Abstract
Mechanotransduction, the pathway by which mechanical forces are translated to biological signals, plays important but poorly characterized roles in physiology. PIEZOs are recently identified, widely expressed, mechanically activated ion channels that are hypothesized to play a role in mechanotransduction in mammals. Here, we describe two distinct PIEZO2 mutations in patients with a subtype of Distal Arthrogryposis Type 5 characterized by generalized autosomal dominant contractures with limited eye movements, restrictive lung disease, and variable absence of cruciate knee ligaments. Electrophysiological studies reveal that the two PIEZO2 mutations affect biophysical properties related to channel inactivation: both E2727del and I802F mutations cause the PIEZO2-dependent, mechanically activated currents to recover faster from inactivation, while E2727del also causes a slowing of inactivation. Both types of changes in kinetics result in increased channel activity in response to a given mechanical stimulus, suggesting that Distal Arthrogryposis Type 5 can be caused by gain-of-function mutations in PIEZO2. We further show that overexpression of mutated PIEZO2 cDNAs does not cause constitutive activity or toxicity to cells, indicating that the observed phenotype is likely due to a mechanotransduction defect. Our studies identify a type of channelopathy and link the dysfunction of mechanically activated ion channels to developmental malformations and joint contractures.
Keywords: congenital contractures, neuromuscular system, whole exome sequencing, whole genome sequencing
Arthrogryposis, or multiple congenital contractures, is a characteristic of over 300 different disorders of highly variable etiology (1–3). Such malformations can be secondary to environmental factors such as decreased intrauterine movement or result from disorders of neurological, muscle, or connective tissue development. Some of these neuromuscular and connective tissue disorders are classified as distal arthrogryposes (DAs). Currently, DAs are subdivided into 10 types, depending on the number and nature of additional features (2, 4). Of these, DA Type 5 (DA5) [online Mendelian inheritance in man (OMIM) 108145] has been described in the literature for more than 70 y and can be further divided into several subtypes based on additional phenotypic features (1, 2, 4–9). DA5 itself is an autosomal dominant multisystem disorder characterized by multiple distal contractures, characteristic facies, ophthalmoplegia with ptosis, (2), and in some cases restrictive lung disease with pulmonary hypertension (4, 9). Although DA5 is often dominantly inherited and several large families have been described (4, 5, 9), molecular insight is lacking. Recently, loss-of-function mutations in endothelin converting enzyme-like 1 (ECEL1) have been shown to be responsible for a specific, recessively inherited subtype of DA (10, 11). Meanwhile, other types of DAs have been shown to involve mutations in the myosin heavy chain genes MYH3 and MYH8, in the myosin-binding protein C gene, MYBPC1, and in the genes TNNI2, TNNT3 and TPM2 that encode the muscle regulatory proteins troponin I, troponin T and beta-tropomyosin, respectively (2, 12–14). Here, we show that a subtype of DA5 that includes restrictive pulmonary disease is caused by gain-of-function mutations in the mechanically activated (MA) cation channel PIEZO2 (15).
Results
Clinical Assessment.
To identify the genetic cause of DA5, we ascertained two affected unrelated kindreds for next generation DNA sequencing. In the first kindred, the index case (individual 1) presented with DA5 and subsequently gave birth to a son with left talipes equinovarus (club foot) (individual 2; Fig. 1A). Both mother and son exhibited generalized arthrogryposis, deep-set hypermetropic eyes, ptosis and ophthalmoplegia, and restricted movements of the jaw, neck and spine, thorax and lungs, and large and small joints of the extremities (Table 1, Fig. 1 A and B). The contractures respond to physiotherapy, but severe contractures remain despite such treatment. During childhood, the mother was operated on for hypertrophic, constricted neck muscles, and in adulthood, the mother developed significant musculoskeletal pain resulting in disability. However, muscle strength and structure were normal on clinical investigation, muscle biopsy, and computed tomography (CT) scan. Both mother and son exhibited shortness of breath upon exercise, and the mother had pulmonary function tests of [forced expiratory capacity in 1 second (% normal value) / forced vital volume (% normal value)] = 60%/53%, characteristic of moderately severe restrictive lung disease. More detailed examination of major joint movements revealed severely limited joint extension but largely normal joint flexion (Table S1). Other findings were severe scoliosis in individual 2, and relatively short stature, both in individual 1 (length 158 cm, i.e., 10th centile; head circumference 55 cm, i.e., 50th centile; her mother’s length was 174 cm and her father’s length was 176 cm) and individual 2 (his growth has followed the 2.5 centile, weight and head circumferences on the 50th centile). In the mother, recurrent knee subluxations were found to be associated with an absence of anterior cruciate ligaments and small menisci in both knees. The son also has unstable knees compatible with similar intra-articular ligament agenesis (Table 1).
