Abstract
Oxidative stress is a widely recognized cause of cell death associated with neurodegeneration, inflammation, and aging. Tyrosine nitration in these conditions has been reported extensively, but whether tyrosine nitration is a marker or plays a role in the cell-death processes was unknown. Here, we show that nitration of a single tyrosine residue on a small proportion of 90-kDa heat-shock protein (Hsp90), is sufficient to induce motor neuron death by the P2X7 receptor-dependent activation of the Fas pathway. Nitrotyrosine at position 33 or 56 stimulates a toxic gain of function that turns Hsp90 into a toxic protein. Using an antibody that recognizes the nitrated Hsp90, we found immunoreactivity in motor neurons of patients with amyotrophic lateral sclerosis, in an animal model of amyotrophic lateral sclerosis, and after experimental spinal cord injury. Our findings reveal that cell death can be triggered by nitration of a single protein and highlight nitrated Hsp90 as a potential target for the development of effective therapies for a large number of pathologies.
Keywords: apoptosis, peroxynitrite, PC12 cells
It is generally accepted that biomarkers of oxidative damage such as nitrated tyrosine residues accumulate even during the early stages of neurodegeneration, inflammation, and aging and that scavenging of reactive oxygen and nitrogen species can ameliorate injury in many pathological conditions (1). Tyrosine nitration is a widely used marker for the formation of peroxynitrite and other reactive nitrogen species. We have shown previously that the endogenous production of peroxynitrite induces motor neuron apoptosis, either after trophic factor deprivation or when the cells express the amyotrophic lateral sclerosis (ALS) mutant superoxide dismutase (SOD) in the presence of nitric oxide (2–4). Furthermore, inhibition of tyrosine nitration prevented this peroxynitrite-induced apoptosis in motor neurons and PC12 cells (4, 5), suggesting that peroxynitrite may nitrate specific targets responsible for activating specific signaling pathways upstream of caspase activation (3, 6). However, the multiplicity of potential oxidative reactions including tyrosine nitration thus far has precluded linking specific molecular targets of nitration with causal mechanisms of cell death (7).
The 90-kDa heat-shock protein (Hsp90) is a member of a highly conserved family of chaperones present in all eukaryotes (8). This chaperone is involved in both the regulation of cellular homeostasis and the stress response (9–11). As a chaperone, Hsp90 participates in folding and stabilizing client proteins, induces conformational changes that can lead to activation or inactivation of its clients, and prevents the unspecific aggregation of nonnative proteins (9, 10, 12). Hsp90 has more than 200 client proteins, many of which are involved in the regulation of cell survival and death (13, 14). Vertebrates express two cytosolic isoforms, Hsp90α and -β, that share 86% homology (15). Hsp90β is constitutively expressed at high levels and generally is the more abundant isoform, whereas Hsp90α is inducible under stress conditions and is expressed at high levels in many cancers (16, 17). Because of Hsp90’s pleiotropic functions (14), the simultaneous genetic deletion of both isoforms is lethal to eukaryotic cells (8, 18). Despite the high degree of homology shared by the two isoforms, the inactivation of Hsp90β cannot be compensated fully by Hsp90α and is fatal during embryonic development in the mouse (19).
Here, we report that Hsp90 can be converted from a prosurvival protein into a potent mediator of motor neuron death after nitration of a single tyrosine residue. Moreover, the toxic form of nitrated Hsp90 is present in vivo in pathological conditions such as ALS and spinal cord injury. The data presented here provide conclusive evidence that selective and limited tyrosine nitration plays a causal role in the induction of cell death.
Results
Peroxynitrite-Treated Hsp90 Induces Cell Death.
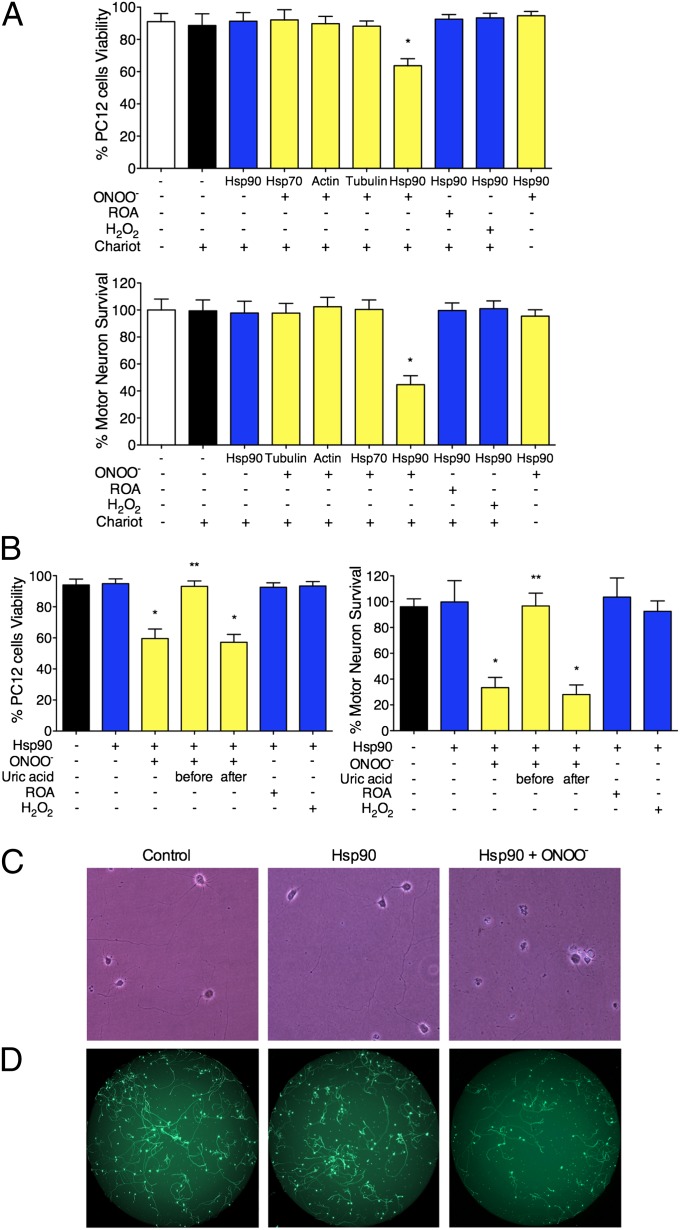
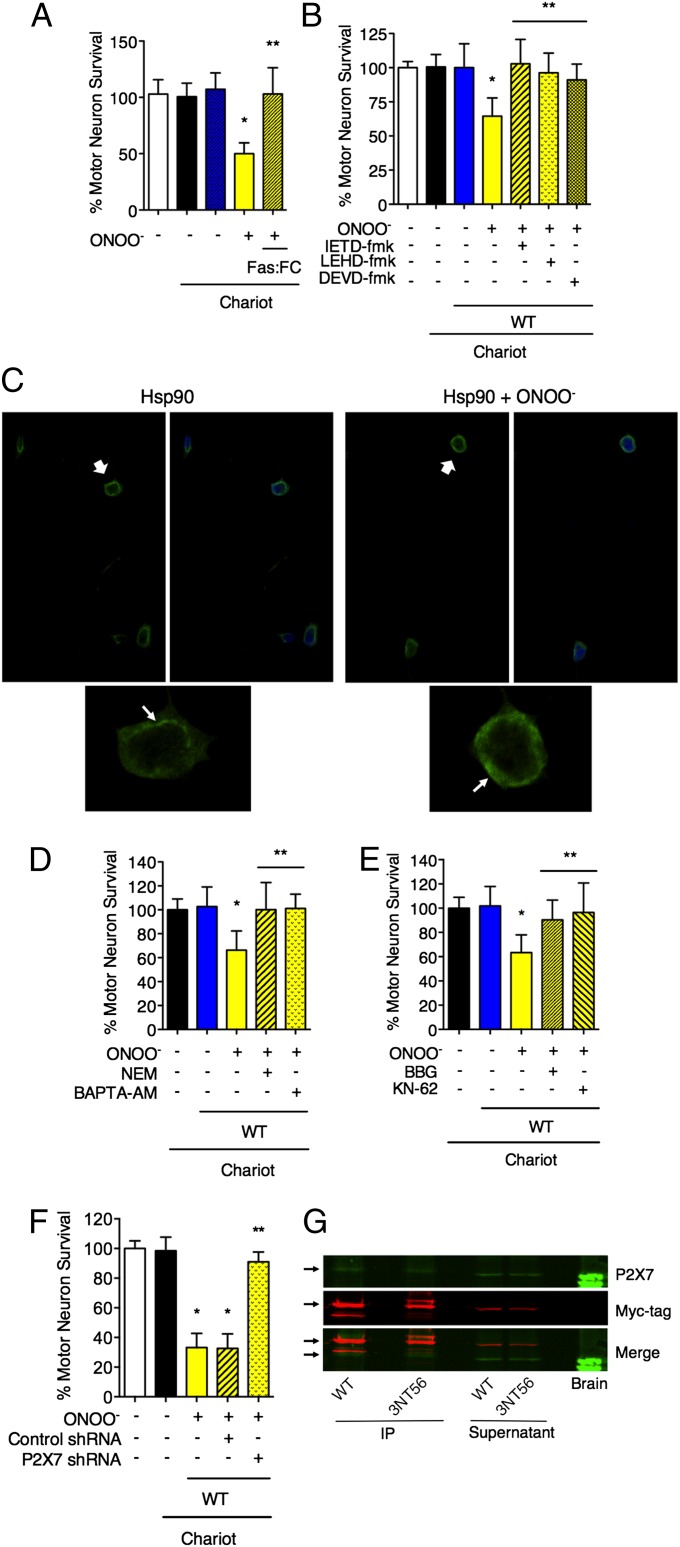
We have shown previously that tyrosine-containing peptides prevent peroxynitrite-induced apoptosis in motor neurons and PC12 cells (4, 5), suggesting that tyrosine nitration activates cell-signaling pathways that lead to cell death. To identify potential targets of peroxynitrite able to stimulate cell death upon tyrosine nitration, we first characterized the putative nitrated proteins present in homogenates from PC12 cells after treatment with peroxynitrite by mass spectrometric analysis (Table S1). Four of the identified proteins with the highest scores were actin, tubulin, 70-kDa heat-shock protein (Hsp70), and Hsp90. We delivered these peroxynitrite-treated proteins intracellularly using the membrane-permeating agent Chariot (Active Motif) into PC12 cells and motor neurons before assaying for cell survival. Peroxynitrite-treated Hsp70, actin, and tubulin were nontoxic for motor neurons and PC12 cells (Fig. 1A). In contrast, peroxynitrite-treated Hsp90 induced death in ∼40% of PC12 cells and 60% of motor neurons (Fig. 1 A–D). Nitrated Hsp90 was toxic to the cells only when delivered intracellularly, as evidenced by its lack of effect on cell survival in the absence of Chariot (Fig. 1A). Similarly, no toxicity was observed when Hsp90 was treated with hydrogen peroxide or decomposed peroxynitrite (Fig. 1A) or with peroxynitrite in the presence of uric acid (Fig. 1B). Because uric acid does not react with peroxynitrite directly but efficiently scavenges the nitrating products of peroxynitrite decomposition (20, 21), these results suggest that nitration of Hsp90 is necessary to induce cell death. Equivalent results were obtained by counting all neurons with neurites longer than three soma diameters (Fig. 1C) or by using high-throughput image capture and analysis (Fig. 1D) (4, 22).
