Abstract
In the field of regenerative medicine, one of the ultimate goals is to generate functioning organs from pluripotent cells, such as ES cells or induced pluripotent stem cells (PSCs). We have recently generated functional pancreas and kidney from PSCs in pancreatogenesis- or nephrogenesis-disabled mice, providing proof of principle for organogenesis from PSCs in an embryo unable to form a specific organ. Key when applying the principles of in vivo generation to human organs is compensation for an empty developmental niche in large nonrodent mammals. Here, we show that the blastocyst complementation system can be applied in the pig using somatic cell cloning technology. Transgenic approaches permitted generation of porcine somatic cell cloned embryos with an apancreatic phenotype. Complementation of these embryos with allogenic blastomeres then created functioning pancreata in the vacant niches. These results clearly indicate that a missing organ can be generated from exogenous cells when functionally normal pluripotent cells chimerize a cloned dysorganogenetic embryo. The feasibility of blastocyst complementation using cloned porcine embryos allows experimentation toward the in vivo generation of functional organs from xenogenic PSCs in large animals.
Keywords: apancreatic pig, organ reconstitution, transplantation, somatic cell nuclear transfer, chimera
Rapid progress in stem cell science promises novel therapeutic approaches for a number of intractable diseases. In particular, recent developments in induced pluripotent stem cell (iPSC) technology has enabled reprogramming of somatic cells that will offer new ways to treat a myriad of diseases with regenerative medicine using individual patients’ own cells. Although millions of patients suffer from end-stage organ failure that can be cured only by organ transplantation, treatment options are limited due to a shortage of donor organs. Therefore, generation of organs from stem cells is one of the ultimate goals of regenerative medicine. Current stem cell therapy, however, mainly targets diseases that can be treated by transplantation of cells of a single type [e.g., dopaminergic neurons for Parkinson disease (1), oligodendrocytes for spinal cord injury (1), pigment epithelial cells for retinal degenerative diseases (2)]. This is partly because generation of organs from iPSCs has been considered impractical due to difficulty in the replication in vitro of the complex interactions between cells and tissues during organogenesis.
We recently demonstrated that functional organs can be generated from pluripotent stem cells (PSCs) in vivo by blastocyst complementation in organogenesis-disabled mouse embryos (3, 4). To apply this principle in generating human organs, large nonrodent mammals must be used. This entails overcoming several difficulties, such as making organ-deficient large animals and supplying them in large numbers to collect embryos for blastocyst complementation procedures. More fundamental, however, is whether functional organs can be generated from exogenous PSCs in nonrodent large mammals. To address this, we used pigs, a species in which somatic cell nuclear transfer (SCNT) is feasible. We have shown that the level of Hes1 (hairy and enhancer of split 1) expression is critical for development of the biliary system (5). Proceeding from the assumption that overexpression of Hes1 under the promoter of Pdx1 (pancreatic and duodenal homeobox 1) inhibits pancreatic development, we have generated Pdx1 promoter-Hes1 transgenic pigs with an apancreatic phenotype. Here, we demonstrate that as in rodent models, donor pluripotent cell complementation of cloned blastocysts that would otherwise give rise to apancreatic animals yields pigs with pancreata of normal configuration and function that survive to adulthood. Blastocyst complementation using cloned porcine embryos thus may permit use of a large animal for the generation of functional organs from xenogenic PSCs, including human iPSCs.
Results
Creation of Pancreatogenesis-Disabled Pigs by a Transgenic Approach.