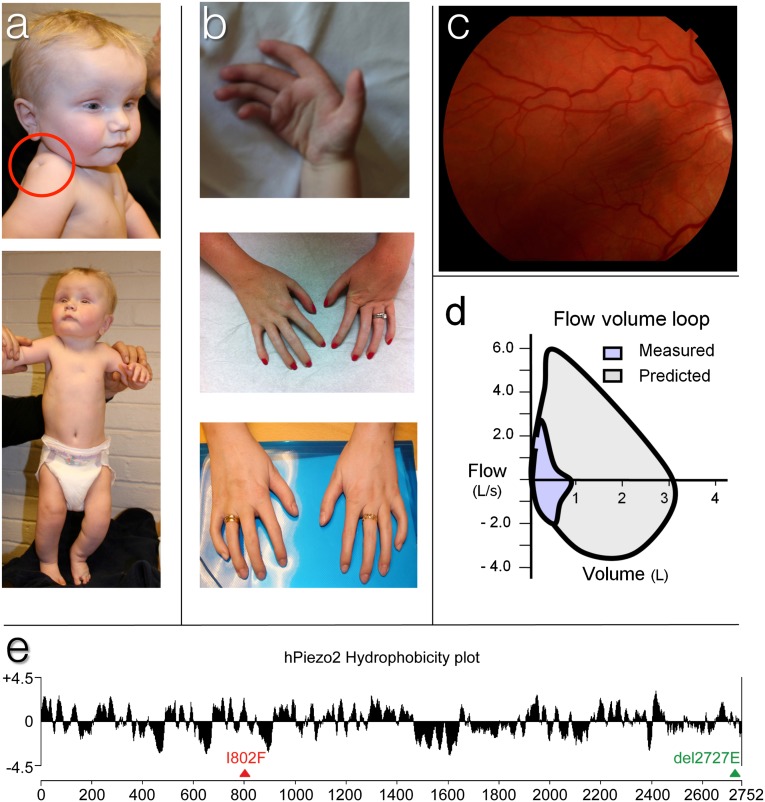
Fig. 1.
Dysmorphic features of DA5 individuals. (A) Infant photographs of individual 2 showing deep-set small eyes with mild ptosis, restricted shoulder abduction, and flexion contractures in the knees. Deep skin dimples can be seen on the shoulder (red circle) and sternum. (B) The DA of individual 2, 3, and 1 (from top to bottom). Note flexion contractures of the interphalangeal joints, increasing in severity from thumb to little finger. (C) Retinal photograph of individual 3 shows pigmented macular striae. This was not found in individual 1. (D) Flow volume loop of individual 3 showing markedly reduced total lung capacity (∼1 L) and restricted flow dynamics during both inspiration and expiration. (E) Hydrophobicity plot of human PIEZO2 showing Kyte–Doolittle hydrophobicity analysis (19-residue window) done with ProtScale program (Expasy). Triangles indicate the position of I802F and E2727del mutations.
Table 1.
Clinical features in three cases of DA5
| Phenotype | Individual 1 (I802F) (35-y-old mother) | Individual 2 (I802F) (5-y-old son) | Individual 3 (Δ2727E) (38-y-old female) |
| Short stature | Yes | Yes | Yes |
| Narrow/high palate | Yes | Yes | Yes |
| Reduced ability to open mouth | Yes | Yes | Yes |
| Decreased facial expression | Yes | Yes | Yes |
| Ophthalmoplegia | Yes | Yes | Yes |
| Deep-set eyes | Yes | Yes | Yes |
| Blepharophimosis | No | Yes | No |
| Ptosis | Yes | Yes | Yes |
| Hypermetropia | Yes (+7 o.u.) | Yes (+8/+10) | Yes |
| Duane anomaly | Nd | Nd | Yes |
| Abnormal retinal pigmentation | No | Nd | Yes |
| Macular retinal folds | No | Nd | Yes |
| Hunched anteverted shoulders | Yes | Yes | Yes |
| Restrictive lung disease | Yes | Likely | Yes |
| Spine stiffness | Yes | Yes | Yes |
| Hypermobile first metacarpophalageal | Yes | Yes | Yes |
| Absent phalangeal creases | Yes | Yes | Yes |
| Poorly formed palmar creases | Yes | Yes | Yes |
| Limited wrist extension | Yes | Yes | Yes |
| Absent ACL (knee) | Yes | Probably | No |
| Camptodactyly | Yes | Yes | Yes |
| Clinodactyly | Digits III–V | Digit V | Yes |
| Club feet | No | Yes, left | No |
| Dimples over large joints | Yes | Yes | No |
| Exertional dyspnea | Yes | Yes | Yes |
| Constriction of urethra | Yes | No | No |
| FEV1/FVC | 60%/53% | Nd | 28%/26% |
| Increased muscle tone | Yes | No | Yes |
| Tendon reflexes | Weak and absent in knees and ankles | Weak | Weak |
| Normal intelligence | Yes | Yes | Yes |
| Hearing loss | No | Nd | Yes (subjective) |
| Musculoskeletal pain | Yes, major problem | Nd | No |
| Altered pain sensation | Possibly as child | High threshold suspected | Nd |
ACL: anterior cruciate ligaments; Nd: not determined; o.u.: oculus uterque.