Fig. 1.
Peroxynitrite-treated Hsp90 induces cell death. (A) Actin, tubulin, Hsp70, and Hsp90 (1 mg/mL) were treated with 0.5 mM peroxynitrite (ONOO−), decomposed peroxynitrite (reverse order addition, ROA), or 0.5 mM H2O2 and were delivered into PC12 cells and motor neurons using the permeating agent Chariot. PC12 cell viability was determined 24 h later using fluorescein diacetate/propidium iodide, and motor neuron survival was established by counting neurons with neurites longer than four soma diameters after 24 h in culture. (B) Uric acid (0.5 mM) was added to Hsp90 5 min before or after the addition of peroxynitrite, and the treated protein was added to PC12 cells or motor neurons in the presence of Chariot. Columns represent the mean ± SD of 4–10 experiments performed in quadruplicate. Statistical analyses were performed by ANOVA followed by Bonferroni multiple comparison test. *P < 0.05 versus Chariot, **P < 0.05 versus ONOO−. (C) Representative images of motor neurons 24 h after the intracellular delivery of either Hsp90 or peroxynitrite-treated Hsp90 (Hsp90+ONOO−) and in the presence of Chariot alone (control). The number of motor neurons per well was counted (A and B) as described in Materials and Methods. (D) Representative images of motor neurons 24 h after the intracellular delivery of either Hsp90 or peroxynitrite-treated Hsp90 (Hsp90+ONOO−) and in the presence of Chariot alone (control). The motor neurons were stained with calcein-AM, and the images were captured with a Runner HD (Trophos). The number of motor neurons per well in each condition was quantified using Tina software, as described in Materials and Methods. The results were similar to the quantitation showed in A.
Incubation of Hsp90 with Peroxynitrite Activates a Toxic Gain of Function.
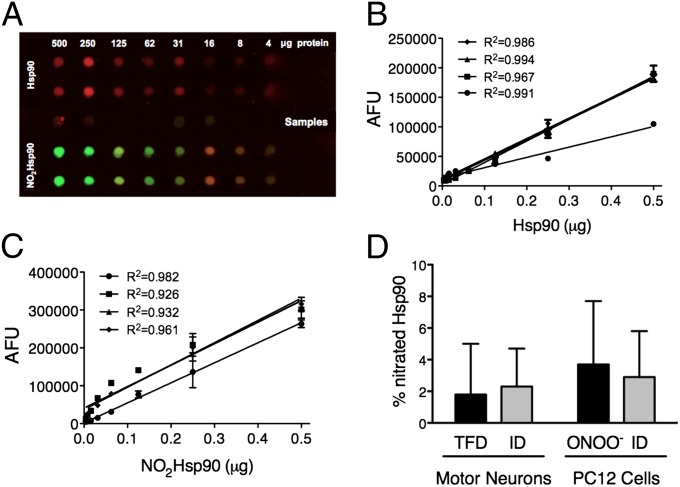
Hsp90 constitutes 1–2% of total cytoplasmic protein (18). The previous results strongly suggest that tyrosine nitration activates a gain of function in Hsp90, because the cells still have the endogenous unmodified chaperone after the delivery of the nitrated protein. The proportion of nitrated Hsp90 to the total amount of intracellular Hsp90 in motor neurons and PC12 cells was determined by quantitative dot blot using a monoclonal antibody against nitrated Hsp90 (5) (Fig. 2 A–C). Approximately 4% of Hsp90 was nitrated in motor neurons subjected to trophic factor deprivation. In contrast, none was detected in control cells (Fig. 2D). The proportion of peroxynitrite-treated Hsp90 released using Chariot in Fig. 1 constituted only 2–3% of the endogenous cellular Hsp90 (Fig. 2D). In PC12 cells treated with peroxynitrite, ∼4% of Hsp90 was nitrated (Fig. 2D). Intracellular release of peroxynitrite-treated Hsp90 in these proportions had no effect on proteasome activity (Fig. S1). Because cell death was induced when only a small percentage of total Hsp90 was peroxynitrite-modified Hsp90, we investigated whether this toxic gain of function could result specifically from tyrosine nitration.
Fig. 2.
Only a small percentage of total Hsp90 is endogenously nitrated. (A) The proportion of nitrated Hsp90 with respect to total Hsp90 in PC12 cells and motor neurons was determined by quantitative dot blot. For the standard curves, peroxynitrite-treated Hsp90 (NO2Hsp90, green) or untreated Hsp90 (red) purified from rat liver was loaded at the indicated amounts (upper and lower sections of the dot blot). Samples were collected from PC12 cells and motor neurons upon treatment as described in D and were loaded on the dot blot (middle section). Infrared detection allowed the merging of the two signals (yellow) (LiCor Biosciences). (B and C) Calibration curves were blotted for Hsp90 (B) and NO2Hsp90 (C) infrared signals. The graphs show the standard curves for Hsp90 and NO2Hsp90 from four independent experiments (R2 > 0.92 for all calibration curves). The intensity of the signal (expressed as arbitrary fluorescence units, AFU) was quantified as the integrated intensity of fluorescence for each dot in the dot blot using the Odyssey software. (D) The percentage of NO2Hsp90 versus total cytosolic Hsp90 was determined in trophic factor-deprived motor neurons (TFD), peroxynitrite-treated PC12 cells (ONOO−), and motor neurons and PC12 cells 2 h after the intracellular delivery of nitrated Hsp90 (ID) by interpolating the infrared signal of the samples on the corresponding standard curves. Columns represent the mean ± SD (n = 4, in triplicate).
Five Tyrosine Residues on Hsp90 Are Targets for Nitration.
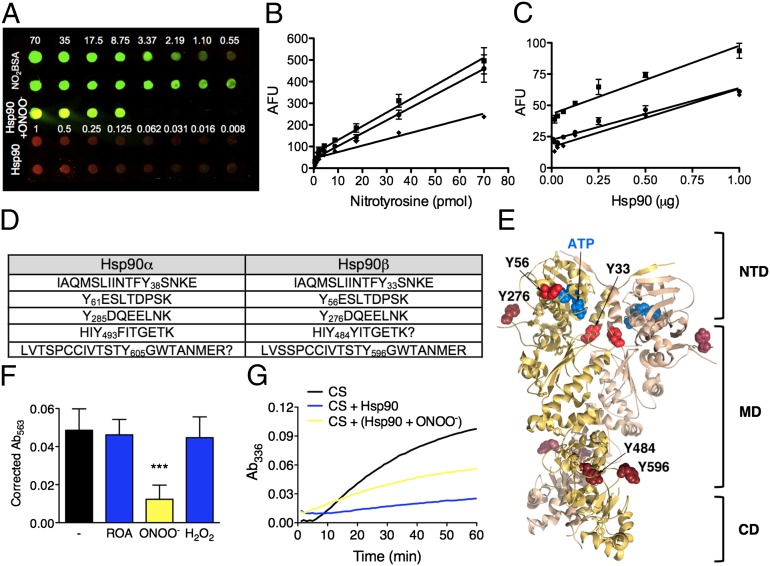
Both isoforms of Hsp90 contain 24 tyrosine residues. By quantitative dot blot, we determined that only five of these residues were prone to nitration on the chaperone (Fig. 3 A–C). Mass spectrometry revealed the nitrated tyrosines to be 38, 61, 285, 493, and 605 in Hsp90α and 33, 56, 276, 484, and 596 in Hsp90β (Fig. 3D). The two first tyrosine residues were located in the amino-terminal domain of Hsp90, tyrosine 276 and 484 were located in the middle domain, and tyrosine 596 was located in the carboxy-terminal domain (Fig. 3E).
Fig. 3.
Tyrosine nitration is necessary and sufficient for the conversion of Hsp90 to a toxic protein. (A) The number of nitrated tyrosine residues on Hsp90 was assayed by quantitative dot blot. Nitrated BSA (NO2BSA) was used as the standard for nitrotyrosine, and Hsp90 purified from rat liver was the standard for Hsp90. NO2BSA and Hsp90 were loaded at the indicated amounts. Purified Hsp90 was treated with peroxynitrite and loaded at different amounts in the linear range of detection (middle of the dot blot, Hsp90+ONOO−). The dot blot was probed with antibodies against Hsp90 (in red) and nitrotyrosine (in green). (B and C) The standard curve regressions for nitrotyrosine (B) and Hsp90 (C) were performed in duplicate and were repeated three times (R2 > 0.91 for all calibration curves). The molar ratio of nitrotyrosine to Hsp90 was 5.3 ± 0.5 nitrated residues per Hsp90 molecule. (D) Table showing the positions of the nitrated tyrosine residues on peroxynitrite-treated Hsp90 as determined by mass spectrometry analysis. (E) Model of the crystal structure of human Hsp90β [based on the yeast Hsp82 structure PDB 2CG9 (24)] showing the location of the tyrosine residues prone to nitration (red) including the two tyrosine residues located in the amino terminal domain (bright red). CD, carboxy-terminal domain; MD, middle domain; NTD, amino-terminal domain. (F) Treatment with peroxynitrite inhibits the intrinsic ATPase activity of Hsp90. Geldanamycin (5 µM), a specific inhibitor of Hsp90 ATPase activity, was used to verify Hsp90 ATP hydrolysis. Columns show the mean ± SD (n = 5). ***P < 0.05 versus ROA. (G) Treatment with peroxynitrite reduces 50% of Hsp90 activity to prevent heat-induced aggregation of citrate synthase (CS) at 43 °C.