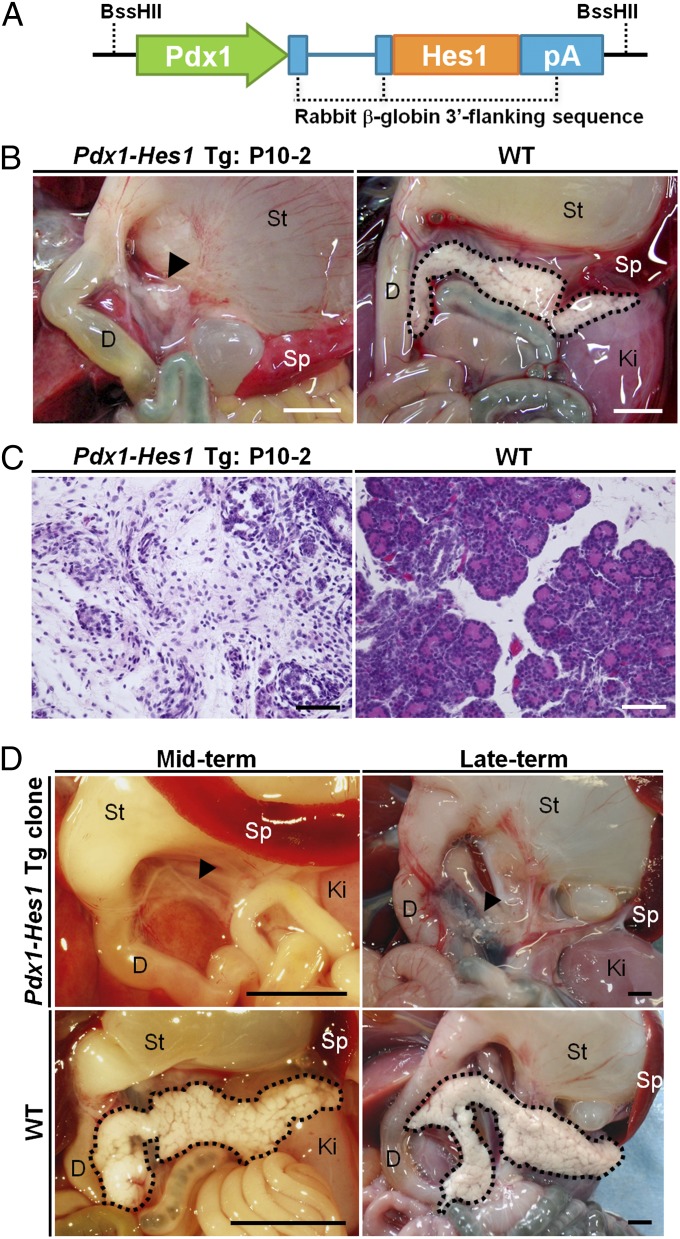
We introduced a Pdx1-Hes1 transgene construct into in vitro matured pig oocytes by intracytoplasmic sperm injection (ICSI)-mediated gene transfer (6) and produced transgenic pig fetuses by embryo transfer (Fig. 1 and Table S1). Among the five transgenic fetuses obtained, the pancreatogenesis-disabled phenotype was observed in one male fetus (day 74) and one female fetus (day 80), each of which had a vestigial pancreas (Fig. 1B and Fig. S1). These vestigial pancreata consisted of loose connective tissue dotted with ductal structures and small islands of epithelial cells (Fig. 1C). No cells showed acinar differentiation; such cells were observed in the pancreas of a normal fetus (Fig. 1C) at the same developmental stage.
Fig. 1.
Generation of pancreatogenesis-disabled pigs. (A) Construction of a Pdx1-Hes1 expression vector consisting of the mouse Pdx1 promoter, mouse Hes1 cDNA, and rabbit β-globin 3′ flanking sequence, including the polyadenylation signal (pA). (B and C) Macroscopic and microscopic appearances of the vestigial pancreas (arrowhead) of a Pdx1-Hes1 transgenic (Tg) male fetus and the pancreas of a WT fetus at the same gestational age. (D, Upper) Faithful reproduction of the pancreatogenesis-disabled phenotype of the Pdx1-Hes1 Tg fetus in fetuses cloned from it. (D, Lower) Normal pancreata of age-matched WT fetuses. D, duodenum; Ki, kidney; Sp, spleen; St, stomach. (Scale bars: B and D, 5 mm; C, 50 μm.)
Reproduction of Pancreatogenesis-Disabled Pigs by Somatic Cell Cloning.
We established primary cultures of fibroblast cells from the male fetus with a vestigial pancreas (Fig. 1 and Fig. S1) to use as nucleus donor cells for somatic cell cloning. Using SCNT from these Pdx1-Hes1 transgenic cells, we produced cloned fetuses. Observations in five midterm (day 59) and four late-term (day 110) cloned fetuses confirmed that the pancreatogenesis-disabled phenotype in the original male transgenic fetus was reproduced in its clones (Fig. 1D and Table S2). These findings demonstrate that transgenic pigs expressing Pdx1-Hes1 displayed a pancreatogenesis-disabled phenotype and that somatic cell cloning could faithfully reproduce this phenotype. In addition, they hold out the prospect of large-scale production of such embryos via SCNT from Pdx1-Hes1 transgenic fibroblasts.
Apancreatic Phenotype in Pdx1-Hes1 Cloned Pigs Rescued by Blastocyst Complementation.
