Abstract
Based on a unique dataset comprising all 325,000 Austrian patients that were under pharmaceutical treatment for diabetes during 2006 and 2007, we measured the excess risk of developing diabetes triggered by undernourishment in early life. We studied the percentage of all diabetes patients in the total population specifically for each year of birth, from 1917 to 2007. We found a massive excess risk of diabetes in people born during the times of the three major famines and immediately after, which occurred in Austria in the 20th century: 1918–1919, 1938, and 1946–1947. Depending on the region, there was an up to 40% higher chance of having diabetes when born in 1919–1921, compared with 1918 or 1922, where age-specific typical diabetes ratios are observed. The excess risk for diabetes was practically absent in those provinces of Austria that were less affected by the famines. We show that diabetes rates exhibit nontrivial, age-specific sex differences, and correlate with the economic wealth of the region. Our results might be of relevance for establishing higher awareness in the health system for those born in high-risk years, and underline the importance of ensuring sufficient nutrition in prenatal and early stages of life.
Keywords: epidemic, glucose tolerance, intrauterine programming, massive data analysis, fetal development
In 1918, the Austro-Hungarian Empire spectacularly collapsed from a population of 52 million to the remaining modern state of Austria with 7 million inhabitants (1). The new state lost all food supplies from its former member states and was, at the same time, subject to a food embargo used by the Allies, the so-called “starvation embargo.” This situation led to a massive famine in Austria, the first of three experienced in the 20th century. The second period of hunger occurred in 1938 as a consequence of continued economic crisis, unemployment, bad harvests, and economic boycotts from Nazi Germany. The third period of acute hunger occurred in the aftermath of World War II, in 1946 and 1947 (for more information, see SI Text).
Since the 1930s it has been argued that the environment of early development can have long-lasting effects on health (2). Developmental origins of health and disease are often studied through “natural experiments,” where the impact of a clearly defined exposure to noncontrollable factors (such as famines) on the health of a subpopulation is studied. Crucial findings in such a natural experiment were obtained by studying the Dutch Hunger Winter (for a recent review, see ref. 3), where the situation of pregnancy in times of hunger was investigated in terms of the health of the offspring, not only in the immediate postnatal period but throughout the offspring’s adulthood. Using the Dutch Famine Birth Cohort, late adulthood diabetes and impaired glucose tolerance was associated with fetal growth and early gestation exposure (4, 5) modulated by the individual’s genetic background. The influence of hunger on type 2 diabetes in adulthood was also confirmed in the natural experiment of the Chinese Famine (1959–1961). Here it was shown that incidences of diabetes are related with fetal exposure to famines, and that a nutritionally rich environment in later life can amplify this effect (6). Fetal and infant undernourishment were also associated with adult impaired glucose tolerance in the Biafran Famine, during the Nigerian Civil War (1967–1970) (7). These findings are in line with results of the Helsinki Birth Cohort Study, focusing on the effects of malnutrition in early life on the development of diabetes in adulthood (8). The cohort sizes in these studies are limited to several thousand subjects, e.g., the Dutch Famine Birth Cohort consists of 2,414 persons, the Helsinki Birth Cohort 8,760 persons, and 1,339 and 7,874 persons for the Biafran and Chinese Famine, respectively. No difference in glucose tolerance was found between a group of 169 persons exposed to the Siege of Leningrad (1941–1944) and another group of 188 born outside the siege area (9). This dissimilar finding may hint at the importance of catch-up growth during early childhood, which was absent in Leningrad in contrast to the Dutch famine (9).
In light of this epidemiological data, the so-called “thrifty phenotype hypothesis” was proposed (10), stating that under nutritional deprivation, the developing fetus adopts a number of strategies to maximize the postnatal survival rate, assuming continued poor nutrition after birth. Several epidemiological studies show an intriguing relation between birth weight and subsequent disease development, often decades later in life. For type 2 diabetes in adulthood, the molecular mechanisms for intrauterine programming are not fully understood; however, several studies suggest the effect of low birth weight on reduced muscle expression of protein kinase Cζ, GLUT4, phosphoinositide 3-kinase p85α, and p110β subunits that precede the onset of impaired glucose tolerance (11, 12). Fetal and neonatal life are critical periods for pancreatic β-cell development, because in the first year of life, already about half the number of adult β-cells is present (13). The first glucose tolerance studies were performed in 59- to 70-y-old men (14), indicating that birth weight is directly related to an increased risk of either impaired glucose tolerance or diabetes. In addition to type 2 diabetes (15), diseases associated with low fetal and childhood growth include a central distribution of body fat (16), increased stroke rates (15), insulin resistance (17), metabolic syndrome (18), ischemic cardiovascular disease (19), and even cognitive (20) and psychiatric disorders (21, 22).