In the second kindred, the only affected individual (individual 3), a female, was born at 7 mo to a healthy mother and diagnosed with DA5 as a newborn. Between the ages of 2–15 y, she required multiple orthopedic procedures on her hips, knees, and feet to improve function. As an adult, she exhibited decreased facial expression with deep-set eyes and ptosis (Table 1); she also exhibited ophthalmoplegia that was most severe upon upward gaze, globally decreased muscle mass and spinal stiffness, limited forearm rotation, long fingers with congenital contractures, and poorly formed phalangeal and palmar creases (Fig. 1B). Fundoscopic examination revealed bilateral macular striae (Fig. 1C). She also had severe restrictive lung disease (FEV1/FVC 28%/26%) (Fig. 1D) and required chronic supplemental nocturnal oxygen. Taken together, affected individuals in both kindreds exhibit generalized arthrogryposis with characteristic ophthalmoplegia and ptosis, and with moderately severe to severe restrictive lung disease as a key feature of this particular DA5 subtype.
Whole Genome and Exome Sequencing.
Individuals 1 and 2 and their respective unaffected parents gave consent for clinical whole exome and whole genome sequencing, respectively. Exome sequencing of individual 1 identified a de novo c.2404A > T [p.Ile802Phe] missense variant in PIEZO2 (Fig. 1E) that was confirmed by Sanger sequencing in both individuals 1 and 2. In individual 3’s genome sequence, analyzed independently, 62 potential de novo single nucleotide variants (SNVs) were discovered along with 75 potential de novo indels. Two of these de novo variants were predicted to alter the respective protein sequences: one was a three base pair deletion c.8179_8181del in PIEZO2 (Ref. seq. NM_022068.2), resulting in an in-frame single amino acid deletion p.Glu2727del (Fig. 1E); the other was a missense variant [p.Arg341Gln] in the acyl-CoA synthetase medium-chain family member 4 gene, ACSM4. Sanger sequencing confirmed both the in-frame deletion and missense variant as authentic de novo changes in individual 3. Interestingly, the E2727del PIEZO2 variant falls within the C-terminal portion of PIEZO2, in a highly conserved pentapeptide motif Leu-Glu-Glu-Asp-Leu (LEEDL) embedded within a ∼550 residue domain of unknown function (DUF3595) that is highly conserved across metazoa from human to hydra. The I802F variant falls within one of ∼37 predicted transmembrane domains in PIEZO2, and this mutation is also in a region of the protein that is conserved in all mammals and chicken. The occurrence of these unique de novo I802F and E2727del variants in PIEZO2 is consistent with autosomal dominant inheritance and suggests pathogenic roles for both variants in the DA5 patients.
Electrophysiologic Studies of I802F and E2727del.
Piezos in mice are expressed in many tissues that respond to mechanical force (15). Drosophila Piezo functions in nociception (16), and human PIEZO1 missense mutations were recently identified in a disorder of erythrocyte volume homeostasis, hereditary xerocytosis (OMIM 194380) (17). However, the spectrum of PIEZO physiologic functions and the biological consequences of PIEZO mutations remain unknown. To investigate the functional effects of the two presumptive PIEZO2 mutations identified in DA5 patients, we generated the I802F and E2727del point mutations in full-length human PIEZO2 cDNA and characterized their effects on MA currents. Cells transfected with wild-type, I802F, or E2727del PIEZO2 cDNAs were stimulated by applying force to the cell surface while patch clamp recording in the whole cell configuration was at –80 mV, as previously described (15, 18, 19).
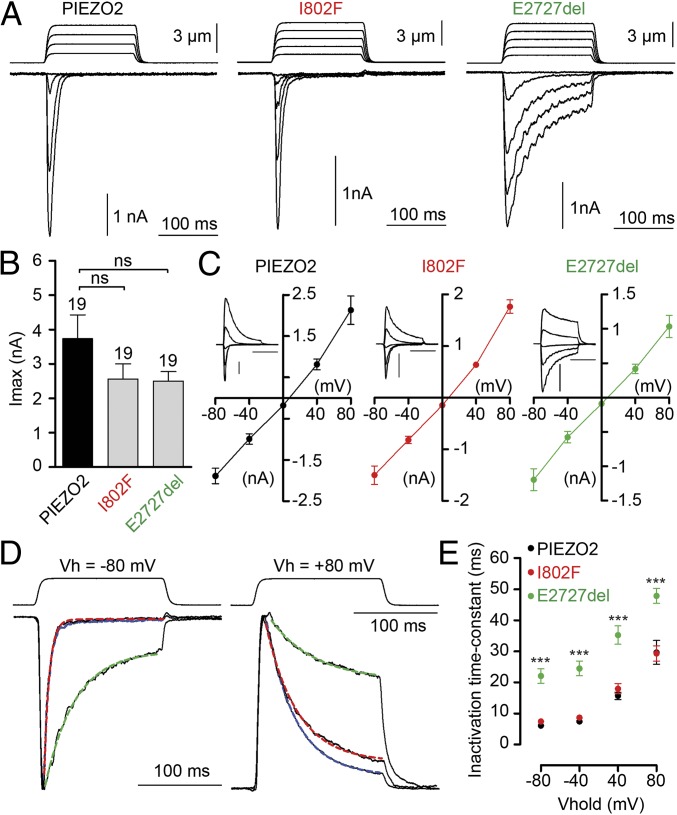
Large MA currents were present in cells transfected with wild-type, I802F, and E2727del (Fig. 2A). I802F and E2727del MA currents have similar amplitudes compared with wild-type PIEZO2 currents, as well as a linear current voltage relationship with a reversal potential close to 0 mV under the recording conditions (Fig. 2 B and C). Human PIEZO2 MA currents have a time constant of inactivation τ of 6.2 ± 0.6 ms (n = 12 cells) at –80 mV when fitted with a monoexponential equation, which is comparable to mouse Piezo2 (∼7 ms). I802F-induced MA current inactivation kinetics are similar to wild-type; however, E2727del-induced MA current inactivation kinetics are clearly slower at all potentials tested from –80 mV to +80 mV (Fig. 2 D and E). These differences in the kinetics of inactivation suggest that for a given mechanical stimulation that elicits currents of similar amplitude, the current charge will be larger for E2727del than for wild-type. Interestingly, I802F currents were similar to wild-type currents, suggesting another causal mechanism for this putative mutation.