Next, we investigated whether in pancreatogenesis-disabled pigs, as in rodents (3), blastocyst complementation could generate pancreata (Fig. 2). Using cloned embryos carrying Pdx1-Hes1 (white coat color) as hosts and cloned embryos carrying the gene encoding orange fluorescent protein humanized Kusabira-Orange (huKO) as donors (7), we produced chimeric embryos (Table S3). Host embryos at the morula stage were injected with ∼10 blastomeres of morula-stage donor embryos (Fig. 3A). Chimeric blastocysts (n = 96) obtained after culture for 1 or 2 d were transferred to the uteri of two estrus-synchronized recipient gilts (Fig. 3B). We obtained 14 late-term fetuses (day 110 or 111) in which we assessed pancreas formation (Fig. 3 C–E and Fig. S2).
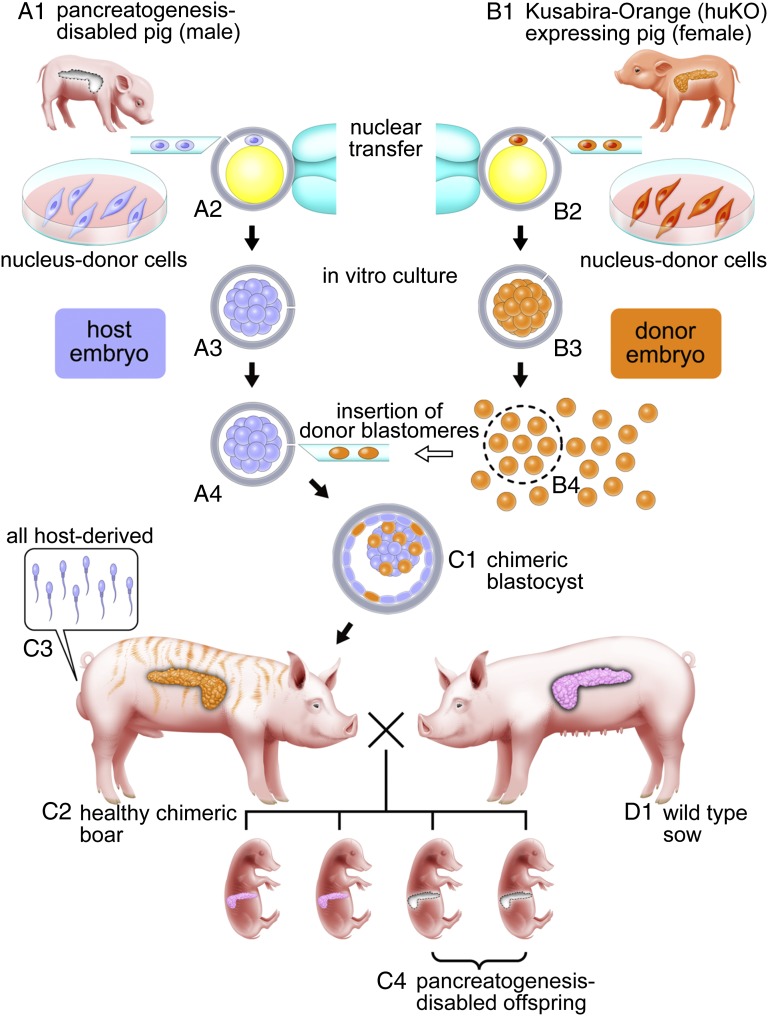
Fig. 2.
Schematic representation of complementation for Pdx1-Hes1 cloned pig embryos with a pancreatogenesis-disabled phenotype using cloned embryos expressing huKO. Primary fibroblast cells as nucleus donor cells for somatic cell cloning were established from a pancreatogenesis-disabled cloned pig with Pdx1-Hes1 transgene expression (A1) and a cloned pig with systemic orange fluorescence conferred by huKO transgene expression (B1). (A2–A4) Host embryos reconstructed by nuclear transfer from male Pdx1-Hes1 transgenic cells yielded pancreatogenesis-disabled piglets. (B2 and B3) Donor embryos were reconstructed by nuclear transfer from female cells expressing huKO. Blastomeres isolated from donor embryos at the morula stage (B4) were inserted into host embryo morulae (A4) to produce chimeric blastocysts (C1) and pigs (C2). (C2) All the chimeric pigs obtained developed into fertile males as a result of intersex chimerism between the male host embryos and female donor embryos. (C3) Sperm of the chimeric boars theoretically originate from male host embryos carrying the Pdx1-Hes1 transgene. After mating of chimeric boars with WT sows (D1), the pancreatogenesis-disabled phenotype of the Pdx1-Hes1 host embryos was transmitted to the next generation (C4).