In this work we quantify the nationwide excess risk of developing diabetes (mainly type 2) later in life when born in times of hunger. We address the case of three famines in Austria, based on a unique nationwide dataset containing 42 million medical treatments of all pharmaceutically treated diabetes patients in the country (23) (325,998 people born between 1917 and 2007) in the time period 2006–2007. Our effective cohort size is Austria’s entire population of 8.3 million (www.statistik.at/web_en/statistics/population/population_censuses/index.html), representing an increase of about three orders of magnitude compared with similar previous studies. We control for the date of birth, residency, and sex of the subjects. In the time period 1917–2007, large and traditional discrepancies and gradients in economic wealth between the eastern and western parts of Austria existed. Classifying the subjects by residency allows for a socioeconomic stratification of the dataset at an unprecedented precision. We then report the nationwide and regional excess risk differences for females and males. Provinces studied have been abbreviated as follows: Burgenland (B), Carinthia (K), Lower Austria (N), Upper Austria (O), Salzburg (Sa), Styria (St), Tyrol (T), Vorarlberg (V), and Vienna (W).
Results
Increased Prevalence of Diabetes at Times of Austrian Famines.
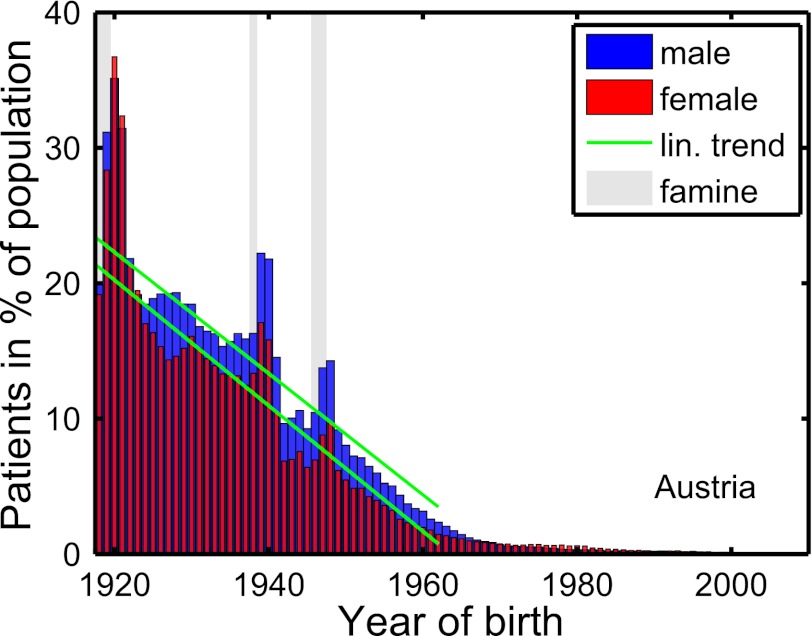
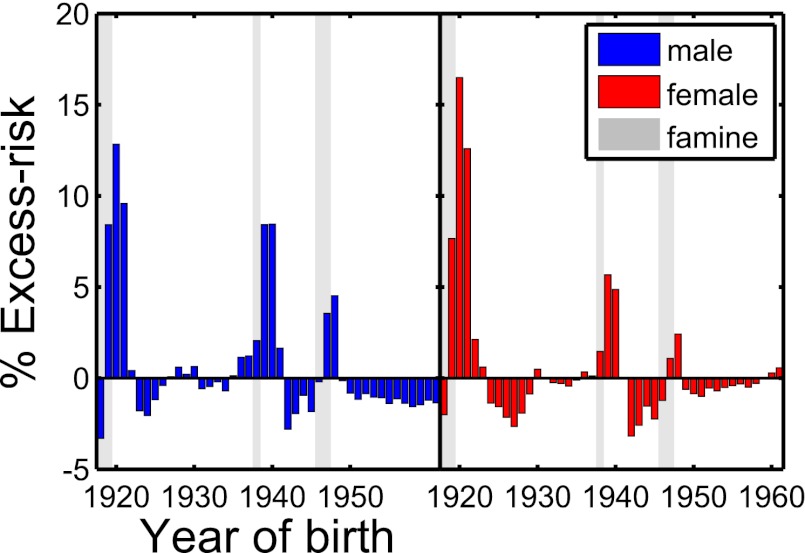
The nationwide dataset (Materials and Methods) reveals an increased rate of (for the most part, type 2) diabetes at the times of the three Austrian famines. The situation is depicted in Fig. 1 for males (blue) and females (red). There exist three clear peaks located exactly at and slightly after the famines, 1918–1919, 1938, and 1946–1947. The excess risk (difference to the green linear trend line in Fig. 1; Materials and Methods) is plotted in Fig. 2 for males and females. The slope of the trend line (Materials and Methods) for males and females is 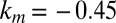 and
and 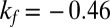 , respectively (Table 1). The nationwide risk for a male (female) to develop diabetes when born in 1919–1921 is ∼13% (16%) higher than for people born in 1918 or 1922. For the second famine, 1938, the excess risk is somewhat less pronounced, at 9% for males and 8% for females, respectively. The third famine, 1946–1947, corresponds to excess risks of 5% for males and 3% for females.