Fig. 2.
E2727del PIEZO2 channels display slower inactivation kinetics compared with wild type. (A) Representative traces of MA inward currents at –80 mV in cells transfected with the indicated constructs and subjected to a series of mechanical steps in 1 µm increments. (B) Average maximal current amplitude of MA inward currents at –80 mV. The number of cells tested is shown above bars. (C) Averaged current-voltage relationships of MA currents in cells transfected with hPiezo2 (n = 7 cells), I802F (n = 8 cells), or E2727del (n = 8 cells). The insets show representative MA currents evoked at holding potentials ranging from –80 to +80 mV; inset scale bars are 1 nA and 100 ms. (D) Representative traces of MA currents elicited at –80 and +80 mV holding potentials. Traces were normalized to the peak current, and blue, red, and green dashed lines represent fits of inactivation with a monoexponential equation of human PIEZO2, I802F, and E2727del currents, respectively. (E) Time-constant of inactivation (tau) of PIEZO2 (black dots, n = 7 cells), I802F (red dots, n = 8 cells), and E2727del (green dots, n = 8 cells) currents at different holding potentials. Dots and bars represent mean ± SEM. ns, not statistically different; **P < 0.01; ***P < 0.001; Mann–Whitney test.
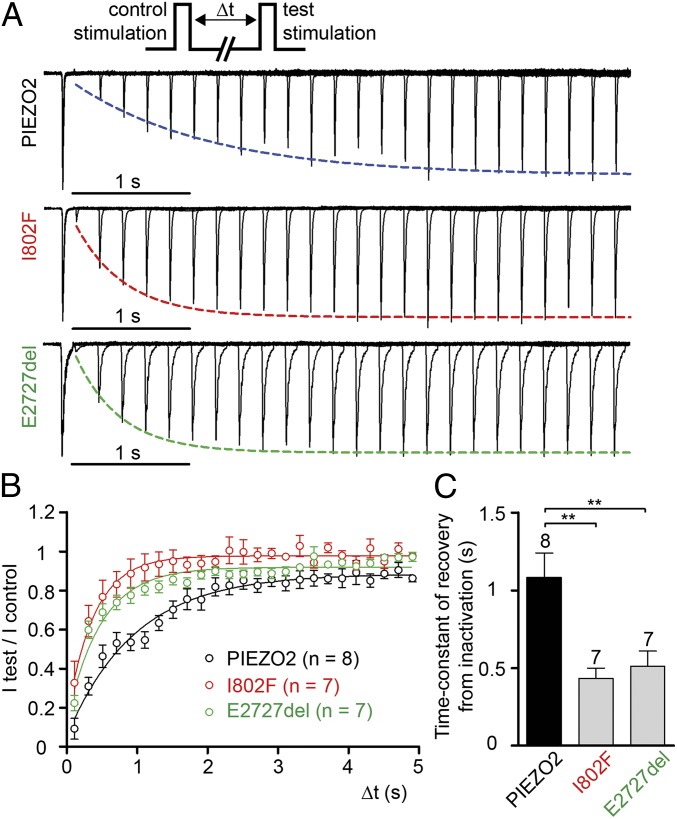
We next assayed the kinetics of recovery from inactivation of MA currents, which describe the ability of MA channels to respond to repetitive stimulation (Fig. 3). Interestingly, both I802F and E2727del recover more quickly from inactivation than wild-type, with recovery from inactivation time constants, τ, of 0.43 ± 0.07 and 0.51 ± 0.10 s, respectively, versus 1.08 ± 0.16 s for wild type (Fig. 3). These results suggest that both I802F and E2727del channels spend about twofold less time in an inactivated state following mechanical stimulation than wild-type channels, and thus can be reactivated quicker.
Fig. 3.
E2727del and I802F PIEZO2 channels recover faster from inactivation compared with wild type. (A) Typical recording traces of PIEZO2, I802F, and E2727del MA currents recorded at –80 mV. The protocol depicted on the top consists of two consecutive mechanical stimulation steps separated by increasing delay and aims at testing recovery from inactivation. The traces for each construct represent superimposition of multiple test-control stimulation pairings at different delta-t intervals. The test current traces are each normalized to the peak of control currents. Peak of test currents are fitted with a monoexponential equation. (B) Average of test peak current/control peak current for PIEZO2 (black, n = 8 cells), I802F (red, n = 7 cells), and E2727del (green, n = 7 cells) are fitted with monoexponential equation. (C) Average of time-constant (tau) of recovery from inactivation determined for each individual cell exemplified in A. The number of cells tested is shown above bars. Dots and bars represent mean ± SEM. **P < 0.01; ***P < 0.001; Mann–Whitney test.