Functionally, the binding of ATP to the amino-terminal domain of Hsp90 regulates a cycle of conformational changes responsible for the chaperone activity (23). Interestingly, the two tyrosine residues located in the amino-terminal domain, Tyr38 and Tyr61 in Hsp90α and Tyr33 and Tyr56 in Hsp90β, are required for the intrinsic ATPase activity of the chaperone. Tyr61 and Tyr56 are located in the ATPase-active site cleft (Fig. 3E) (24), whereas Tyr38 and Tyr33 are necessary to stabilize the close conformation of the chaperone needed to stimulate ATP hydrolysis (25).
Intrinsic ATPase Activity of Hsp90 Is Inhibited After Nitration.
The localization and structural function of these tyrosine residues suggest that nitration should inhibit Hsp90 intrinsic ATPase activity. Hsp90 ATPase activity was determined using a coupled enzyme system that provides the high sensitivity needed to measure the inorganic phosphate produced by the ATP hydrolysis catalyzed by Hsp90 (26). Treatment with peroxynitrite inhibited more than 80% of geldanamycin-inhabitable Hsp90 intrinsic ATPase activity (Fig. 3F).
Two of the other three tyrosine residues prone to nitration are located in the middle domain, which also is the intermediate client-binding domain. The effect of nitration on Hsp90’s ability to interact with other proteins and prevent unfolding was determined by the citrate synthase inactivation and aggregation assay (27). Peroxynitrite treatment reduced Hsp90 capacity to prevent heat-induced unfolding of citrate synthase by only 50% (Fig. 3G), suggesting that nitrated Hsp90 still retains the ability to interact with other proteins.
Tyrosine Nitration Is Necessary for the Conversion of Hsp90 into a Toxic Protein.
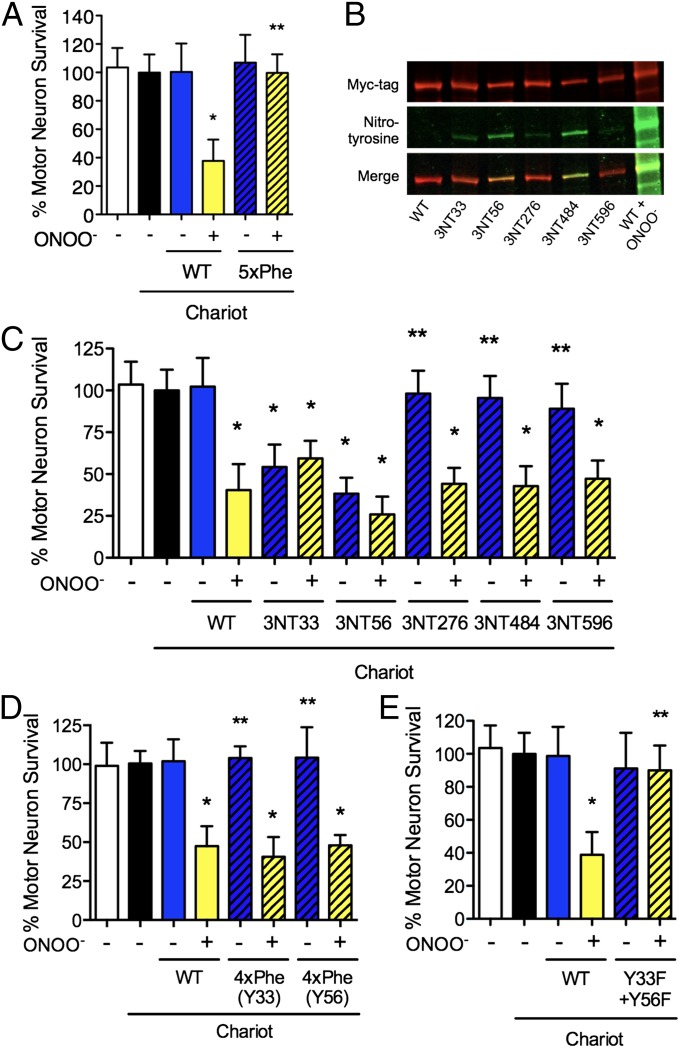
In addition to tyrosine residues, peroxynitrite can cause oxidative modifications to other amino acids such as cysteine, methionine, histidine, and tryptophan (28–31) and also can lead to lysine formylation (31). To investigate whether tyrosine nitration is required for the induction of Hsp90 toxicity or other oxidative modifications are responsible for the conversion of the chaperone into a toxic protein, the five tyrosine residues prone to nitration were replaced by phenylalanine (5xPhe-Hsp90). Phenylalanine is the most conservative substitution for tyrosine and also is resistant to nitration (29). The replacement was made in the highly conserved sequence of human Hsp90β (Fig. S2A), the more abundant isoform in normal cells (16, 17). Recombinant wild-type Hsp90β was nontoxic to motor neurons when delivered with Chariot at the concentrations previously described for purified Hsp90 but became toxic when treated with peroxynitrite (Fig. 4A). In contrast, 5xPhe-Hsp90 remained nontoxic to motor neurons after incubation with peroxynitrite (Fig. 4A), demonstrating that tyrosine nitration is necessary for peroxynitrite-treated Hsp90 toxic gain of function.
Fig. 4.
Nitration of a single tyrosine residue of Hsp90 is sufficient to induce cell death. (A) The replacement of the tyrosine residues prone to nitration on Hsp90 (WT) by phenylalanine (5xPhe) prevented peroxynitrite-treated Hsp90 toxicity. (B) The presence of nitrotyrosine (3NT) in the recombinant proteins carrying a single nitrated residue was assayed by Western blot probed with antibodies against myc-tag (green) and nitrotyrosine (red). The merged image is shown in yellow. (C) Assay for the toxicity of Hsp90 with nitrotyrosine at position 33, 56, 276, 484, or 596 with (yellow bars) or without (blue bars) peroxynitrite treatment. Nitrotyrosine at positions 33 or 56 was sufficient to induce motor neuron death. (D and E) The relevance of tyrosine nitration was confirmed by directed mutagenesis of the relevant residues. Peroxynitrite-treated Hsp90 remained toxic with tyrosine only at position 33 or 56 and the other four residues replaced by phenylalanine [4xPhe(Y33) and 4xPhe(Y56)] (D), whereas replacement of residues 33 and 56 of Hsp90β by phenylalanine (Y33F + Y56F) prevented toxicity induced by peroxynitrite treatment (E). *P < 0.05 versus WT, **P < 0.05 versus WT + ONOO− by ANOVA followed by Bonferroni multiple comparison test.
Nitration of One Tyrosine Residue on the Amino-Terminal Domain of Hsp90 Is Sufficient for the Toxic Gain of Function.
The previous results reveal that nitration of Hsp90 is necessary for the toxic activity of the chaperone. However, oxidation of other residues on Hsp90 also may play a role in the toxic gain of function. To determine the relevance of tyrosine nitration in the conversion of Hsp90 into a toxic protein, we performed recombinant expression using site-specific unnatural amino acid replacement to encode nitrotyrosine genetically in the positions prone to nitration (32, 33). Five different Hsp90β proteins were produced containing a single nitrotyrosine at position 33, 56, 276, 484, or 596 as the sole oxidative modification on the protein. The incorporation of nitrotyrosine in these positions of Hsp90 was verified by Western blot (Fig. 4B) and mass spectrometry (Fig. S2 B–G). The intracellular delivery of the recombinant protein with nitrotyrosine in either position 33 or 56 induced motor neuron death, reproducing the effects of peroxynitrite on Hsp90 (Fig. 4C). In contrast, the release of Hsp90 with nitrotyrosine in position 276, 484, or 596 had no effect on motor neuron survival, but these proteins became toxic after incubation with peroxynitrite (Fig. 4C).
The role of tyrosine 33 and 56 on the toxic gain of function was confirmed by intracellular delivery of Hsp90 with tyrosine at positions 33 or 56 and phenylalanine in the other four positions. Both proteins became toxic to motor neurons after treatment with peroxynitrite (Fig. 4E). Conversely, peroxynitrite-treated Hsp90 in which tyrosine 33 and 56 had been replaced by phenylalanine had no effect on motor neuron survival (Fig. 4E). These results demonstrate that nitration of tyrosine 33 or 56 in the amino terminal domain of Hsp90 is both necessary and sufficient to induce the toxic gain of function responsible for motor neuron death.
Nitrated Hsp90 Induces Activation of the Fas Pathway in Motor Neurons.
Trophic factor deprivation-induced motor neuron apoptosis requires activation of the Fas death receptor (34, 35). The activation of Fas leads to the activation of two parallel pathways. One involves the activation of Fas-associated protein with death domain FADD and caspase 8, followed by release of cytochrome c from the mitochondria. The other pathway has been shown to be exclusive for motor neurons and involves activation of the Fas-associated protein, death-associated protein 6 (DAXX), which leads to the activation of apoptosis signal-regulating kinase 1 (Ask1) and p38 MAPK and the expression of the neuronal isoform of nitric oxide synthase (NOS), which in turn leads to production of nitric oxide and peroxynitrite (3, 34, 36, 37).
To investigate the role of the Fas pathway in the motor neuron death induced by the intracellular delivery of peroxynitrite-treated Hsp90, the cells were cultured for 24 h in the presence of the chimeric fusion protein Fas:Fc, which acts as a Fas ligand decoy competing with Fas. In the presence of Fas:Fc, motor neurons were completely protected from cell death induced by peroxynitrite-treated Hsp90 (Fig. 5A), suggesting that the Fas pathway is activated downstream of the toxic protein.
Fig. 5.