, respectively (Table 1). The nationwide risk for a male (female) to develop diabetes when born in 1919–1921 is ∼13% (16%) higher than for people born in 1918 or 1922. For the second famine, 1938, the excess risk is somewhat less pronounced, at 9% for males and 8% for females, respectively. The third famine, 1946–1947, corresponds to excess risks of 5% for males and 3% for females.
Fig. 1.
Percentage of diabetes patients in the total population of Austria as a function of birth year for females (red) and males (blue). An approximately linear rise of diabetes patients is seen for birth years before 1961. The green lines represent the trend lines of this linear increase. The three peaks above the trend lines indicate an excess risk of developing diabetes. The gray areas represent the three periods of severe hunger in Austria.
Fig. 2.
Excess risk of diabetes patients divided into genders between 1917 and 1961 for the Austrian data. The excess risk is defined as the difference of the value of the diabetes risk (Fig. 1) and the trend line for any given year of birth. The gray areas indicate the three famines.
Table 1.
Patient data and demographic indicators by sex and province, and regional economic indicators
| B | K | N | O | Sa | St | T | V | W | Austria | |
| Population size in 2009 | 283,506 | 560,056 | 1,606,615 | 1,411,041 | 529,314 | 1,207,588 | 704,792 | 368,061 | 1,692,067 | 8,363,040 |
| Treated patients* | 14,580 | 19,206 | 70,934 | 49,171 | 16,459 | 45,874 | 19,323 | 10,267 | 80,184 | 325,998 |
| Treatments/population | 0.0514 | 0.0343 | 0.0442 | 0.0348 | 0.0311 | 0.0380 | 0.0274 | 0.0279 | 0.0474 | 0.0390 |
| Females | 144,873 | 288,435 | 819,692 | 717,855 | 272,306 | 617,807 | 360,087 | 186,756 | 882,363 | 4,290,174 |
| Treated patients, female | 7,843 | 9,935 | 36,037 | 25,090 | 8,400 | 23,959 | 9,885 | 5,157 | 40,128 | 166,434 |
| Treatments/population, female | 0.0541 | 0.0344 | 0.0440 | 0.0350 | 0.0308 | 0.0388 | 0.0275 | 0.0276 | 0.0455 | 0.0388 |
| Males | 138,633 | 271,621 | 786,923 | 693,186 | 257,008 | 589,781 | 344,705 | 181,305 | 809,704 | 4,072,866 |
| Treated patients, male | 6,737 | 9,271 | 34,897 | 24,081 | 8,059 | 21,915 | 9,438 | 5,110 | 40,056 | 159,564 |
| Treatments/population, male | 0.0486 | 0.0341 | 0.0443 | 0.0347 | 0.0314 | 0.0372 | 0.0274 | 0.0282 | 0.0495 | 0.0392 |
| Sex ratio, treatment/population† | 0.8976 | 0.9909 | 1.0087 | 0.9939 | 1.0165 | 0.9582 | 0.9974 | 1.0207 | 1.0878 | 1.0103 |
| kf‡ | −0.69 | −0.42 | −0.50 | −0.42 | −0.41 | −0.53 | −0.45 | −0.30 | −0.43 | −0.46 |
| km‡ | −0.62 | −0.36 | −0.48 | −0.35 | −0.34 | −0.45 | −0.37 | −0.29 | −0.60 | −0.45 |
| df§ | 1,354 | 823 | 986 | 830 | 814 | 1,045 | 881 | 590 | 855 | 911 |
| dm§ | 1,222 | 707 | 949 | 681 | 674 | 879 | 720 | 578 | 1190 | 882 |
| GRP pp in €1,000 | 1.1 | 1.5 | 1.6 | 1.8 | 1.9 | 1.6 | 1.7 | 1.9 | 2.5 | 1.8 |
| Unemployment rate, % | 11.5 | 5.2 | 3.5 | 2.6 | 2.3 | 3.6 | 2.8 | 0.8 | 1.8 | 2.9 |
| Income pp, € | 210 | 340 | 360 | 410 | 370 | 390 | 400 | 420 | 750 | 407 |
Economic indicators include GRP in units of €1,000 for the year 1961 (the last year of the fit of the trend lines), the regional unemployment rates in 1961, and the income per person in EUR of the year 1957. Provinces: B, Burgenland; K, Carinthia; N, Lower Austria; O, Upper Austria; Sa, Salzburg; St, Styria; T, Tyrol; V, Vorarlberg; W, Vienna.
*Total number of patients who received a drug treatment for diabetes.
†Sex ratio is defined in Materials and Methods.
‡Slopes of the trend lines for males (m) and females (f).
§Intercepts.
Regional Differences Between Western and Eastern Provinces.
There is a wide variation of excess risk and diabetes increase rates (slopes) across the provinces. The eastern provinces B, N, St, and W show significantly higher excess risk in the years of famine: B, N, St, and W show pronounced peaks in the famine years, whereas in V and T they are barely detectable above the statistical noise level. The situation is most drastic in the province of B, where the excess risk is 38% for males and 28% for females, respectively, whereas for V the excess risk is at 10% for both males and females (see Figs. S1 and S2).