These results suggest that increased response to mechanical force may explain the phenotype of DA5 patients. However, it is also possible that PIEZO2 mutants are active without mechanical stimuli, and that cation influx from constitutively open ion channels could contribute to cell toxicity (20), or could cause DA5-related phenotypes. However, cells transfected with the mutant PIEZO2 constructs do not show stimulus-independent PIEZO-like channel activity (Fig. 2 and Fig. 3) nor enhanced cytotoxicity (Fig. S1). Therefore, the DA5 phenotype in patients with PIEZO2 mutations may reflect ion channel hyperresponsiveness to forces engendered during development, and later during postnatal life.
Discussion
MA ion channels, also termed stretch-activated ion channels, are present in a variety of cell types, including somatosensory neurons, inner ear hair cells, muscle cells, endothelial cells, and osteoblasts (21). The role of such ion channels in touch, pain, and hearing—where mechanical forces are transduced to biological signals within milliseconds—are relatively clear. During fetal development and during the normal physiological function of many biological systems, including the neuromuscular, musculoskeletal, and respiratory systems, mechotransduction has been proposed to be mediated by a variety of molecules, including integrins, kinases, and ion channels (22). However, the identify and function of MA ion channels in most of these biological systems remains unknown.
Our results show that mutations in PIEZO2 can cause a subtype of DA5 that restricts respiratory function, as well as cause the generalized contractures, ptosis, and ophthalmoplegia that characterize DA5 in general (Table 1). In particular, electrophysiological analysis of mutant PIEZO2 proteins suggests that these channels are more efficient at transducing repetitive mechanical signals. In addition, we found no evidence that these channels are constitutively active (without mechanical force), arguing that the observed phenotype most likely reflects dysregulated mechanotransduction. Since DA5 is manifest as a congenital disorder present at birth, this implies that MA ion channels play a crucial role in the integrated development of the joints and neuromuscular and respiratory systems during embryogenesis. Further insight into the nature of this embryonic requirement for mechanotransduction (which cell types and at what embryonic time points) awaits analyses of loss-of-function mutations in PIEZO2.
The identification of mutations in PIEZO2 in a subtype of DA5 affords further insight into the potential pathogenic mechanisms in arthrogryposis. In the adult mouse, PIEZO2 RNA is expressed in a variety of tissues including dorsal root ganglion (DRG) sensory neurons and the lung (15). Therefore, this syndrome could result from altered behavior of stretch sensors like muscle spindles and Golgi tendon organs that are important for proprioception and muscle tone. Interestingly, mutation of ECEL1 was also recently shown to cause a DA subtype (10, 11). However, patients with mutations in ECEL1 are phenotypically distinct from the patients reported here, as the former exhibit knee extensions and micrognathia but not ophthalmoplegia or shoulder girdle contractures. Nonetheless, like PIEZO2, ECEL1 is expressed in neuronal cell types, and knockout of Ecel1 in mice reveals that Ecel1 (also called DINE, or damage-induced neuronal endopeptidase) is required for the proper intramuscular axonal branching of motor neurons in skeletal muscle during embryogenesis (23,24). These results suggest that mutations in neuronally expressed genes such as PIEZO2 and ECEL1, as well as in the already known DA genes that are expressed in muscle, can perturb the neuromuscular pathway that controls the development of muscle tone during embryogenesis.
Arthrogryposis could also result from abnormal tissue modeling during development, such that hyperactive PIEZO2 signaling regulates morphogenesis in ways that constrain joint extension, lung or thorax expansion, and oculomotor movement. Expression analyses of PIEZO2 in human or mouse embryos might shed light on how this ion channel causes arthrogryposis. Interestingly, anterior cruciate ligament agenesis was identified in the first kindred, and similar DA5 patients have been described before (25). Perturbations in PIEZO2 function may therefore hold relevance for structural syndromes involving abnormal joint mobility. Furthermore, the delineation of two different types of PIEZO2 gain-of-function mutations [viz., slower inactivation and accelerated recovery from inactivation (E2727del) vs. only accelerated recovery (I802F)] may prove to have genotype–phenotype implications, with composite gain-of-function mutations (E2727del) having more severe clinical consequences.
It has been proposed that congenital contractures could result from decreased fetal movement brought about by primary neural or muscular disorders, or from other abnormalities in utero (3, 7, 26). Interestingly, previously identified DA mutations reside in MYH3 and MYH8 that encode sarcomeric proteins, or in TNNI2, TNNT3 and TPM2, respectively (2, 12–14). These proteins are expressed in fast twitch fibers, and functional studies have shown that DA (DA1 and DA2) mutations in TNNI2, TNNT3, and TPM2 produce increased contractile function in troponin-replaced rabbit psoas fibers (13). This could suggest that these specific DA mutations cause congenital contractures on the basis of hypercontractility and increased tension in developing fast twitch skeletal muscles, resulting in diminished muscle movement in utero. The possibility that PIEZO2 senses forces associated with muscle contraction, combined with the finding that mutant PIEZO2 proteins have increased activity, is likewise consistent with the view that the integrated development of muscles and joints during embryogenesis is an active rather than a passive process (13). For example, increased ion flux could result in increased neural firing during fetal muscle movement, and this in turn could inhibit overall muscle movement via a negative feedback loop that integrates muscle contraction and movement. This model could predict that as yet unidentified PIEZO2 variants might have the opposite effect—that is, diminished PIEZO2 function may be associated with joint hypermobility, a common condition of suspected multifactorial etiology and dominant inheritance pattern.