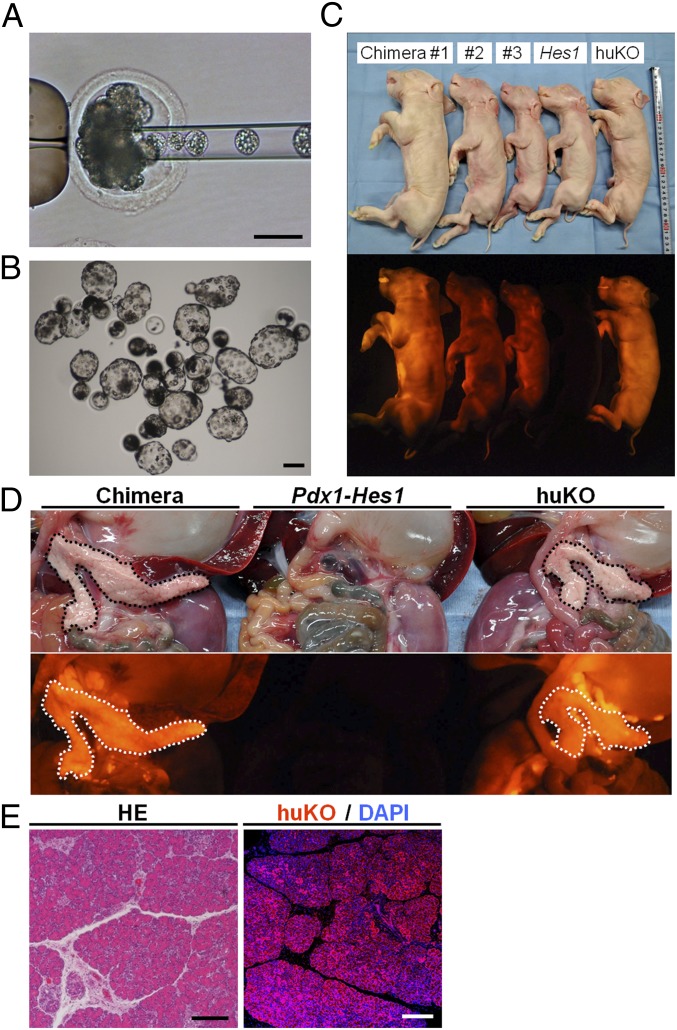
Fig. 3.
Construction of chimeric embryos and fetuses from Pdx1-Hes1 cloned and huKO cloned embryos. (A) Cloned embryo derived from the Pdx1-Hes1 transgenic fetus via microinjection with donor morula blastomeres. (B) Chimeric blastocysts. (C) Full-term chimeric fetuses (chimeras 1–3) and sibling nonchimeric cloned fetuses derived from host (Hes1) or donor (huKO) embryos. Note that nonchimeric fetuses derived from host embryos (Hes1) showed no fluorescence. (D, Center) Fetuses derived from host embryos were pancreatogenesis-disabled. Pancreata of the chimeric fetuses (Left) appeared normal and brightly fluoresced orange throughout, as did pancreata of huKO clone fetuses (Right), indicating that pancreata of the chimeric fetuses were generated from donor embryo cells. (E) Almost all pancreatic tissue of chimeric fetuses stained with anti-huKO antibody. HE, H&E. (Scale bars: A, B, and E, 100 μm.)
In this study, we complemented cloned embryos derived from the male pancreatogenesis-disabled fetus with blastomeres from female cloned embryos expressing huKO fluorescent protein (huKO transgenic embryos). We have confirmed that when male and female embryos are combined to produce a chimeric pig embryo, the chimera develops as a male (8). Fetuses with the host embryo’s male sex that expressed donor cells’ orange fluorescence were accordingly viewed as likely chimeric.
Of the 14 full-term fetuses, 5 male fetuses (35.7%) appeared chimeric because they systemically displayed orange fluorescence derived from donor cells (Fig. 3C, chimeras 1–3). Chimerism was substantiated by detection of Pdx1-Hes1 and huKO sequences on PCR analysis of fetal genomic DNA. The remaining 9 fetuses, 5 male fetuses derived from Pdx1-Hes1 cells and expressing the pancreatogenesis-disabled phenotype (Fig. 3C, Hes1) and 4 female fetuses derived from huKO cells and expressing orange fluorescence (Fig. 3C, huKO), were not chimeric, as substantiated by PCR analysis.