Because a patient’s current residence and place of birth need not be the same, migration issues become important on the regional scale. If inhomogeneities of excess risk exist on the regional scale at the time of famine, these will be washed out over time. To understand how migration between the provinces affects regional excess risk, we correct the observed excess risks (in 2006–2007) as discussed in SI Text. As expected, the correction amplifies the effects in the uncorrected data. For example, the highest observed excess risk, males in B born during the 1918–1919 famine, now increases from 38% to 42%. The province-specific values for observed and migration-corrected data are given in Fig. S3.
Similarly, the slopes of the trend lines (diabetes-increase rates) vary considerably across provinces (Table 1). In Fig. 3, we plot the averaged negative slope  for each province as a function of the gross regional product (GRP) of that province in 1961. We find a high correlation coefficient of
for each province as a function of the gross regional product (GRP) of that province in 1961. We find a high correlation coefficient of 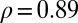 between the (negative) slopes and the GRP. The correlation coefficients for the slopes and the per capita income are
between the (negative) slopes and the GRP. The correlation coefficients for the slopes and the per capita income are 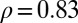 , and for the unemployment rates
, and for the unemployment rates 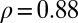 , respectively. The number of physicians per 100,000 inhabitants in the various provinces does not correlate with the negative slopes,
, respectively. The number of physicians per 100,000 inhabitants in the various provinces does not correlate with the negative slopes, 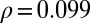 .
.
Fig. 3.
Scatter plot of the (negative) trend line slopes k vs. the gross regional product of 1961 (in €1,000 per person per month) for the Austrian provinces, except the city of Vienna. The data indicates a strong correlation of economic wealth and overall diabetes rates. The traditionally rich provinces T, V, O, and Sa show lower rates than the poorest province of B. Vienna as the capital city does not follow the pattern—it has a much higher GRP (€2,500) than any of the other provinces; however, due to its higher fraction of poor inhabitants it maintains a high slope of 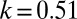 , averaged over males and females (Table 1).
, averaged over males and females (Table 1).
Sex-Specific Analysis.
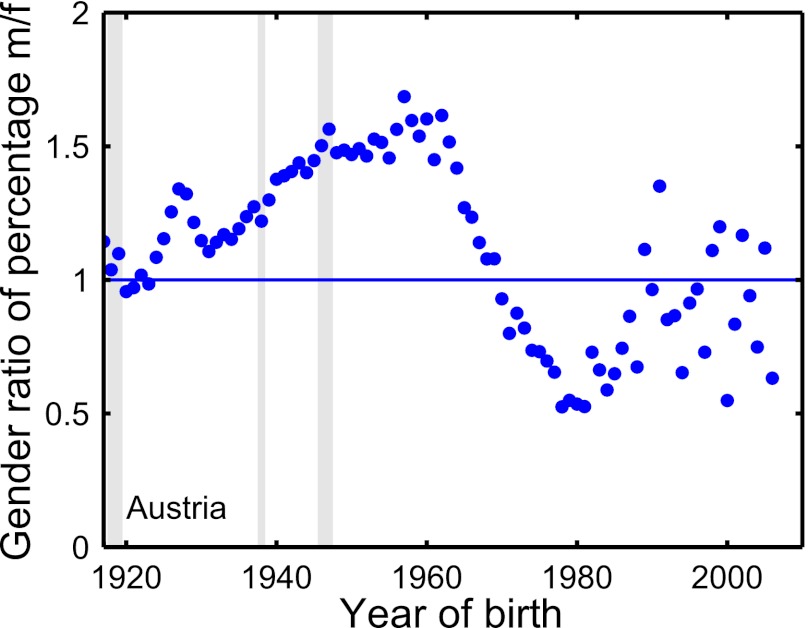
The three peaks indicating excess risk are observed for both males and females. However, in the all-Austrian data, males show a consistently higher overall risk of diabetes over the entire interval from 1917 to around 1970 (Fig. 4). From 1917 to 1965 we observe a male-to-female sex ratio (Materials and Methods) that increases from ∼1 (male and female rates are equal) in 1917 to 1.6 from about 1960 to 1965. For the birth year of about 1962 we observe the maximum sex disparity, where the fraction of males having diabetes is ∼60% higher than females. The male-to-female ratio becomes less than 1 from about 1970 on, reflecting an increased diabetes rate in women. The minimum at 0.5 is realized shortly before 1980 (Fig. 3), and coincides roughly with the most likely year of giving birth of Austrian women, which is ∼30 y (www.statistik.at/web_en/statistics/population/births/index.html). We repeat the sex-specific analyses for the provinces. In SI Text, the age-related diabetes probability, the excess risk, and the sex ratios are shown in Figs. S1, S2, and S4, respectively.
Fig. 4.
Time dependence of the sex ratio, defined as the quotient of the percentage of male diabetes patients over the percentage of female diabetes patients for a given birth year (Materials and Methods). The ratio increases from about equal percentages in 1917 (ratio = 1) to a male dominance of almost 60% at the year 1962. For birth years after 1970 the situation reverses and females become more likely to develop diabetes. This reversal may be related to the well-known phenomenon of gestational diabetes (35).