Mechanotransduction has been implicated in several physiological processes, including the sensing of hemodynamic forces during development and the function of various organs (22), but not as a cause of defective muscle development and arthrogryposis. Here, we show that a mechanosensitive ion channel plays a key role in the development and function of both the neuromuscular and respiratory systems. Further analyses will be required to elucidate the frequency of PIEZO2 mutations in other individuals with DA and to determine whether these mutations affect other classical mechanotransduction-dependent processes such as hearing and pain perception.
Methods
Whole Genome Sequencing.
Whole genome sequencing was performed by the Illumina Clinical Services Laboratory (Illumina, Inc.). Briefly, genomic DNA was randomly fragmented and then sequenced using 100 base pair paired-end reads on an Illumina HiSeq Sequencer (27). The resulting output was converted to FASTQ format. The paired-end FASTQ files were aligned to the human reference sequence [University of California Santa Cruz (UCSC) HG19 (Human Genome version 19) build] using the Burroughs-Wheeler Alignment tool (28) in paired-end mode followed by base quality recalibration and targeted local realignment focused around known short insertions and deletions (indels) using the Genome Analysis Toolkit (GATK) (29, 30). Duplicated reads from sequencing the same DNA fragment were discarded.
Single nucleotide substitutions and indels were identified for all three samples simultaneously using the Unified Genotyper tool from the GATK in multisample calling mode. Variant quality score recalibration was performed using the GATK to identify a set of high-confidence variants. The functional consequence of the resulting set of variants was predicted using the Variant Effect Predictor from Ensembl (31).
Novel variants were defined as those not found in dbSNP (Single Nucleotide Polymorphism Database) build 135 (32). A stringent set of putative de novo variants was defined as those found to be heterozygote in the proband without evidence of any sequencing read supporting the alternate allele in both parents and absence of the alternate allele in both dbSNP build 135 and the Exome Sequencing Project (http://evs.gs.washington.edu/EVS/).
For individual 3 and her unaffected parents, an average of 1.15 × 1011 bases of sequence were generated per individual, resulting in an average of 43-fold coverage across the genome. A total of 4,165,074 SNVs were identified in the family along with 817,283 short insertions or deletions (indels). Of these SNVs, 45,626 were novel. By comparing individual 3’s genome sequence with that of her unaffected parents, 62 potential de novo SNVs were discovered along with 75 potential de novo indels; as not all of these variants were validated, some may represent false positives. However, two of the de novo variants were predicted to alter their respective protein sequences: one was a three base pair deletion resulting in an in-frame single amino acid deletion in the PIEZO2 (p.E2727del); the other was a missense variant identified in the acyl-CoA synthetase medium-chain family member 4 gene (ACSM4, p.R341Q). Sanger sequencing confirmed that the in-frame deletion and missense variant were both de novo changes in individual 3—that is, present in the heterozygous state and absent in her parents. Consent to perform whole genome sequencing under Clinical Laboratory Improvement Amendments (CLIA) conditions was obtained under protocols approved by the Institutional Review Board (IRB) of Partners HealthCare, Inc. Explicit permission to publish the findings contained herein was obtained from individual 3.
Whole Exome Sequencing.
Whole exome sequencing for trio 1 was in principle performed as previously described (33). Briefly, massively parallel sequencing of genomic DNA from an affected individual–parent trio was performed at the Radboud University Nijmegen Medical Centre by using the SOLiD 5500xl platform (Life Technologies). Enrichment of exonic sequences was achieved by using the human SureSelect 50 Mb set (Agilent, v2), which targets ∼21,000 human genes. On average, we obtained >136 million mappable sequencing reads (50 bp single-end) and 6.6 Gb of mappable sequence data per individual after multiplex sequencing, using six exomes per flowchip. Color space reads were mapped to the hg19 reference genome with SOLiD LifeScope software version 2.1, which uses an iterative mapping approach. On average, >88% of bases mapped on or near (50 bp proximity) the targets, resulting in a mean coverage of 104-fold (median of 82-fold). In total, >81% of the targeted exons were covered ≥20 times (>86% ≥10 times) (Table S2). SNVs were subsequently called by the DiBayes algorithm using high-stringency calling settings, and small insertions and deletions were detected using the SOLiD Small Indel Tool.
We identified a total of 44,606 variants in the proband of trio 1 (individual 1). All variants were annotated using an in-house annotation pipeline built in Nijmegen. These variants were filtered and analyzed for potential de novo occurrence using an automated prioritization scheme (34) involving an automated check of all private, nonsynonymous variants identified in the affected individuals with high quality (>4 variant reads, >25% variation) for occurrence in the respective parental bam files. Candidate de novo events (Table S3) were then verified using conventional Sanger sequencing. The only validated de novo mutation was the I802F missense variant in PIEZO2. The regional ethical review board as well as the Norwegian health authorities have approved whole exome sequencing as a method to find de novo disease-causing mutation. The patients have given informed consent to the publishing of clinical information and pictures.