Nitrated Hsp90 induces cell death through the activation of the P2X7 receptor that leads to exposure of FasL on the cellular membrane and the activation of the Fas pathway. (A) Incubation with the FasL decoy Fas:FC (1 μg/mL) for 24 h completely protected motor neurons from nitrated Hsp90-induced cell death. (B) Motor neurons were incubated for 24 h with inhibitors of caspase 8 (IEDT-fmk; 10 μM), 9 (LEHD-fmk; 20 μM), and 3 (DEVD-fmk; 20 μM) after the intracellular delivery of peroxynitrite-treated Hsp90. *P < 0.05 versus WT, **P < 0.05 versus WT + ONOO− by ANOVA followed by Bonferroni multiple comparison test. (C) Motor neurons show mobilization of FasL to the plasma membrane upon treatment with peroxynitrite-treated Hsp90. The motor neurons were incubated for 16 h after the intracellular delivery of unmodified (Hsp90) or peroxynitrite-treated Hsp90 (Hsp90 + ONOO−). The cells then were stained for FasL (green). The cell nuclei are stained in blue. (Upper) The white arrows in indicate the cells that are shown magnified in the lower panels. (Lower) The white arrows indicate the cellular location of FasL signal. See also Fig. S3. (D) The motor neurons were cultured in the presence of NEM (2 μM) or the intracellular calcium chelator BAPTA-AM (5 μM) for 24 h after the intracellular delivery of peroxynitrite-treated Hsp90. The inhibition of the last steps of exocytosis by NEM and the chelation of intracellular calcium by BAPTA-AM completely protected motor neurons from peroxynitrite-treated Hsp90-induced cell death. (E and F) Both the inhibition of the purinergic receptor P2X7 by BBG (10 μM) and KN-62 (1 μM) (E) and the receptor knockdown by lentiviral particles expressing P2X7 shRNA (F) prevented motor neuron death induced by peroxynitrite-treated Hsp90. Motor neurons were transduced with lentiviral particles expressing P2X7 or control shRNA for 84 h before the intracellular delivery of nitrated Hsp90. *P < 0.05 versus Chariot, **P < 0.05 versus WT + ONOO− by ANOVA followed by Bonferroni multiple comparison test. (G) Nitrated Hsp90 coimmunoprecipitated with the P2X7 receptor. Homogenates from PC12 were incubated for 1 h with Hsp90β-myc (WT) or with Hsp90β-myc nitrated at position 56 (3NT56), and the recombinant proteins were immunoprecipitated with an anti-myc antibody before SDS/PAGE. The membranes then were blotted for P2X7 (green) and the myc-tag (red). Brain homogenate was used as a positive control for P2X7.
We next examined the role of the two pathways downstream of the Fas receptor, the DAXX and FADD components of the Fas pathway. After intracellular delivery of peroxynitrite-treated Hsp90, motor neurons were incubated with inhibitors of caspase 8, 9, and 3 (IETD-fmk, LEHD-fmk, and DEVD-fmk, respectively, from Sigma-Aldrich, St. Louis, MO), which are activated downstream of FADD. All the inhibitors completely prevented motor neuron death (Fig. 5B). Conversely, incubation of motor neurons with either the p38 inhibitor SB253580 (5 μM) or the NOS inhibitor l-NG-nitroarginine methyl ester (100 μM) after intracellular delivery of nitrated Hsp90 was not protective (Fig. S3 A and B). These results suggest that the DAXX component of the Fas pathway is not activated downstream of peroxynitrite-treated Hsp90.
Induction of Apoptosis by Nitrated Hsp90 Is Calcium Dependent and Involves Activation of the P2X7 Receptor.
Trophic factor deprivation in motor neurons causes inactivation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway with the subsequent dephosphorylation and translocation to the nucleus of the transcription factor Forkhead box O3a (FOXO3a), which induces the expression of Fas ligand (FasL), activating the Fas pathway (35). There were no differences in the expression of Fas receptor or FasL in motor neurons up to 16 h after intracellular delivery of either the unmodified Hsp90 or the peroxynitrite-treated chaperone (Fig. S3 C and D).
Several mechanisms control FasL expression in the cell membrane. Mobilization of lysosomes containing FasL from the Golgi network to the membrane is one such mechanism (38, 39). To investigate the possible role of the mobilization of FasL to the plasma membrane in peroxynitrite-treated Hsp90-dependent activation of the Fas pathway, motor neurons were stained with antibodies to FasL. Peroxynitrite-treated Hsp90 induced the translocation of FasL immunofluorescence from a perinuclear vesicular distribution to the plasma membrane (Fig. 5C). Further evidence for this mechanism was obtained by using N-ethylmaleimide (NEM), the inhibitor of the NEM-sensitive factor, to prevent the last steps of exocytosis (40, 41). The incubation with NEM completely prevented motor neuron death induced by peroxynitrite-treated Hsp90 (Fig. 5D). Membrane fusion during exocytosis is calcium dependent (42). The intracellular calcium chelator 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM) decreased the mobilization of FasL to the cell membrane (Fig. S3E) and prevented the death of motor neurons after the intracellular delivery of peroxynitrite-treated Hsp90 (Fig. 5D).
In neurons and in the immune system, the ATP-gated ion channel P2X7 is responsible for calcium influx upon activation and can initiate the early steps of exocytosis (43, 44). The P2X7 receptor is present in motor neurons (Fig. S3F), and the cells incubated with the P2X7 inhibitors Brilliant Blue G (BBG) and KN-62 were protected from apoptosis induced by peroxynitrite-treated Hsp90 (Fig. 5E). Similar results were obtained by knocking down the expression of the P2X7 receptor in motor neurons with shRNA before the intracellular delivery of the peroxynitrite-treated chaperone (Fig. 5F and Fig. S3 G and H). There were no changes in P2X7 expression upon delivery of peroxynitrite-treated Hsp90 (Fig. S3I).
Together, these results suggest that peroxynitrite-treated Hsp90 stimulates the P2X7 receptor to induce an influx of calcium that in turn mobilizes FasL-containing intracellular vesicles to transfer FasL to the cellular membrane and thereby activates cell death. Immunoprecipitation studies of P2X7 with nitrated Hsp90 further support the association of P2X7 receptors in nitrated Hsp90-induced motor neuron apoptosis (Fig. 5G).
Nitrated Hsp90 Is Found in Vivo in Pathological Conditions.
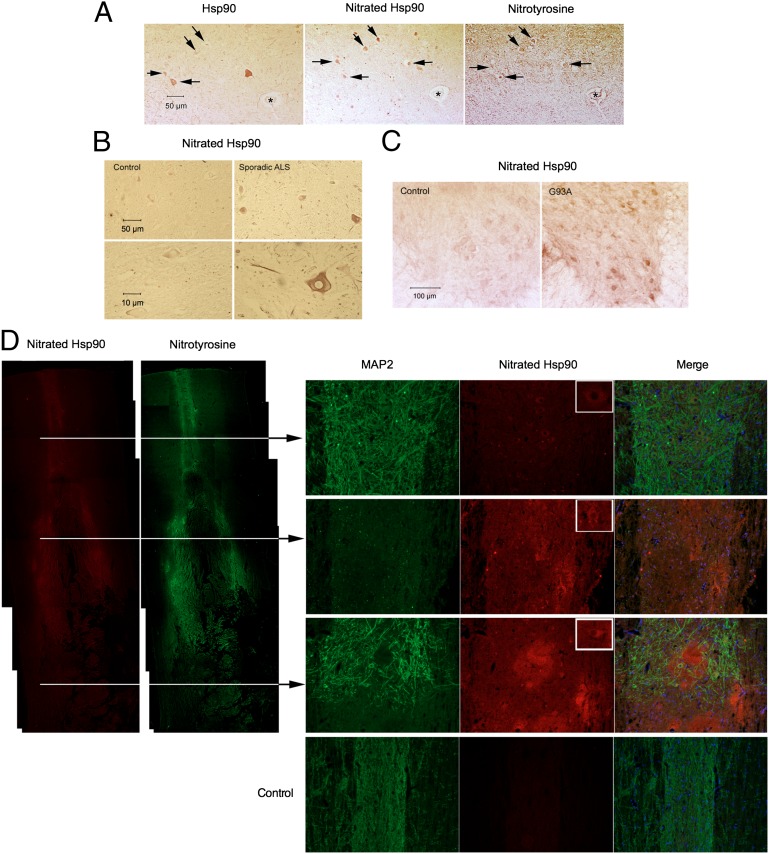
We sought to characterize the relevance of nitrated Hsp90 in vivo. Protein nitration in motor neurons correlates with the progressive degeneration in ALS in human patients as well as in SOD-transgenic animals, whether measured by immunohistology (45–49) or by mass spectrometry (50, 51). The presence of nitrated Hsp90 in human and animal pathology was investigated using a monoclonal antibody we developed against nitrated Hsp90 (5). This antibody specifically recognizes nitrotyrosine-56 and thus identifies one of the toxic forms of nitrated Hsp90 (Fig. S4 A–C). Immunohistology with this antibody revealed intensely stained motor neurons for nitrated Hsp90 in spinal cords from sporadic ALS patients (Fig. 6 A and B) and disease-affected ALS SOD-mutant mice (Fig. 6C).
Fig. 6.
The toxic form of nitrated Hsp90 is present in vivo in neuropathological conditions. (A) Hsp90, Hsp90 nitrated at tyrosine-56 (nitrated Hsp90), and nitrotyrosine immunoreactivity in adjacent sections (7 µm thick) of spinal cord from a human ALS patient. Black arrows show the immunoreactivity for Hsp90, nitrated Hsp90, and nitrotyrosine in motor neurons. The star indicates a blood vessel. (B) Intense immunoreactivity for nitrated Hsp90 is present in the spinal cord of sporadic ALS patient but not in a control patient. The lower panels at higher magnification show a motor neuron stained for nitrated Hsp90 in the ALS patient. (C) Intense nitrated Hsp90 immunoreactivity was found in the spinal cord of fully symptomatic ALS mutant G93A mice (110 d old) but not in age-matched control mice. Remaining large neurons in the anterior horn of G93A spinal cord sections were densely stained with the antibody recognizing nitrated Hsp90, compared with the control spinal cord sections. (D) Nitrated Hsp90 is present in the spinal cord after spinal cord injury. (Left) Immunoreactivity for nitrotyrosine and nitrated Hsp90 is detected in the spinal cord as early as 6 h after spinal cord contusion injury in rat. (Right) Nitrated Hsp90 is still present at the sections indicated in the figure 24 h after the spinal cord injury. MAP2 was used as a neuronal marker. The cell nuclei are stained in blue. As indicated by the arrows, the top section is rostral to the injury, the middle section corresponds to the place of contusion, and the bottom section is caudal from the injury site. Nitrated Hsp90 is present in all sections, is localized mostly in neuronal somas (upper right corners of nitrated Hsp90 panels), and is absent in uninjured control animals (Bottom Right).