Discussion
We quantify the excess risk to develop (type 2) diabetes caused by famines in the population of an entire country. The history of Austria provides an excellent source of three well-documented nationwide famines during the past century. The records of more than 325,000 patients were analyzed in terms of birth date, sex, and location of current treatment over a 2-y observation period in 2006–2007.
We observe a nationwide higher risk of diabetes for those born in and immediately after periods of massive hunger. For a male (female) born in 1919–1921, the risk of developing diabetes is ∼13% (16%) higher than for people born in 1918 or 1922. For the 1938 famine the excess risks are 9% (8%), and for the 1946–1947 famine they are 5% (3%) for males (females). We find regional differences within Austria, particularly between western (V and T) and eastern provinces (W, B, N). The highest rates are observed in the eastern part of Austria, again in W, B, and N, with an excess risk of up to 38% (28%) for males (females). Lowest rates are found in the western provinces, in particular T and V, down to ∼10% for both sexes. Note that the patients’ locations are the provinces of current residency and are thus not necessarily the same as the provinces of birth. After correcting for internal migration flows between Austria’s provinces, we observe even higher regional excess risks in the eastern provinces, with values up to 42% (30%) for males (females).
Historically, the western provinces had a strong economical and cultural relationship to Switzerland, which led to better nutrition during the hunger periods—a fact well documented for the first famine, 1918–1919 (24). In contrast, B has been by far the poorest province in Austria, with a comparably very low average income until the 1990s. We find that economic wealth correlates strongly with the overall diabetes rates (Fig. 3, slopes). We can exclude that this strong correlation results from regional differences of medical coverage. For this reason, we computed the correlation of slopes with the regional density of medical doctors (in 2007) and found practically no correlation.
We study the age dependence of the sex ratio of male and female diabetes patients within the total population of Austria and for its provinces. We consistently find equal percentages of male and female patients around 1919. After this time, however, two different sex gaps emerge. The first gap exists in persons aged 40 and older, where male patients dominate the females by almost 60% for a birth year of about 1962. This dominance may be caused by sex-related differences in lifestyle and health literacy (SI Text). A recent survey (23) suggests that there are a higher number of women claiming to have type 2 diabetes than the number of women under actual pharmaceutical treatment, which indicates that women are more likely to be treated by nutrition therapy than by medications.
Although the overall lifelong diabetes prevalence is comparable in men and women, there exist some differences in various countries that depend on the ethnicity and age of the population under study. In both sexes there is a clear relationship between body mass index (BMI) and diabetes risk, with a steeper increase of diabetes for obese females than for males; this follows from a comparison of the Nurses’ Health Study and the Physicians’ Health Study (25). However, it was shown that in males, diabetes is diagnosed at a lower BMI than in females (26). Males are more insulin resistant and have more visceral and liver fat, which can contribute to their higher diabetes risk (27–29). These findings could partly explain the higher prevalence of diabetes seen in middle-aged and elderly men (30). Further, risk factors differ between men (e.g., more likely to be overweight) and women (e.g., higher risk for smoking) (30). Finally, females—in particular at the early stage of diabetes—more often exhibit impaired glucose tolerance and postprandial glycemia, which may be less often detected than fasting hyperglycemia (31).
Except for 1920, the famine-related peaks in diabetes seen in the Austrian population are more pronounced in males than in females. Recent studies seem to yield evidence for sex-dimorphic epigenetic effects. In a rat model of fetal programming, protein shortage during pregnancy leads to insulin resistance and higher lipids in male offspring (32). It was shown that male sex has greater impact on hyperglycemia in middle-aged adults with very low birth weight (33). In addition, male sex is a risk factor of perinatal complications and adverse outcome in early life (34). Therefore, it may be that males are particularly vulnerable to malnutrition during pregnancy.
The second sex gap exists for those aged 15–40 y, where female patients outnumber males. This second gap may be explained by a higher risk of diabetes that is related to reproductive factors and the pregnant state, as well as weight gain and retention postpartum in females (35). Whereas universal screening for gestational diabetes was unusual in the past, it is now clinical practice in all parts of Austria (36).
The pronounced peaks of diabetes in the postwar periods of World War I and World War II clearly show the importance of external determinants, such as sufficient nutrition of the overall population and socioeconomic factors during the time of conception, pregnancy, and early childhood, in addition to genetic disposition or shared socioeconomic, family, or lifestyle factors.
Could it be that it is not hunger but other effects that drive the excess risk to develop diabetes? The time of the first famine coincides with the Spanish flu, caused by an unusually virulent strain of the A/H1N1 virus (37), which hit Austria in three main waves from spring 1918 to spring 1919. This flu had an exceptionally high death rate, but otherwise was not much different from the annual seasonal influenza (38). If there exists a mechanism that relates viral infection during pregnancy or early childhood with increased type II diabetes rates, this should be observable in other influenza epidemics also. The strong influenza epidemics in Austria of the years 1928–1929, 1932–1933, 1936–1937, and 1943–1944 do not show higher excess risk levels in our data.