Cell Culture and Transient Transfection.
Human embryonic kidney 293T (HEK293T) cells were grown in Dulbecco’s Modified Eagle Medium containing 4.5 mg⋅mL−1 glucose, 10% (vol/vol) FBS, 50 U⋅mL−1 penicillin, and 50 mg⋅mL−1 streptomycin. Cells were plated onto poly-lysine-coated 12-mm round glass coverslips placed in 24-well plates and transfected using lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. A total of 800 ng⋅mL−1 of plasmid DNA was cotransfected with 300 ng⋅mL−1 of GFP plasmid, and GFP positive cells were recorded 12–48 h later.
Electrophysiology.
Patch-clamp experiments were performed in standard whole-cell recordings using an Axopatch 200B amplifier (Axon Instruments). Patch pipettes had a resistance of 1.5–2.5 MΩ when filled with an internal solution consisting of (in mM) 133 CsCl, 10 Hepes, 5 EGTA, 1 CaCl2, 1 MgCl2, 4 MgATP, and 0.4 Na2GTP (pH adjusted to 7.3 with CsOH). The extracellular solution consisted of (in mM) 130 NaCl, 3 KCl, 1 MgCl2, 10 Hepes, 2.5 CaCl2, 10 glucose (pH adjusted to 7.3 with NaOH). All experiments were done at room temperature. Currents were sampled at 20 kHz and filtered at 2 kHz. Voltages were not corrected for a liquid junction potential. Leak currents before mechanical stimulations were subtracted off-line from the current traces.
Mechanical Stimulation.
Mechanical stimulation was achieved using a fire-polished glass pipette (tip diameter 3–4 μm) positioned at an angle of 80° to the cell being recorded. Downward movement of the probe toward the cell was driven by a Clampex controlled piezo-electric crystal microstage (E625 LVPZT Controller/Amplifier; Physik Instrumente). The probe had a velocity of 1 µm⋅ms−1 during the ramp segment of the command for forward motion, and the stimulus was applied for 150 ms. To assess the mechanical sensitivity of a cell, a series of mechanical steps in 1 μm increments was applied every 10 s, which allowed for full recovery of mechanosensitive currents. Inward MA currents were recorded at a holding potential of –80 mV. For I–V relationship recordings, voltage steps were applied 0.7 s before the mechanical stimulation from a holding potential of –60 mV. The protocol used to characterize the recovery from inactivation kinetics was applied every 15 s, and each of the two consecutive mechanical stimulus steps lasted 100 ms.
Toxicity/Viability Assay.
To exclude the possibility that the PIEZO2 mutations tested are constitutively active and cause cellular toxicity on that basis, we directly tested if human PIEZO2 mutants incurred toxicity or affected the viability of HEK cells. We used wild-type and constitutively active (F640L) versions of TRPV1, the latter as a positive control. The F640L TRPV1 mutation exhibited an increased cytotoxicity ratio compared with wild-type TRPV1, whereas PIEZO2 I802F and E2727del did not (Fig. S1). HEK293T cells were transfected with various cDNAs and seeded in a 384-well plate (TransIT-LT1, Mirus, 9,000 cells/well; 62.5 ng cDNA/well; eight wells per cDNA). Two days after transfection, cells were tested using the MultiTox-Fluor Multiplex Cytotoxicity Assay per manufacturer’s instructions (Promega; measures two protease activities correlated with number of dead and live cells). Briefly, reagents were incubated with cells for 2–3 h at 37 °C after which fluorescence at Ex485/Em520 (dead-cell fluorescence or cytotoxicity) and Ex405/Em492 (live-cell fluorescence or viability) were measured using EnVision Multilabel Plate Reader (Perkin-Elmer). Average cytotoxicity–viability fluorescence ratio was calculated and normalized to the average ratio of pcDNA3.1 controls. Results of four independent experiments were analyzed for significance using Prism 6 (Graphpad). Rat TRPV1 cloned in pcDNA5-FRT and the toxic TRPV1-F640L mutant (Quikchange, Agilent Technologies) were used as controls (20).
Supplementary Material
Acknowledgments
We thank the patients and their families for participation in this study. We also thank Stuart Cahalan for cloning contributions. We thank Christian Gilissen and Peer Arts from the genomic disorders group Nijmegen for the technical support in analyzing the exomes from kindred 2. This work was supported by Helse Vest, National Institutes of Health (NIH) Grants R01HD060050, R01MH084676, and R01DE022358, and by funds from Brigham and Women’s Hospital. N.S. was supported in part by a career development award from the NIH/National Heart, Lung, and Blood Institute (HL114642). A.H. was supported by a grant from the Netherlands Organization for Health Research and Development (ZonMW 917-66-363). J.C. was supported by a Medical Research Council Career Development Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221400110/-/DCSupplemental.
