.1016/j.neuron.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 58.Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol. 1965;22(7):770–783. [PubMed] [Google Scholar]
- 59.Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim Behav. 2000;59(6):1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- 60.Soha JA, Shimizu T, Doupe AJ. Development of the catecholaminergic innervation of the song system of the male zebra finch. J Neurobiol. 1996;29(4):473–489. doi: 10.1002/(SICI)1097-4695(199604)29:4<473::AID-NEU5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 61.Bottjer SW. The distribution of tyrosine hydroxylase immunoreactivity in the brains of male and female zebra finches. J Neurobiol. 1993;24(1):51–69. doi: 10.1002/neu.480240105. [DOI] [PubMed] [Google Scholar]
- 62.Fuster JM. The Prefrontal Cortex. 2008. (Academic Press, London), 4th Ed. [Google Scholar]
- 63.Vu ET, Mazurek ME, Kuo YC. Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci. 1994;14(11 Pt 2):6924–6934. doi: 10.1523/JNEUROSCI.14-11-06924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jarvis ED, et al. Avian Brain Nomenclature Consortium Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci. 2005;6(2):151–159. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.