From our data it is not possible to strictly differentiate between effects from undernutrition from those caused by, e.g., war- and famine-related stress. It was argued that stress in childhood war evacuees (average age 4.8, SD 2.4) is linked to increased risk of diabetes later in life (39). Maybe the highest population-wide stress levels during the last century in Austria were reached by the bombing of Austrian cities between December 1943 and April 1945. If effects from stress in childhood related to bombing would be detectable in the data, we would expect a peak at the birth years between 1940 and 1943, which we do not observe. Assuming that stress during pregnancy has an effect on higher diabetes prevalence, we would expect a peak for the years around 1944–1945. The observed peak appears in 1946–1947. We think that these facts, in combination with the large existing body of literature on early-life famine-induced development of diabetes in the Netherlands (4, 5), China (6), Nigeria (7), and Helsinki (8), provide solid evidence that the hunger hypothesis is indeed the leading explanatory factor for the observations shown.
Materials and Methods
Diabetes Patient Dataset.
We used in this study a database of the Main Association of Austrian Social Security Institutions (40), which contains pseudonymized claims data of all persons receiving outpatient as well as inpatient care in Austria between January 1, 2006, and December 31, 2007. Apart from those 3% of Austrians who are covered by alternative insurance carriers (such as municipalities, unemployment service, religious orders, and notaries), data of all Austrians who received outpatient care from a general practitioner or a specialist, and/or received inpatient care at a hospital, and/or received pharmaceuticals at a pharmacy during the 2-y period are covered by the database. Therefore, most of the ∼8.3 million Austrians, of all age groups, are represented in the database, which was safeguarded and maintained by the Main Association of Austrian Social Security Institutions and accessible only for selected research partners under a strict data protection policy (23). In the present investigation we focused on diabetes patients who received pharmaceutical treatment. To define our patient collective, we identified all patients who received one or more diabetes-specific medications indexed by an Anatomic Therapeutic Chemical Classification System (ATC) code A10A (insulins and analogues), A10B (oral antidiabetics), or A10X (other antidiabetics) between 2006 and 2007. For more detailed information on the incidence of diabetes in Austria, including estimates for undiagnosed cases, see SI Text. We retrieved a total of 42 million medical treatments for our patient collective in the database. We excluded all patients who died in 2006 and 2007. In this way we selected 328,120 patients; from these, we omitted those born before 1917 due to the low abundance (less than 1,000 patients), leading to our final data set of 325,998 people (166,434 females, 159,564 males). From these patients, we gathered their year of birth, sex, and residency in one of the nine provinces of Austria. The numbers of treated diabetes mellitus patients for a given birth year  we denote by
we denote by 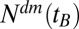 . The numbers of patients in each province are listed in Table 1.
. The numbers of patients in each province are listed in Table 1.
Census Data.
From the Austrian census database (www.statistik.at/web_en/statistics/population/population_censuses/index.html) we obtained the population size of the year 2009, 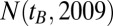 , denoting the number of living people with year of birth
, denoting the number of living people with year of birth 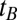 as observed in 2009 for given sex and region. For the year 2007 there was no such census data available. To estimate
as observed in 2009 for given sex and region. For the year 2007 there was no such census data available. To estimate  , we use the following iterative scheme. We use (again from census data; www.statistik.at/web_en/statistics/population/deaths/index.html) the male and female mortality rates
, we use the following iterative scheme. We use (again from census data; www.statistik.at/web_en/statistics/population/deaths/index.html) the male and female mortality rates 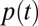 as a function of age t. We then estimate
as a function of age t. We then estimate 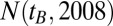 as
as  , and apply this correction iteratively one more time to get the estimate for
, and apply this correction iteratively one more time to get the estimate for 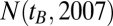 . Dividing the number of treated patients of a given birth year
. Dividing the number of treated patients of a given birth year 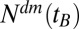 by the population size
by the population size 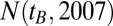 provides us with the percentage of the total population who were currently (2006–2007) treated as diabetes patients in Austria. The percentage of treated diabetes patients in terms of the total population of Austria as a function of the birth year is shown for females and males in Fig. 1. Data by individual provinces is given in Fig. S1. The percentage of treated diabetes patients serves as a direct approximation to the risk of having developed diabetes at any point in life, given a specific birth year.
provides us with the percentage of the total population who were currently (2006–2007) treated as diabetes patients in Austria. The percentage of treated diabetes patients in terms of the total population of Austria as a function of the birth year is shown for females and males in Fig. 1. Data by individual provinces is given in Fig. S1. The percentage of treated diabetes patients serves as a direct approximation to the risk of having developed diabetes at any point in life, given a specific birth year.
For rough proxies for the regional economic performance of the provinces, we obtained the unemployment rates (41) and the gross regional product for the year 1961, and the regional income per person for 1957 (from WKÖ-Stabsabteilung Statistik, http://wko.at/statistik). (We use data from 1961 because this is the last point in the fit for the trend lines, and before the full onset of the Wirtschaftswunder, or “economic miracle,” which restructured the Austrian economy considerably.) Until the 1990s, a significant disparity in the distribution of economic wealth in Austria existed, the western provinces being traditionally wealthier than the eastern provinces. The number of medical doctors per 100,000 inhabitants was assessed for the individual provinces for the year 2007 (www.statistik.at/web_en/statistics/health/health_care/index.html).