Spinal cord trauma is another condition in which the presence of nitrotyrosine is well documented (52–54). Nitrated Hsp90 immunoreactivity also was found in the spinal cords of rats 6 and 24 h following experimental spinal cord contusion injury, but the staining was absent in uninjured animals (Fig. 6D). These results show that nitrated Hsp90 is present in vivo both in the cell type more affected in conditions of chronic degeneration, such as ALS, and after acute damage of the nervous tissue.
Discussion
Many types of stress including trophic factor deprivation or exposure to nitric oxide when the cells are expressing mutant SOD, result in motor neurons producing peroxynitrite before undergoing apoptosis (2, 3). Peroxynitrite decomposes to form the strongest oxidants known to be produced in biology and is well documented to oxidize and nitrate many different cellular targets (1). Tyrosine nitration has been reported in a discrete number of proteins and leads to functional inactivation in a limited number of specific residues (1, 55). The site specificity for tyrosine nitration results from differences in the ionization and solvent exposure of the tyrosine residues as well as surrounding neighbors that stabilize the tyrosyl radical intermediate (55, 56). Although the intracellular concentration of a protein and the content of tyrosine are not predictors of the level of nitration (55, 57–59), certain abundant proteins in the cells, such as actin, tubulin, neurofilament L, myosin, and manganese-SOD, are preferred targets for peroxynitrite (1). The nitration of a small fraction of Hsp90 may be explained by its abundance, which accounts for 1–2% of total cytosolic protein (18). We found that nitrated Hsp90 was among the proteins nitrated by peroxynitrite in PC12 cells and in motor neurons deprived of trophic factors (5). Nitrated Hsp90 also was one of ∼20 nitrated proteins identified in a proteomic survey of spinal cords of both ALS SOD-mutant mice and ALS patients (51). Here we show that only a small fraction (less than 5%) of the total chaperone was nitrated by exogenous peroxynitrite in PC12 cells and by endogenous peroxynitrite in trophic factor-deprived motor neurons.
In most cases, inactivation of a small fraction of a protein might have little or no biological significance, unless tyrosine nitration could lead to the activation of a gain of function responsible for the induction of cell death. The challenge then is to distinguish nitrated proteins acting as causal drivers of pathology from collateral damage. Our results demonstrate that nitration of a specific protein can induce a gain of function that causes cell death. The intracellular delivery of a small amount of exogenous nitrated Hsp90 is sufficient to induce cell death even though the endogenous prosurvival Hsp90 is still fully active. These results strongly suggest that a toxic function is activated in Hsp90 upon tyrosine nitration and that this gain of function cannot be overcome by the unmodified Hsp90. Indeed, although complete genetic deficiency of Hsp90β is lethal during embryonic development in the mouse, the heterozygous animals appear normal despite a 50% decrease in Hsp90β levels (19).
Several tyrosines on Hsp90 are particularly prone to nitration. The replacement of two tyrosine residues near the ATP binding site by phenylalanine demonstrated that the nitration of these residues is necessary for Hsp90 to become toxic after treatment with peroxynitrite. However, other amino acids, such as cysteine and tryptophan, also are targets for oxidation and could participate in the activation of the toxic gain of function (29, 30). Indeed, nitrotryptophan is found in Hsp90α after treatment of PC12 cells with peroxynitrite (60). The use of unnatural amino acid mutagenesis to encode nitrotyrosine genetically (32) allowed the direct examination of the consequences of nitration at specific tyrosine residues on Hsp90 in the absence of any other oxidative modification. Using this approach, we demonstrated here that nitration of a single tyrosine residue on the amino-terminal domain of Hsp90, as the sole modification on the protein, is both sufficient and necessary to induce motor neuron death (Fig. 4). These results also validate the use of unnatural amino acid mutagenesis as a powerful tool for the identification of mediators of oxidative damage and make possible the screening new therapeutic agents directed to specifically modified targets found in pathology. Moreover, the toxic form of nitrated Hsp90 was present in motor neurons in pathological conditions such as ALS and spinal cord injury (Fig. 6), suggesting that the identification of specific inhibitors of nitrated Hsp90 may have therapeutic relevance.
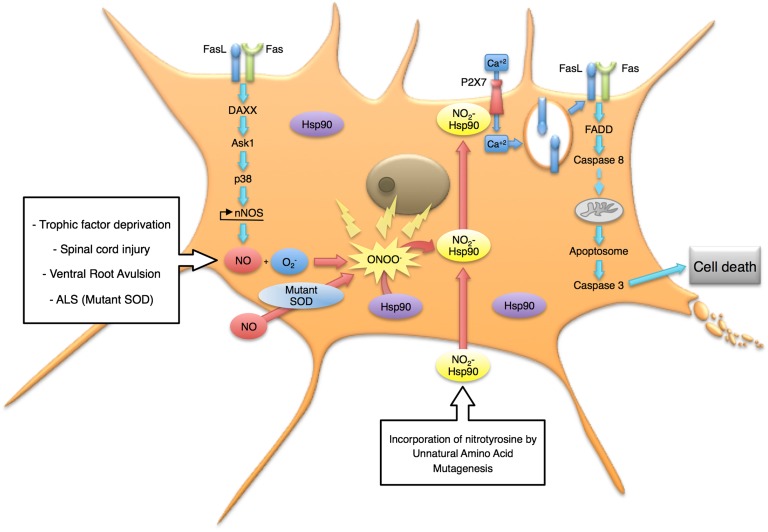
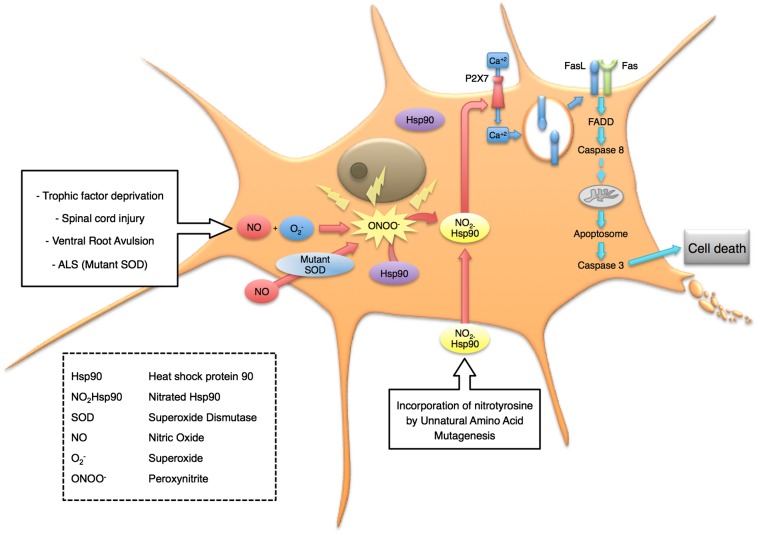
The identification of the death pathway activated by nitrated Hsp90 also may lead to new, effective therapeutic approaches. Our results suggest that nitrated Hsp90 induces apoptosis by activating the purine receptor P2X7, which triggers the calcium-dependent mobilization of FasL-containing vesicles to the plasma membrane followed by activation of the FADD component of the Fas pathway (Figs. 5 and 7). Tyrosine-phosphorylated Hsp90 is a P2X7 repressor (61, 62). Nitrated Hsp90 also interacts with P2X7. Deletion of the P2X7 receptor prevented the effect toxic effects of nitrated Hsp90 in motor neurons. In aggregate these results suggest that nitrated Hsp90 either may fail to repress P2X7 activity or may activate the receptor. Interestingly, the local injection and the systemic administration of P2X7 antagonists improve recovery in models of spinal cord injury (63, 64) where nitrated Hsp90 is present (Fig. 6).
Fig. 7.
Model for the induction of cell death by nitrated Hsp90 in motor neurons. Many types of stress, including trophic factor deprivation, exposure to nitric oxide when the cells also are expressing mutant SOD, and activation of the DAXX-mediated Fas pathway, result in motor neurons producing peroxynitrite before undergoing apoptosis. Nitration of one of two tyrosine residues on Hsp90 is sufficient to activate a toxic gain of function involving stimulation of the P2 P2X7 receptor. The influx of calcium through the receptor in turn mobilizes FasL to the plasma membrane and activates the FADD-mediated Fas pathway leading to cell death by apoptosis.
Incubation of motor neurons with Fas ligand activates two pathways dependent on DAXX and FADD, which enhance each other to trigger apoptosis (3). In contrast, nitrated Hsp90 activated only the FADD-caspase 8 pathway. Blocking of the DAXX-p38-nNOS pathway, which leads to peroxynitrite production, had no effect on nitrated Hsp90 induced motor neuron death (Fig. 5B), suggesting that nitrated Hsp90 is downstream of peroxynitrite production. These results further suggest that the activation of the DAXX component of the pathway is responsible for the endogenous nitration of Hsp90. This critical step may amplify the Fas signal to trigger cell death by activating the P2X7 receptor to increase further Fas stimulation and activation of caspases (Fig. 7).
Oxidative stress traditionally is defined as an imbalance between the cellular defense mechanisms and conditions capable of triggering oxidative reactions, resulting in damage to the molecular cellular components (65). Defining the role of oxidative stress in neurodegeneration, and particularly the role of nitrative stress, has been extremely difficult because of the multiplicity of potential targets that can be damaged by oxidation and nitration. As a consequence, oxidative stress generally is treated as a “black box,” which includes generic damage to multiple cellular components leading to inactivation of vital cellular functions, such as mitochondrial activity, and finally causing cell death (66, 67). In contrast to the concept of generalized oxidative damage, biological oxidants can attack certain targets with vastly greater efficiency, providing specificity to the modifications and thus leading to the concept of redox signaling (68, 69). The results presented here demonstrate that a widely reactive oxidant activates a gain of function that leads to cell death by introducing an apparently irreversible protein modification. The abundance of Hsp90 and its induction under stress conditions make the chaperone a potential sensor for peroxynitrite. In this scenario, nitration of Hsp90 over a certain threshold would mediate the activation of death pathways to remove highly damaged and subfunctional cells.