Formation of morphologically normal pancreata in the chimeric fetuses was confirmed by necropsy (Fig. 3D). Orange fluorescence of the donor cells was evident throughout the generated pancreata (Fig. 3D). The weights of the generated pancreata were 0.056–0.102% (average, 0.082%) of respective body weights, values like those for normal pancreata and cloned fetuses at the same gestational age (0.081–0.101%; average, 0.085%) (Table S4). The generated pancreatic tissue was densely cellular; acini had developed, and acinar cells were replete with zymogen granules. Additionally, many islets of Langerhans (“islets”) were scattered throughout the pancreas, as in normal pigs. We concluded that the generated pancreata were histologically and cytologically normal (Fig. 3E). Almost all pancreatic tissues, including islets, acinar tissue, and ducts, stained with anti-huKO antibody (Fig. 3E). This result indicates that almost all pancreatic tissues of the chimeric fetuses were huKO-expressing cells derived from donor cells. Orange fluorescence was observed in skin over the whole body and in every internal organ of these fetuses, indicating that chimerism was present throughout the entire body (Fig. S2A). Immunostaining of skin, lung, and kidney of four chimeric fetuses revealed that ∼40–60% of each sample by area expressed huKO (Fig. S2B).
Chimeric Pigs Generated by Complementation of Pdx1-Hes1 Embryos Have Functionally Normal Pancreata and Grow into Fertile Adults.
To assess whether these chimeric pigs could survive until term, be born alive, and grow normally after birth, we again used Pdx1-Hes1 clone embryos as hosts and huKO clone embryos as donors (Fig. 2). In addition, we used cloned embryos derived from a colored-coat WT Duroc × Berkshire hybrid sow as donors. Blastocyst complementation with either sort of donor gave rise to viable chimeric piglets (Fig. 4 and Tables S5 and S6). Production efficiencies for chimeric piglets were 4 (30.8%) of 13 with huKO and 1 (16.7%) of 6 with hybrid donor embryos. Blastocyst complementation also produced nonchimeric male cloned piglets derived from Pdx1-Hes1 host embryos and nonchimeric female clones derived from donor embryos (Fig. 4A); these were farrowed in the same litters as the chimeric offspring (Fig. 4 A and B and Tables S5 and S6).
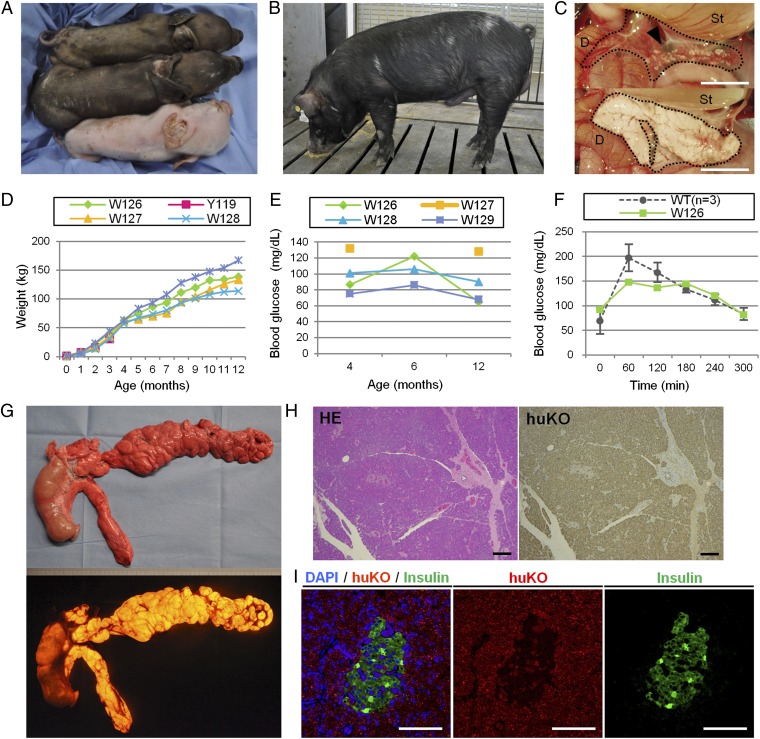
Fig. 4.
Production of chimeric offspring by complementation of Pdx1-Hes1 pancreatogenesis-disabled embryos. (A) Chimeric pig (middle pig) was obtained after complementation of host Pdx1-Hes1 embryos with embryonic cells from a coat-colored WT donor. Sibling nonchimeric cloned pigs were derived from donor (top pig, brown coat) and host (bottom pig, white coat) embryos. (B) Mature chimeric boar exhibits WT (donor) coat-color chimerism. (C) Vestigial pancreas (Upper, arrowhead) of a host embryo-derived cloned piglet and normal pancreas (Lower) of a donor-embryo derived piglet. D, duodenum; St, stomach. (D and E) Normal growth and serum glucose concentrations were observed in chimeric pigs. Pig W127 did not undergo blood sampling at the age of 6 mo due to a leg injury. (F) Serum glucose concentrations in a chimeric pig (W126) and WT pig during oral glucose tolerance testing. (G) Normally formed pancreas generated in a chimeric pig (W128) exhibits orange fluorescence derived from the donor huKO embryo. (H) Histological appearance of pancreas generated in a chimeric pig, with staining throughout by anti-huKO antibody. HE, H&E. (I) Islet of Langerhans in the pancreas generated in a chimeric pig marks immunohistochemically for insulin. (Scale bars: C, 1 cm; H and I, 100 μm.)