References
- 1.Altman H, Davidson L. Amyoplasia congenita (arthrogryposis mutiplex congenita) J Pediatr. 1939;15(4):551–557. [Google Scholar]
- 2.Bamshad M, Van Heest AE, Pleasure D. Arthrogryposis: A review and update. J Bone Joint Surg Am. 2009;91(Suppl 4):40–46. doi: 10.2106/JBJS.I.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall JG. Arthrogryposis multiplex congenita: Etiology, genetics, classification, diagnostic approach, and general aspects. J Pediatr Orthop B. 1997;6(3):159–166. [PubMed] [Google Scholar]
- 4.Bamshad M, Bohnsack JF, Jorde LB, Carey JC. Distal arthrogryposis type 1: Clinical analysis of a large kindred. Am J Med Genet. 1996;65(4):282–285. doi: 10.1002/(SICI)1096-8628(19961111)65:4<282::AID-AJMG7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 5.Beals RK, Weleber RG. Distal arthrogryposis 5: A dominant syndrome of peripheral contractures and ophthalmoplegia. Am J Med Genet A. 2004;131(1):67–70. doi: 10.1002/ajmg.a.30289. [DOI] [PubMed] [Google Scholar]
- 6.Castori M, et al. Juvenile macular dystrophy and forearm pronation-supination restriction presenting with features of distal arthrogryposis type 5. Am J Med Genet A. 2009;149A(3):482–486. doi: 10.1002/ajmg.a.32668. [DOI] [PubMed] [Google Scholar]
- 7.Hall JG, Reed SD, Greene G. The distal arthrogryposes: Delineation of new entities—review and nosologic discussion. Am J Med Genet. 1982;11(2):185–239. doi: 10.1002/ajmg.1320110208. [DOI] [PubMed] [Google Scholar]
- 8.Schrander-Stumpel CT, et al. Arthrogryposis, ophthalmoplegia, and retinopathy: Confirmation of a new type of arthrogryposis. J Med Genet. 1993;30(1):78–80. doi: 10.1136/jmg.30.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams MS, Elliott CG, Bamshad MJ. Pulmonary disease is a component of distal arthrogryposis type 5. Am J Med Genet A. 2007;143(7):752–756. doi: 10.1002/ajmg.a.31648. [DOI] [PubMed] [Google Scholar]
- 10.Dieterich K, et al. The neuronal endopeptidase ECEL1 is associated with a distinct form of recessive distal arthrogryposis. Hum Mol Genet. 2012 doi: 10.1093/hmg/dds514. 10.1093/hmg/dds514. [DOI] [PubMed] [Google Scholar]
- 11.McMillin MJ, et al. the University of Washington Center for Mendelian Genomics Mutations in ECEL1 Cause Distal Arthrogryposis Type 5D. Am J Hum Genet. 2013;92(1):150–156. doi: 10.1016/j.ajhg.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurnett CA, et al. Myosin binding protein C1: A novel gene for autosomal dominant distal arthrogryposis type 1. Hum Mol Genet. 2010;19(7):1165–1173. doi: 10.1093/hmg/ddp587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson P, et al. Mutations in fast skeletal troponin I, troponin T, and beta-tropomyosin that cause distal arthrogryposis all increase contractile function. FASEB J. 2007;21(3):896–905. doi: 10.1096/fj.06-6899com. [DOI] [PubMed] [Google Scholar]
- 14.Sung SS, et al. Mutations in genes encoding fast-twitch contractile proteins cause distal arthrogryposis syndromes. Am J Hum Genet. 2003;72(3):681–690. doi: 10.1086/368294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coste B, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330(6000):55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483(7388):209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarychanski R, et al. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood. 2012;120(9):1908–1915. doi: 10.1182/blood-2012-04-422253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coste B, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483(7388):176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubin AE, et al. Inflammatory signals enhance piezo2-mediated mechanosensitive currents. Cell Rep. 2012;2(3):511–517. doi: 10.1016/j.celrep.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers BR, Bohlen CJ, Julius D. A yeast genetic screen reveals a critical role for the pore helix domain in TRP channel gating. Neuron. 2008;58(3):362–373. doi: 10.1016/j.neuron.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sachs F. Stretch-activated ion channels: What are they? Physiology (Bethesda) 2010;25(1):50–56. doi: 10.1152/physiol.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10(1):63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagata K, et al. Damage-induced neuronal endopeptidase is critical for presynaptic formation of neuromuscular junctions. J Neurosci. 2010;30(20):6954–6962. doi: 10.1523/JNEUROSCI.4521-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schweizer A, et al. Neonatal lethality in mice deficient in XCE, a novel member of the endothelin-converting enzyme and neutral endopeptidase family. J Biol Chem. 1999;274(29):20450–20456. doi: 10.1074/jbc.274.29.20450. [DOI] [PubMed] [Google Scholar]
- 25.Kwan K, Ross K. Arthrogryposis and congenital absence of the anterior cruciate ligament: a case report. Knee. 2009;16(1):81–82. doi: 10.1016/j.knee.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Gordon N. Arthrogryposis multiplex congenita. Brain Dev. 1998;20(7):507–511. doi: 10.1016/s0387-7604(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 27.Bentley DR, et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456(7218):53–59. doi: 10.1038/nature07517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DePristo MA. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKenna A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLaren W, et al. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics. 2010;26(16):2069–2070. doi: 10.1093/bioinformatics/btq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherry ST, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29(1):308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoischen A, et al. De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat Genet. 2010;42(6):483–485. doi: 10.1038/ng.581. [DOI] [PubMed] [Google Scholar]
- 34.Vissers LE, et al. A de novo paradigm for mental retardation. Nat Genet. 2010;42(12):1109–1112. doi: 10.1038/ng.712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.