Materials and Methods
Peroxynitrite Synthesis.
Peroxynitrite was synthesized by rapidly mixing acidified hydrogen peroxide with sodium nitrite and quenching the reaction with sodium hydroxide as previously described (70). Residual hydrogen peroxide was eliminated with manganese dioxide, and the peroxynitrite concentration was determined spectrophotometrically using ɛ302 = 1,700 M−1⋅cm−1. The concentration of peroxynitrite was measured immediately before each experiment.
PC12 Cell Culture and Treatment with Peroxynitrite.
PC12 cells were cultured on collagen-coated dishes in RPMI medium (Gibco/Invitrogen), supplemented with 10% (vol/vol) horse serum, 5% (vol/vol) FetalClone II (HyClone), and antibiotics. The treatment with peroxynitrite was performed as previously described (71). Briefly, the cells were plated in the same medium at a density of 1.44 × 105 per 35-mm dish and incubated overnight. Following three washings with warm PBS, the cultures were incubated with peroxynitrite (0.5 mM) for 3 min in 1 mL of 50 mM phosphate buffer saline. The buffer was replaced by complete RPMI medium.
Immunoprecipitation and Mass Spectrometry Analysis of Proteins.
The immunoprecipitation of nitrated proteins in PC12 cell (2 x107) homogenates was performed using polyclonal nitrotyrosine antibodies linked to AminoLink gel (Pierce) immediately after incubation with 0.5 mM peroxynitrite as previously described (5). Briefly, after separation of the proteins by SDS/PAGE [12% (vol/vol)], the proteins were transferred to nitrocellulose membranes for Western blot analysis or stained in gel using GelCode Blue (Pierce). Stained gels were use for mass spectrometry analysis (MALDI-TOF) of trypsinized peptides at the University of Alabama at Birmingham Mass Spectrometry Facility. The protein was identified by analysis of the tryptic fragments and comparison with the National Center for Biotechnology Information (NCBI) database. The peptide masses were entered into Mascot database search engine (www.matrixscience.com/), and the NCBI database was searched to identify the protein. Molecular weight search (MOWSE) scores above 73 were considered statistically significant. For the coimmunoprecipitation of Hsp90 and P2X7, homogenates from PC12 cells (1 mg/mL) were incubated with recombinant human Hsp90-myc-tag or with Hsp90-myc-tag with nitrotyrosine at position 56 (purified as described below) for 1 h at 4 °C. The immunoprecipitation of Hsp90-myc was performed using an anti-myc antibody conjugated to Sepharose beads (Cell Signaling Technology) according to the manufacturer’s instructions. The membrane was blotted for P2X7 (1:500; Chemicon) and myc-tag (1:2,000; Cell Signaling).
Motor Neuron Isolation and Culture.
Rat embryo motor neurons were purified using a combination of cushion centrifugation and immunoaffinity and then were cultured in Neurobasal medium enriched with glutamine, glutamate, β-mercaptoethanol, and B27 supplement (Gibco-Invitrogen) as previously described (2, 34, 36, 72) in the presence or absence of brain-derived neurotrophic factor (BDNF; 1 ng/mL), glial-derived neurotrophic factor (GDNF; 0.1 ng/mL), and cardiotrophin 1 (CT-1, 10 ng/mL). More than 95% of the cells were immunoreactive for p75 neurotrophin receptor and Islet1, two early markers of motor neurons (73–75).
Purification of Rat Hsp90.
Rat liver Hsp90 was purified by a combination of ion exchange, gel filtration, and hydroxyapatite chromatography. Fractions were collected and analyzed with SDS/PAGE. The protein was more than 90% pure in all the preparations. Detailed methods are provided in SI Materials and Methods.
Expression and Purification of Recombinant Human Hsp90β.
The human Hsp90β sequence was optimized for bacterial expression (GenScript USA) and cloned in a pBad/Myc-Hisx6 vector (Invitrogen). The replacement of two, four, and five tyrosine residues by phenylalanine (Y33F+Y56F, 4xPhe(Y56), and 5xPhe-Hsp90, respectively) was performed on the optimized wild-type human Hsp90β sequence (GenScript). The proteins were expressed after induction of the bacterial culture with 0.2% l-arabinose for 2 h at 37 °C and 220 rpm. Recombinant wild-type and mutant Hsp90β were purified from the bacterial culture using the Ni-NTA purification system (Invitrogen) according to the manufacturer’s instructions.
Expression of Hsp90β with Nitrotyrosine in Selected Positions.
The incorporation of a nitrotyrosine residue in a selected position of recombinant human Hsp90β was performed as previously described (32) but with a newly selected orthogonal nitrotyrosine-bearing suppressor tRNA and engineered tRNA synthase pair. Briefly the codon codifying for each of the five tyrosine residues in the human Hsp90β sequence optimized for bacterial expression was replaced by an Amber stop codon (TAG), which was recognized by the orthogonal nitrotyrosine-bearing suppressor tRNA and tRNA synthase. The modified proteins were expressed and purified as described above for recombinant human Hsp90β.
Intracellular Delivery of Proteins in PC12 Cells and Determination of Cell Survival.
For the intracellular delivery of Hsp90, Hsp70, actin, and tubulin, 200 µL of a mixture containing 10 μg of the protein in 100 µL PBS and 4 μL of the lipophilic Chariot permeation agent in 100 µL of H2O was incubated for 30 min at room temperature, according to the manufacturer’s instructions (Active Motif). A pellet corresponding to 1 × 106 PC12 cells was resuspended gently in the protein/Chariot mixture, and 400 µL of RPMI 1640 was added. After 1 h incubation at 37 °C in 5% CO2/air, 1.5 mL of RPMI medium supplemented with 10% horse serum, 5% FetalClone II, and antibiotics was added, and the cells were incubated in the same conditions for an additional hour. After incubation, 4 × 104 cells per 35-mm dish were plated in RPMI medium supplemented with 10% horse serum, 5% FetalClone II, and antibiotics to reach a final volume of 2 mL Viability was determined using fluorescein diacetate/propidium iodide after 24 h in culture (Molecular Probes) (71, 76, 77).
Intracellular Delivery of Proteins in Motor Neuron and Determination of Cell Survival.
For the intracellular delivery of Hsp90, Hsp70, actin, and tubulin, 100 µL of a mixture containing 1 μg of the protein in 50 µL PBS and 1 μL of the lipophilic permeation agent Chariot solution in 50 µL of H2O was incubated for 30 min at room temperature, as described above. About 1,500 motor neurons were plated in 100 µL of Neurobasal medium (Invitrogen/Gibco) per well in four-well plates precoated with polyornithine/lamin. The protein/Chariot mixture was added to each well, incubated for 1 h at 37 °C in a humidified CO2 incubator, and then supplements plus trophic factors (BDNF, GDNF, and CT-1) in 800 µL of the complete neurobasal medium were added to reach a final volume of 1 mL The cultures were incubated for an additional 24 h; then motor neuron survival was determined by counting all neurons with neurites longer than three soma diameters as previously described (2) or by high-throughput image capture and analysis in 96-well plates using a Runner microscope (Trophos) as reported previously (4). For high-throughput image capture, motor neurons plated in black 96-well plates coated with poly-d-ornithine and laminin (Greiner Bio-one) were treated with calcein-AM (Molecular Probes-Invitrogen) in L15 medium (Invitrogen) for 1 h at 37 °C. Image analysis was performed using the Tina software (Trophos) (4, 22). The survival of motor neurons cultured in the presence of trophic factors or trophic factors plus Chariot was considered 100% and was used to standardize the experiments.
Transduction of Motor Neuron Lentiviral Particles.
The transduction of motor neurons with lentiviral particles expressing P2X7 shRNA, control shRNA, or coGFP (Santa Cruz Biotechnology) was performed at multiplicity of infection 20. The lentiviral particles were added to the culture medium 2 h after plating the motor neurons. The cells were incubated for additional 84 h before the intracellular delivery of the proteins. The efficiency of transduction was 90–95%.
Proteasome Activity.
The proteasome chymotrypsin-like activity was determined in PC12 cells using a cell-based luminescent assay (Promega Corp.) according to the manufacturer’s instructions.
RT-PCR.
RNA was extracted from 50,000 motor neurons using TRIzol (Invitrogen) and then was purified using PureLink RNA micro kit (Invitrogen) according to the manufacturer’s instructions. The cDNA was synthesized using the SuperScript III First Strand Synthesis Supermix kit (Invitrogen). The PCRs for P2X7 and β-actin were performed using Platinum PCR SuperMix High Fidelity (Invitrogen) and the following primer sequences: P2X7, sense (5′-AAGGGAAAGAAGCCCCACGG-3′) and antisense (5′-CCGCTTTTCCATGCCATTTT-3′); β-actin, sense (5′-CCCTAAGGCCAACCGTGAA-3′) and antisense (5′-GCCATCTCTTGCTCGAAGTC-3′).
Western, Slot, and Quantitative Dot Blotting Analysis.
Western blots were performed as previously described (5, 6). PC12 cells incubated with peroxynitrite were harvested and rinsed and process for Western blotting. All Western blots were visualized and the bands quantified using the Odyssey System (Li-Cor Biosciences).
Dot-blot quantification of nitrated Hsp90 was performed by harvesting total protein from 30,000 motor neurons or from 40,000 PC12 cells plated in a 35-mm dish for 24 h. Proteins were harvested, and samples containing equal amounts of protein were blotted on a PVDF membrane by gravity flow using a Bio-Blot Microfiltration apparatus (Bio-Rad) as previously described (78). Slot blots of Hsp90 peptides synthesized to contain nitrotyrosine at selected positions were performed by blotting the indicated amounts of peptide on a PVDF membrane as described for dot blot. Membranes then were processed as described for Western blotting.
Hsp90 Functional Assays.
The intrinsic ATPase activity of Hsp90 was determined as previously described for the optimized Hsp90 ATPase assay without modifications (26). Geldanamycin (5 µM), a specific inhibitor of Hsp90 ATPase activity, was used to verify ATP hydrolysis by the chaperone. The citrate synthase aggregation assay was performed as previously described (27).