Two of the 10 nonchimeric Pdx1-Hes1 cloned piglets produced were stillborn. These animals were confirmed at necropsy to be pancreatogenesis-disabled with vestigial pancreata, as observed in full-term Pdx1-Hes1 cloned fetuses (Fig. 4C, Upper). Two of the 8 surviving newborn piglets had undetectably low serum insulin concentrations. Their serum glucose concentrations were 12 and 33 mg/dL; these levels suggest a hypoglycemic condition common in newborn pigs (9).
These piglets were killed after blood collection. Of the six other piglets, four died within 24 h but two survived for 3 d with serum glucose concentrations >600 mg/dL. These piglets also had undetectably low serum insulin concentrations. Their body weights fell drastically, and clinical-biochemistry findings strongly suggested severe catabolism (Table S7). The pancreatogenesis-disabled status of all Pdx1-Hes1 cloned piglets in the litter was confirmed at necropsy.
In contrast to the pancreatogenesis-disabled piglets, the chimeric piglets developed normally (Fig. 4 B and D). As shown in Fig. 4E, every chimeric pig had normal serum glucose concentrations at the ages of 4, 6, and 12 mo. Clinical-biochemistry findings at the ages of 6 and 12 mo supplied no evidence for pancreatic, hepatobiliary, or renal injury, and serum electrolyte concentrations were all normal (Table S8). Oral glucose tolerance test results in one of the chimeric pigs were also normal (Fig. 4F).
At necropsy of a healthy-appearing 12-mo-old chimeric boar killed to examine its intestinal organs, macroscopic examination found no abnormalities. The entire pancreas distinctly fluoresced orange (Fig. 4G). The pancreas (Fig. 4H), the duodenum, and the extrahepatic biliary tract lacked any histopathological abnormalities. On immunostaining for huKO, nearly every pancreatic cell marked for huKO (Fig. 4 H and I), demonstrating that these cells were derived from donor embryos, as seen in the chimeric fetuses. All 19 organs and tissues examined, including testes and brain, contained huKO-expressing cells.
The five chimeric piglets farrowed by three recipient sows all grew normally (except for one, which died accidentally at weaning) and reached sexual maturity ∼7–8 mo after birth. The sexually mature chimeric boars all could mate with WT sows in estrus, and all proved fertile. By examining the fetuses sired by the chimeric boars, we confirmed that the pancreatogenesis-disabled phenotype had been transmitted to their progeny (Fig. 2 and Fig. S3). Furthermore, the chimeric boars sired no fetuses derived from donor embryos (i.e., expressing huKO, with colored coats). Together, these results indicate that sperm of the chimeric boars were derived from host embryos (i.e., male Pdx1-Hes1 clone embryos). They are consistent with earlier failure of intersexual chimeras produced with male and female embryos to generate sperm from cells with an XX chromosomal complement in pigs (8) and mice (10, 11).
Discussion
In this study, we demonstrated that generation of organ-deficient livestock animals is possible and that in pigs, as in rodents, a functional organ derived from exogenic pluripotent cells can be formed when organogenesis-disabled embryos are complemented by allogenic blastomeres. The pancreata derived from exogenic pluripotent cells were normal in their configuration and functions. Thus, the results clearly indicated that the principle we established in rodents also holds in large animals: If an empty developmental niche for an organ is provided, PSC-derived cellular progeny can occupy that niche, producing the appropriate organ in the vacant space. Although blastocyst complementation has been used to study development of a number of tissues and organs (3, 4, 12–14), all these studies were conducted in rodents. In this study, we demonstrate generation of a functional organ from exogenic pluripotent cells in a large animal, which is a very important step toward generation of human organs in large animals.
The chimeric pigs thus generated survived to adulthood, providing a source of sperm for large-scale expansion and production of pancreatogenesis-disabled embryos. Similarly, fibroblasts obtained from the apancreatic pig fetuses can also serve as a source of cloned embryos harboring a pancreatogenesis-disabled phenotype for complementation. SCNT technology thus has resolved initial difficulties associated with the use of large animals, such as pigs, allowing in vitro generation of viable host embryos for blastocyst complementation. It enables, in theory, limitless production of pancreatogenesis-disabled cloned embryos from cultured cells.