Linear Trend Estimation.
The fractions of treated diabetes patients are fitted by a linear regression (least squares) for the birth years 1917–1961. The resulting lines represent the age-related linear trend and are shown as green lines in Fig. 1 and Fig. S1. The (negative) slope of the trend lines is called k, and is reported in Table 1 for males (km), females (kf), and the provinces. These trend lines are approximations to the typical age-related risk of having developed diabetes, as a function of the birth year,
The slopes of the trend lines kf/m are an indication for the overall diabetes-increase rates over time in the population.
Excess Risk.
We define the excess risk for having diabetes by subtracting the linear trend line from the actual percentage of treated diabetes patients, i.e., 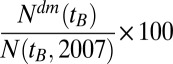 . We present this number as a percent above the linear trends in Fig. 2 for Austria, and in Fig. S2 for its provinces. Positive numbers indicate an excess risk of having diabetes with respect to the expected age-related trend lines.
. We present this number as a percent above the linear trends in Fig. 2 for Austria, and in Fig. S2 for its provinces. Positive numbers indicate an excess risk of having diabetes with respect to the expected age-related trend lines.
Sex Ratios.
For the detection of potential sex differences, we define a sex ratio by dividing the fraction of male by the fraction of female diabetes patients for every birth year (Fig. 3). A value of x indicates that males have an x-fold higher (if 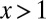 ) risk of having diabetes than females. The data for the provinces (Fig. S4) was produced in the same way.
) risk of having diabetes than females. The data for the provinces (Fig. S4) was produced in the same way.
Supplementary Material
Acknowledgments
We thank Verena Ahne, Klaus Taschwer, and Susanne Wicha-Müller for assistance in historical research on the Austrian famines, and Milan Hronsky for aggregation of the patient data.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215626110/-/DCSupplemental.
References
- 1. Unknown (Nov 1, 1919) Foreign comment: Dark days in Austria and Hungary. Literary Digest, p 21.
- 2.Kermack WO, McKendrick AG, McKinlay PL. Death-rates in Great Britain and Sweden: Expression of specific mortality rates as products of two factors, and some consequences thereof. J Hyg (Lond) 1934;34(4):433–457. doi: 10.1017/s0022172400043230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulz LC. The Dutch Hunger Winter and the developmental origins of health and disease. Proc Natl Acad Sci USA. 2010;107(39):16757–16758. doi: 10.1073/pnas.1012911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Rooij SR, et al. Impaired insulin secretion after prenatal exposure to the Dutch famine. Diabetes Care. 2006;29(8):1897–1901. doi: 10.2337/dc06-0460. [DOI] [PubMed] [Google Scholar]
- 5.Kahn HS, Graff M, Stein AD, Lumey LH. A fingerprint marker from early gestation associated with diabetes in middle age: The Dutch Hunger Winter Families Study. Int J Epidemiol. 2009;38(1):101–109. doi: 10.1093/ije/dyn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Hoek M, Langendonk JG, de Rooij SR, Sijbrands EJ, Roseboom TJ. Genetic variant in the IGF2BP2 gene may interact with fetal malnutrition to affect glucose metabolism. Diabetes. 2009;58(6):1440–1444. doi: 10.2337/db08-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hult M, et al. Hypertension, diabetes and overweight: Looming legacies of the Biafran famine. PLoS ONE. 2010;5(10):e13582. doi: 10.1371/journal.pone.0013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eriksson JG, Forsen TJ, Osmond C, Barker DJ. Pathways of infant and childhood growth that lead to type 2 diabetes. Diabetes Care. 2003;26(11):3006–3010. doi: 10.2337/diacare.26.11.3006. [DOI] [PubMed] [Google Scholar]
- 9.Stanner SA, Yudkin JS. Fetal programming and the Leningrad Siege study. Twin Res. 2001;4(5):287–292. doi: 10.1375/1369052012498. [DOI] [PubMed] [Google Scholar]
- 10.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 11.Terauchi Y, et al. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85 alpha subunit of phosphoinositide 3-kinase. Nat Genet. 1999;21(2):230–235. doi: 10.1038/6023. [DOI] [PubMed] [Google Scholar]
- 12.Ozanne SE, et al. Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia. 2005;48(3):547–552. doi: 10.1007/s00125-005-1669-7. [DOI] [PubMed] [Google Scholar]
- 13.Rahier J, Wallon J, Henquin JC. Cell populations in the endocrine pancreas of human neonates and infants. Diabetologia. 1981;20(5):540–546. doi: 10.1007/BF00252762. [DOI] [PubMed] [Google Scholar]
- 14.Hales CN, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303(6809):1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kensara OA, et al. Hertfordshire Study Group Fetal programming of body composition: Relation between birth weight and body composition measured with dual-energy X-ray absorptiometry and anthropometric methods in older Englishmen. Am J Clin Nutr. 2005;82(5):980–987. doi: 10.1093/ajcn/82.5.980. [DOI] [PubMed] [Google Scholar]