Immunohistochemistry and Immunofluorescence.
Spinal cord sections from ALS patients and the G93A animal model of ALS were collected as free-floating sections and processed for immunohistochemistry as previously described (79). The spinal cord contusion injury in rats was performed as previously described (80). Mice and rats were housed and used following the guidelines of the Weill Cornell Medical College and Rutgers, The State University of New Jersey, respectively. Human samples were obtained and used according with the guidlines of the Weill Cornell Medical College. Following fixation, spinal cord tissue sections were paraffin embedded and processed for immunofluorescence. The sections were incubated with the antibodies anti-nitrotyrosine (1:500), anti–microtubule-associated protein 2 (anti-MAP2) (1:500, Chemicon), and anti-nitrated Hsp90 (1:100). Fluorescence images were acquired using a Zeiss Axiovert 200 epifluorescence microscope. To study the mobilization of FasL to the plasma membrane, motor neurons were culture for 16 h in the presence or absence of 5 μM BAPTA-AM after intracellular delivery of nitrated Hsp90. Cultures were fixed with paraformaldehyde plus glutaraldehyde (Sigma) on ice for 20 min before the staining was performed, as described previously (2). The cells then were incubated with anti FasL (1:250, Santa Cruz Biotechnology).
Statistical Analysis.
For statistical analyses, ANOVA was used with all groups compared by Bonferroni test. All tests were performed using the program Prism (GraphPad Software Inc.).
Supplementary Material
Acknowledgments
We thank M. Kirk and L. Wilson for technical help with the mass spectrometric analysis; C. Dennys for help with the P2X7 PCR; and C. Fernadez-Valle, C. E. Henderson, M. M. Gandelman, and M. Levy for constructive criticism on the manuscript. Funds for the purchase of the mass spectrometers were from National Institutes of Health (NIH)/National Center for Research Resources Shared Instrumentation Grant Awards RR06487 and RR13795. Operation of the Mass Spectrometry Shared Facility is supported by National Cancer Institute Core Support Grant P30 CA13148. The investigations were supported by the Burke Medical Research Institute, the New York State Spinal Cord Injury Center of Research, by NIH Grants NS36761 and NS42834 (to A.G.E.), and by NIH Grants NS058628, AT002034, and ES00210 and grants from the ALS Association (to J.S.B.). M.C.F. was a Goldsmith Fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.J.S. is a guest editor invited by the Editorial Board.
See Author Summary on page 4449 (volume 110, number 12).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215177110/-/DCSupplemental.
References
- 1.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estévez AG, et al. Nitric oxide and superoxide contribute to motor neuron apoptosis induced by trophic factor deprivation. J Neurosci. 1998;18(3):923–931. doi: 10.1523/JNEUROSCI.18-03-00923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raoul C, et al. Motoneuron death triggered by a specific pathway downstream of Fas. potentiation by ALS-linked SOD1 mutations. Neuron. 2002;35(6):1067–1083. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 4.Sahawneh MA, et al. Cu,Zn-superoxide dismutase increases toxicity of mutant and zinc-deficient superoxide dismutase by enhancing protein stability. J Biol Chem. 2010;285(44):33885–33897. doi: 10.1074/jbc.M110.118901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye Y, et al. Prevention of peroxynitrite-induced apoptosis of motor neurons and PC12 cells by tyrosine-containing peptides. J Biol Chem. 2007;282(9):6324–6337. doi: 10.1074/jbc.M610800200. [DOI] [PubMed] [Google Scholar]
- 6.Shacka JJ, et al. Two distinct signaling pathways regulate peroxynitrite-induced apoptosis in PC12 cells. Cell Death Differ. 2006;13(9):1506–1514. doi: 10.1038/sj.cdd.4401831. [DOI] [PubMed] [Google Scholar]
- 7.Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: Cause, effect, or association? J Clin Invest. 2003;111(2):163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borkovich KA, Farrelly FW, Finkelstein DB, Taulien J, Lindquist S. hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989;9(9):3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wandinger SK, Richter K, Buchner J. The Hsp90 chaperone machinery. J Biol Chem. 2008;283(27):18473–18477. doi: 10.1074/jbc.R800007200. [DOI] [PubMed] [Google Scholar]
- 10.Zhao R, et al. Navigating the chaperone network: An integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell. 2005;120(5):715–727. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Dezwaan DC, Freeman BC. HSP90: The Rosetta stone for cellular protein dynamics? Cell Cycle. 2008;7(8):1006–1012. doi: 10.4161/cc.7.8.5723. [DOI] [PubMed] [Google Scholar]
- 12.Wiech H, Buchner J, Zimmermann R, Jakob U. Hsp90 chaperones protein folding in vitro. Nature. 1992;358(6382):169–170. doi: 10.1038/358169a0. [DOI] [PubMed] [Google Scholar]
- 13.Pratt WB, Morishima Y, Osawa Y. The Hsp90 chaperone machinery regulates signaling by modulating ligand binding clefts. J Biol Chem. 2008;283(34):22885–22889. doi: 10.1074/jbc.R800023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z, Moghaddas Gholami A, Kuster B. Systematic identification of the HSP90 candidate regulated proteome. Mol Cell Proteomics. 2012;11(6):M111–, 016675. doi: 10.1074/mcp.M111.016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta RS. Phylogenetic analysis of the 90 kD heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species. Mol Biol Evol. 1995;12(6):1063–1073. doi: 10.1093/oxfordjournals.molbev.a040281. [DOI] [PubMed] [Google Scholar]
- 16.Barnier JV, Bensaude O, Morange M, Babinet C. Mouse 89 kD heat shock protein. Two polypeptides with distinct developmental regulation. Exp Cell Res. 1987;170(1):186–194. doi: 10.1016/0014-4827(87)90128-5. [DOI] [PubMed] [Google Scholar]
- 17.Sreedhar AS, Kalmár E, Csermely P, Shen YF. Hsp90 isoforms: Functions, expression and clinical importance. FEBS Lett. 2004;562(1-3):11–15. doi: 10.1016/s0014-5793(04)00229-7. [DOI] [PubMed] [Google Scholar]
- 18.Didelot C, et al. Heat shock proteins: endogenous modulators of apoptotic cell death. In: Gaestel M, editor. Molecular Chaperones in Health and Disease, Handbook of Experimental Pharmacology. Vol 172. Berlin: Springer; 2006. pp. 171–198. [DOI] [PubMed] [Google Scholar]
- 19.Voss AK, Thomas T, Gruss P. Mice lacking HSP90beta fail to develop a placental labyrinth. Development. 2000;127(1):1–11. doi: 10.1242/dev.127.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Robinson KM, Morré JT, Beckman JS. Triuret: A novel product of peroxynitrite-mediated oxidation of urate. Arch Biochem Biophys. 2004;423(1):213–217. doi: 10.1016/j.abb.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Santos CX, Anjos EI, Augusto O. Uric acid oxidation by peroxynitrite: Multiple reactions, free radical formation, and amplification of lipid oxidation. Arch Biochem Biophys. 1999;372(2):285–294. doi: 10.1006/abbi.1999.1491. [DOI] [PubMed] [Google Scholar]
- 22.Bordet T, et al. Identification and characterization of cholest-4-en-3-one, oxime (TRO19622), a novel drug candidate for amyotrophic lateral sclerosis. J Pharmacol Exp Ther. 2007;322(2):709–720. doi: 10.1124/jpet.107.123000. [DOI] [PubMed] [Google Scholar]
- 23.Mayer MP, Prodromou C, Frydman J. The Hsp90 mosaic: A picture emerges. Nat Struct Mol Biol. 2009;16(1):2–6. doi: 10.1038/nsmb0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali MMMMU, et al. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440(7087):1013–1017. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham CN, Krukenberg KA, Agard DA. Intra- and intermonomer interactions are required to synergistically facilitate ATP hydrolysis in Hsp90. J Biol Chem. 2008;283(30):21170–21178. doi: 10.1074/jbc.M800046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avila C, Kornilayev BA, Blagg BSJ. Development and optimization of a useful assay for determining Hsp90’s inherent ATPase activity. Bioorg Med Chem. 2006;14(4):1134–1142. doi: 10.1016/j.bmc.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 27.Jakob U, Lilie H, Meyer I, Buchner J. Transient interaction of Hsp90 with early unfolding intermediates of citrate synthase. Implications for heat shock in vivo. J Biol Chem. 1995;270(13):7288–7294. doi: 10.1074/jbc.270.13.7288. [DOI] [PubMed] [Google Scholar]
- 28.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266(7):4244–4250. [PubMed] [Google Scholar]
- 29.Alvarez B, Ferrer-Sueta G, Freeman BA, Radi R. Kinetics of peroxynitrite reaction with amino acids and human serum albumin. J Biol Chem. 1999;274(2):842–848. doi: 10.1074/jbc.274.2.842. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez BB, et al. Peroxynitrite-dependent tryptophan nitration. Chem Res Toxicol. 1996;9(2):390–396. doi: 10.1021/tx950133b. [DOI] [PubMed] [Google Scholar]
- 31.Vana L, Kanaan NM, Hakala K, Weintraub ST, Binder LI. Peroxynitrite-induced nitrative and oxidative modifications alter tau filament formation. Biochemistry. 2011;50(7):1203–1212. doi: 10.1021/bi101735m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neumann H, Hazen JL, Weinstein J, Mehl RA, Chin JW. Genetically encoding protein oxidative damage. J Am Chem Soc. 2008;130(12):4028–4033. doi: 10.1021/ja710100d. [DOI] [PubMed] [Google Scholar]
- 33.Hammill JT, Miyake-Stoner S, Hazen JL, Jackson JC, Mehl RA. Preparation of site-specifically labeled fluorinated proteins for 19F-NMR structural characterization. Nat Protoc. 2007;2(10):2601–2607. doi: 10.1038/nprot.2007.379. [DOI] [PubMed] [Google Scholar]