Now that pancreatogenesis-disabled pig embryos are available, one can investigate whether pancreata can be formed from xenogenic PSCs in the pig environment. Most nonrodent PSCs are, however, at the epiblast stage and cannot contribute to chimera formation (15). Our blastocyst complementation system thus may not work with currently available nonrodent PSCs, and it may require establishment of nonrodent PSCs with chimera-forming capability. Embryos of organogenesis-disabled pigs generated via SCNT-mediated cloning will, however, provide a simple but reliable platform for testing the potential of nonrodent PSCs for chimera formation and organ generation. Successful establishment of xenogenic organogenesis systems (cow into pig or monkey into pig) will certainly contribute to obtaining the knowledge necessary to apply this technology to humans.
Efforts to produce pancreata by complementing pig embryos with human PSCs will raise ethical questions. Generation of human germ cells in chimeras generated by xenogenic blastocyst complementation may not be acceptable, and measures to prevent human PSCs from contributing to reproductive cells in chimeric animals may be in order. Our data and those of others (8, 10, 11) suggest that formation of male germ cells from exogenic cells is suppressed when a male host embryo is complemented with female donor cells. Cell fate control (16, 17) also may permit suppression of formation of “unwanted” cells in animals generated by blastocyst complementation using human PSCs.
Cost could be another issue that needs to be considered. In the case of the pancreas, however, once a large amount of sperm is obtained from the complemented transgenic male pigs, generation of pancreatogenesis-disabled embryos is not expensive. As shown in our rodent study (3), autologous islets engrafted well without immunosuppression. This type of approach will eventually reduce medical costs and increase quality of life for those with end-stage organ failure.
These important considerations aside, the results demonstrated in this study provide the basis for potential future application of blastocyst complementation to generate transplantable human organs using livestock animals. The greatest technical challenge will be overcoming the species barrier to achieve chimerism with pig embryos as hosts.
Materials and Methods
Animal Care.
All animal experiments were approved by the Institutional Animal Care and Use Committee of Meiji University (IACUC07-0005).
Chemicals.
All chemicals were obtained from Sigma–Aldrich unless otherwise indicated.
ICSI-Mediated Gene Transfer.
A Pdx1-Hes1 transgene construct (8.8 kb) was introduced into in vitro-matured porcine oocytes using ICSI-mediated gene transfer (6). Freeze-thawed sperm (3.75 × 105 sperm per 10 μL) were coincubated with 2.5 ng/μL DNA for 5 min at room temperature (RT). An isolated sperm head was then injected into each oocyte using a piezo-actuated microinjection unit (PMM-150FU; Primtech) and micromanipulators (MO-102; Narishige). Oocytes were electrically activated 30 min before sperm head injection. Sperm-injected oocytes were cultured in vitro for 1 or 2 d until transfer to the oviducts of estrus-synchronized gilts. Transgenic fetuses were recovered by laparotomy from pregnant recipients.
SCNT.
SCNT was performed using in vitro-matured oocytes as recipient cytoplasts (7). Cultured primary cells from an apancreatic fetus, a huKO-expressing fetus, and a colored-coat WT pig were used as nucleus donor cells after cell cycle synchronization by serum starvation for 48 h. A single donor cell was inserted into the perivitelline space of an enucleated oocyte. Membrane fusion between the donor cell and recipient cytoplast was induced electrically. The reconstructed embryos were then electrically activated, followed by in vitro culture for 1–6 d and subsequent transfer to estrus-synchronized recipient gilts. To generate apancreatic cloned fetuses and piglets, SCNT embryos at the single-cell or early cleavage stage were transferred into recipients’ oviducts. Chimeric pigs were produced by transferring cloned embryos at the blastocyst stage into recipients’ uteri.
Embryo Manipulation for Blastocyst Complementation.
Embryos for blastocyst complementation were prepared using somatic cell cloning technology (discussed above). Fibroblast cells derived from a male Pdx1-Hes1 transgenic fetus were used for nuclear transfer to produce cloned host embryos. Cloned donor embryos were produced by nuclear transfer using female fibroblasts carrying huKO or female fibroblasts isolated from a colored-coat WT pig.
Donor embryos at the morula stage (day 4) were decompacted with 0.1 mM EDTA-2Na (in Ca2+/Mg2+-free PBS supplemented with 0.01% polyvinyl alcohol) for 20 min, followed by removal of zonae pellucidae by digestion with 0.25% pronase solution (in PBS). Blastomeres were isolated from embryos by gentle pipetting using a finely drawn glass capillary. Host embryos at the morula stage were decompacted similarly. Approximately 10 donor embryo blastomeres were injected into the center of each host embryo using micromanipulation. Injected embryos were cultured in vitro for 24 or 48 h to obtain chimeric blastocysts. Developing blastocysts were surgically transferred to uteri of estrus-synchronized recipients on day 5 or 6 (45–80 blastocysts per recipient).