- 17.Ravelli AC, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351(9097):173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 18.Hitman GA, Sudagani J. Searching for genes in diabetes and the metabolic syndrome. Int J Clin Pract Suppl. 2004;58(143):3–8. doi: 10.1111/j.1368-504x.2004.00374.x. [DOI] [PubMed] [Google Scholar]
- 19.Singhal A, Lucas A. Early origins of cardiovascular disease: Is there a unifying hypothesis? Lancet. 2004;363(9421):1642–1645. doi: 10.1016/S0140-6736(04)16210-7. [DOI] [PubMed] [Google Scholar]
- 20.de Rooij SR, Wouters H, Yonker JE, Painter RC, Roseboom TJ. Prenatal undernutrition and cognitive function in late adulthood. Proc Natl Acad Sci USA. 2010;107(39):16881–16886. doi: 10.1073/pnas.1009459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 2004;15(4):183–187. doi: 10.1016/j.tem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Mittendorfer-Rutz E, Rasmussen F, Wasserman D. Restricted fetal growth and adverse maternal psychosocial and socioeconomic conditions as risk factors for suicidal behaviour of offspring: A cohort study. Lancet. 2004;364(9440):1135–1140. doi: 10.1016/S0140-6736(04)17099-2. [DOI] [PubMed] [Google Scholar]
- 23. Dorda W, et al. (2011) Outcome Research Based on Performance Data of the Austrian Health System (Medical Univ of Vienna/Univ of Vienna, Vienna). German.
- 24. Haas H (1989) Austria and the allies: 1918–1919. Scientific Commission to Examine the History of the Republic of Austria, eds Ackerl I, Neck R (Verlag für Geschichte und Politik, Vienna), Vol 11. German.
- 25.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89(6):2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 26.Logue J, et al. Scottish Diabetes Research Network Epidemiology Group Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia. 2011;54(12):3003–3006. doi: 10.1007/s00125-011-2313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kautzky-Willer A, et al. Influence of increasing BMI on insulin sensitivity and secretion in normotolerant men and women of a wide age span. Obesity (Silver Spring) 2012;20(10):1966–1973. doi: 10.1038/oby.2011.384. [DOI] [PubMed] [Google Scholar]
- 28.Westerbacka J, et al. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: Implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47(8):1360–1369. doi: 10.1007/s00125-004-1460-1. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113(11):1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rathmann W, et al. Incidence of type 2 diabetes in the elderly German population and the effect of clinical and lifestyle risk factors: KORA S4/F4 cohort study. Diabet Med. 2009;26(12):1212–1219. doi: 10.1111/j.1464-5491.2009.02863.x. [DOI] [PubMed] [Google Scholar]
- 31.Balkau B. The DECODE study. Diabetes epidemiology: Collaborative analysis of diagnostic criteria in Europe. Diabetes Metab. 2000;26(4):282–286. [PubMed] [Google Scholar]
- 32.Zambrano E, et al. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol. 2006;571(Pt 1):221–230. doi: 10.1113/jphysiol.2005.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato R, et al. A cross-sectional study of glucose regulation in young adults with very low birth weight: Impact of male gender on hyperglycaemia. BMJ Open. 2012;2(1):e000327. doi: 10.1136/bmjopen-2011-000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosen T, Bateman D. Male disadvantage in early life; the effects of gender in neonatal medicine. In: Legato M, editor. Principles of Gender Specific Medicine. New York: Elsevier; 2010. [Google Scholar]
- 35. Gunderson E (2009) Childbearing and obesity in women: Weight before, during and after pregnancy. Obstet Gynecol Clin North Am 36(2):317–332. [DOI] [PMC free article] [PubMed]
- 36.Kautzky-Willer A, et al. The impact of risk factors and more stringent diagnostic criteria of gestational diabetes on outcomes in central European women. J Clin Endocrinol Metab. 2008;93(5):1689–1695. doi: 10.1210/jc.2007-2301. [DOI] [PubMed] [Google Scholar]
- 37.Ansart S, et al. Mortality burden of the 1918–1919 influenza pandemic in Europe. Influenza Other Respir Viruses. 2009;3(3):99–106. doi: 10.1111/j.1750-2659.2009.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taubenberger JK, Morens DM. 1918 influenza: The mother of all pandemics. Emerg Infect Dis. 2006;12(1):15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alastalo H, et al. Cardiovascular health of Finnish war evacuees 60 years later. Ann Med. 2009;41(1):66–72. doi: 10.1080/07853890802301983. [DOI] [PubMed] [Google Scholar]
- 40. Endel G (2011) Health systems research in Austria: Part 1. Social Security Online, Available at www.sozialversicherung.at/mediaDB/831485_Artikel_Gesundheitssystemforschung-in-Oesterreich_Dr.Endel.pdf. German.
- 41.Austrian Institute of Economic Research (WIFO) Regional unemployment in Austria. WIFO-Monatsberichte. 1962;35(2):78–83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.