- 34.Raoul C, Henderson CE, Pettmann B. Programmed cell death of embryonic motoneurons triggered through the Fas death receptor. J Cell Biol. 1999;147(5):1049–1062. doi: 10.1083/jcb.147.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barthélémy CC, Henderson CE, Pettmann BB. Foxo3a induces motoneuron death through the Fas pathway in cooperation with JNK. BMC Neurosci. 2004;5(1):48. doi: 10.1186/1471-2202-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Estévez AG, et al. Liposome-delivered superoxide dismutase prevents nitric oxide-dependent motor neuron death induced by trophic factor withdrawal. Free Radic Biol Med. 2000;28(3):437–446. doi: 10.1016/s0891-5849(99)00261-0. [DOI] [PubMed] [Google Scholar]
- 37.Estévez AG, et al. Role of endogenous nitric oxide and peroxynitrite formation in the survival and death of motor neurons in culture. Prog Brain Res. 1998;118:269–280. doi: 10.1016/s0079-6123(08)63214-8. [DOI] [PubMed] [Google Scholar]
- 38.Blott EJ, Bossi G, Clark R, Zvelebil M, Griffiths GM. Fas ligand is targeted to secretory lysosomes via a proline-rich domain in its cytoplasmic tail. J Cell Sci. 2001;114(Pt 13):2405–2416. doi: 10.1242/jcs.114.13.2405. [DOI] [PubMed] [Google Scholar]
- 39.Linkermann A, Qian J, Janssen O. Slowly getting a clue on CD95 ligand biology. Biochem Pharmacol. 2003;66(8):1417–1426. doi: 10.1016/s0006-2952(03)00492-1. [DOI] [PubMed] [Google Scholar]
- 40.Bivona TG, et al. Rap1 up-regulation and activation on plasma membrane regulates T cell adhesion. J Cell Biol. 2004;164(3):461–470. doi: 10.1083/jcb.200311093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsushita K, et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115(2):139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Südhof TC, Rothman JE. Membrane fusion: Grappling with SNARE and SM proteins. Science. 2009;323(5913):474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qu Y, Dubyak GR. P2X7 receptors regulate multiple types of membrane trafficking responses and non-classical secretion pathways. Purinergic Signal. 2009;5(2):163–173. doi: 10.1007/s11302-009-9132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gutiérrez-Martín Y, et al. P2X7 receptors trigger ATP exocytosis and modify secretory vesicle dynamics in neuroblastoma cells. J Biol Chem. 2011;286(13):11370–11381. doi: 10.1074/jbc.M110.139410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou SM, Wang HS, Komai K. Colocalization of NOS and SOD1 in neurofilament accumulation within motor neurons of amyotrophic lateral sclerosis: An immunohistochemical study. J Chem Neuroanat. 1996;10(3-4):249–258. doi: 10.1016/0891-0618(96)00137-8. [DOI] [PubMed] [Google Scholar]
- 46.Abe K, Pan LH, Watanabe M, Kato T, Itoyama Y. Induction of nitrotyrosine-like immunoreactivity in the lower motor neuron of amyotrophic lateral sclerosis. Neurosci Lett. 1995;199(2):152–154. doi: 10.1016/0304-3940(95)12039-7. [DOI] [PubMed] [Google Scholar]
- 47.Beal MF, et al. Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann Neurol. 1997;42(4):644–654. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- 48.Ferrante RJ, et al. Increased 3-nitrotyrosine and oxidative damage in mice with a human copper/zinc superoxide dismutase mutation. Ann Neurol. 1997;42(3):326–334. doi: 10.1002/ana.410420309. [DOI] [PubMed] [Google Scholar]
- 49.Martin LJ, et al. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: Mechanisms of mitochondriopathy and cell death. J Comp Neurol. 2007;500(1):20–46. doi: 10.1002/cne.21160. [DOI] [PubMed] [Google Scholar]
- 50.Casoni F, et al. Protein nitration in a mouse model of familial amyotrophic lateral sclerosis: Possible multifunctional role in the pathogenesis. J Biol Chem. 2005;280(16):16295–16304. doi: 10.1074/jbc.M413111200. [DOI] [PubMed] [Google Scholar]
- 51.Basso M, et al. Characterization of detergent-insoluble proteins in ALS indicates a causal link between nitrative stress and aggregation in pathogenesis. PLoS ONE. 2009;4(12):e8130. doi: 10.1371/journal.pone.0008130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol. 1996;271(5 Pt 1):C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 53.Liu D, Ling X, Wen J, Liu J. The role of reactive nitrogen species in secondary spinal cord injury: Formation of nitric oxide, peroxynitrite, and nitrated protein. J Neurochem. 2000;75(5):2144–2154. doi: 10.1046/j.1471-4159.2000.0752144.x. [DOI] [PubMed] [Google Scholar]
- 54.Xu J, et al. iNOS and nitrotyrosine expression after spinal cord injury. J Neurotrauma. 2001;18(5):523–532. doi: 10.1089/089771501300227323. [DOI] [PubMed] [Google Scholar]
- 55.Souza JM, Daikhin E, Yudkoff M, Raman CS, Ischiropoulos H. Factors determining the selectivity of protein tyrosine nitration. Arch Biochem Biophys. 1999;371(2):169–178. doi: 10.1006/abbi.1999.1480. [DOI] [PubMed] [Google Scholar]
- 56.Ischiropoulos H, Gow A. Pathophysiological functions of nitric oxide-mediated protein modifications. Toxicology. 2005;208(2):299–303. doi: 10.1016/j.tox.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 57.Ischiropoulos H. Biological tyrosine nitration: A pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. 1998;356(1):1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 58.Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun. 2003;305(3):776–783. doi: 10.1016/s0006-291x(03)00814-3. [DOI] [PubMed] [Google Scholar]
- 59.MacMillan-Crow LA, Greendorfer JS, Vickers SM, Thompson JA. Tyrosine nitration of c-SRC tyrosine kinase in human pancreatic ductal adenocarcinoma. Arch Biochem Biophys. 2000;377(2):350–356. doi: 10.1006/abbi.2000.1799. [DOI] [PubMed] [Google Scholar]
- 60.Kawasaki H, et al. Mass spectrometric identification of tryptophan nitration sites on proteins in peroxynitrite-treated lysates from PC12 cells. Free Radic Biol Med. 2011;50(3):419–427. doi: 10.1016/j.freeradbiomed.2010.10.688. [DOI] [PubMed] [Google Scholar]
- 61.Adinolfi E, Kim M, Young MT, Di Virgilio F, Surprenant A. Tyrosine phosphorylation of HSP90 within the P2X7 receptor complex negatively regulates P2X7 receptors. J Biol Chem. 2003;278(39):37344–37351. doi: 10.1074/jbc.M301508200. [DOI] [PubMed] [Google Scholar]
- 62.Kim M, Jiang LH, Wilson HL, North RA, Surprenant A. Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J. 2001;20(22):6347–6358. doi: 10.1093/emboj/20.22.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10(8):821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 64.Peng W, et al. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci USA. 2009;106(30):12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cadenas E, Wefers H, Müller A, Brigelius R, Sies H. Active oxygen metabolites and their action in the hepatocyte. Studies on chemiluminescence responses and alkane production. Agents Actions Suppl. 1982;11:203–216. [PubMed] [Google Scholar]
- 66.Mammucari C, Rizzuto R. Signaling pathways in mitochondrial dysfunction and aging. Mech Ageing Dev. 2010;131(7-8):536–543. doi: 10.1016/j.mad.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med (Berl) 2010;88(10):993–1001. doi: 10.1007/s00109-010-0663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nathan C. Specificity of a third kind: Reactive oxygen and nitrogen intermediates in cell signaling. J Clin Invest. 2003;111(6):769–778. doi: 10.1172/JCI18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forman HJ. Use and abuse of exogenous H2O2 in studies of signal transduction. Free Radic Biol Med. 2007;42(7):926–932. doi: 10.1016/j.freeradbiomed.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robinson KM, Beckman JS. Synthesis of peroxynitrite from nitrite and hydrogen peroxide. Methods Enzymol. 2005;396:207–214. doi: 10.1016/S0076-6879(05)96019-9. [DOI] [PubMed] [Google Scholar]
- 71.Estévez AG, et al. Peroxynitrite-induced cytotoxicity in PC12 cells: Evidence for an apoptotic mechanism differentially modulated by neurotrophic factors. J Neurochem. 1995;65(4):1543–1550. doi: 10.1046/j.1471-4159.1995.65041543.x. [DOI] [PubMed] [Google Scholar]
- 72.Henderson CE, et al. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363(6426):266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- 73.Yan Q, Johnson EM., Jr An immunohistochemical study of the nerve growth factor receptor in developing rats. J Neurosci. 1988;8(9):3481–3498. doi: 10.1523/JNEUROSCI.08-09-03481.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ericson J, Thor S, Edlund T, Jessell TM, Yamada T. Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science. 1992;256(5063):1555–1560. doi: 10.1126/science.1350865. [DOI] [PubMed] [Google Scholar]
- 75.Tsuchida T, et al. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79(6):957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 76.Spear N, Estévez AG, Barbeito L, Beckman JS, Johnson GV. Nerve growth factor protects PC12 cells against peroxynitrite-induced apoptosis via a mechanism dependent on phosphatidylinositol 3-kinase. J Neurochem. 1997;69(1):53–59. doi: 10.1046/j.1471-4159.1997.69010053.x. [DOI] [PubMed] [Google Scholar]
- 77.Spear N, et al. Enhancement of peroxynitrite-induced apoptosis in PC12 cells by FGF-1 and NGF requires p21Ras activation and is suppressed by Bcl-2. Arch Biochem Biophys. 1998;356:41–45. doi: 10.1006/abbi.1998.0741. [DOI] [PubMed] [Google Scholar]
- 78.Peluffo H, et al. Induction of motor neuron apoptosis by free 3-nitro-L-tyrosine. J Neurochem. 2004;89(3):602–612. doi: 10.1046/j.1471-4159.2004.02363.x. [DOI] [PubMed] [Google Scholar]
- 79.Petri S, Kiaei M, Wille E, Calingasan NY, Flint Beal M. Loss of Fas ligand-function improves survival in G93A-transgenic ALS mice. J Neurol Sci. 2006;251(1-2):44–49. doi: 10.1016/j.jns.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 80.Hasegawa K, et al. Embryonic radial glia bridge spinal cord lesions and promote functional recovery following spinal cord injury. Exp Neurol. 2005;193(2):394–410. doi: 10.1016/j.expneurol.2004.12.024. [DOI] [PubMed] [Google Scholar]