Detection of Chimerism and Genotyping by PCR.
Genomic DNA was extracted from tail biopsy specimens of fetuses and newborn piglets using the DNeasy Blood and Tissue Kit (QIAGEN). To detect chimerism in pigs by genotyping, DNA samples were analyzed using PCR with specific primers for huKO (donor embryo-derived) and Pdx1-Hes1 (host embryo-derived) transgene sequences. Nested PCR analysis to detect donor embryo-derived DNA was performed using the primers 5′-AGCACGAAGTCTGGAGACCTCTG-3′ and 5′-AGGTGGTCTTGAACTGGCACTTGTG-3′ for the first round of PCR and the primers 5′-ACCTTACACAGTCCTGCAGACC-3′ and 5′-GCCAGCTTCAGGAACATGGT-3′ for the second round of PCR. PCR conditions were 95 °C for 60 s, followed by 25 cycles of 95 °C for 30 s, 68 °C for 30 s, and 72 °C for 60 s. PCR primers used to amplify Pdx1-Hes1 transgene sequences were 5′-CAATGATGGCTCCAGGGTAA-3′ and 5′-TGACTTTCTGTGCTCAGAGG-3′. PCR conditions were 95 °C for 60 s, followed by 30 cycles of 95 °C for 30 s, 60 °C for 5 s, and 72 °C for 60 s.
Histological Analysis.
Tissue samples from normally formed pancreata of chimeric pigs and from vestigial pancreata of Pdx1-Hes1 transgenic pigs were fixed in paraformaldehyde, embedded in paraffin, sectioned, stained, and immunostained as described by Kobayashi et al. (3). Sections stained with H&E and immunostained (anti-insulin and anti-huKO) were examined by light microscopy to detect insulin production by pancreatic islets and huKO expression. Each section was incubated with primary antibody for 0.5–3 h at RT, followed by incubation with secondary antibody for 1 h at RT. Primary polyclonal antibodies against huKO (1:300, PM051; Medical and Biological Laboratories) and insulin (1:500, LS-C24686; LifeSpan BioSciences) were used. The secondary antibodies used were Alexa Fluor 488 goat anti-mouse IgG (1:200; Life Technologies Corporation) and Alexa Fluor 594 donkey anti-rabbit IgG (1:200; Life Technologies Corporation). After antibody treatments, sections were mounted in Vectashield mounting medium (Vector Laboratories) containing DAPI for nuclear counterstaining and observed by confocal laser scanning microscopy (FV1000-D; Olympus Corporation). The distribution of donor-derived cells expressing huKO in chimeric pigs’ tissues was determined in immunostained materials with peroxidase chromogen development (Histofine; Nichirei Biosciences) after hematoxylin counterstaining.
Supplementary Material
Acknowledgments
We thank H. Kadoi, T. Kanai, and T. Matsuda for their help with maintenance of pigs and E. Haruyama, S. Takayanagi, T. Fujiwara, Y. Ikezawa, S. Saito, Y. Takeuchi, K. Honda, and M. Maehara for experimental/technical assistance. We also thank A. S. Knisely for critically reading the manuscript. This work was supported by the Japan Science and Technology Agency, Exploratory Research for Advanced Technology, Nakauchi Stem Cell and Organ Regeneration Project, Tokyo, and Meiji University International Institute for Bio-Resource Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222902110/-/DCSupplemental.
References
- 1.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441(7097):1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 2.MacLaren RE, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444(7116):203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi T, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142(5):787–799. doi: 10.1016/j.cell.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 4.Usui JI, et al. Generation of kidney from pluripotent stem cells via blastocyst complementation. Am J Pathol. 2012;180(6):2417–2426. doi: 10.1016/j.ajpath.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Sumazaki R, et al. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36(1):83–87. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
- 6.Kurome M, Ueda H, Tomii R, Naruse K, Nagashima H. Production of transgenic-clone pigs by the combination of ICSI-mediated gene transfer with somatic cell nuclear transfer. Transgenic Res. 2006;15(2):229–240. doi: 10.1007/s11248-006-0004-5. [DOI] [PubMed] [Google Scholar]
- 7.Matsunari H, et al. Transgenic-cloned pigs systemically expressing red fluorescent protein, Kusabira-Orange. Cloning Stem Cells. 2008;10(3):313–323. doi: 10.1089/clo.2008.0024. [DOI] [PubMed] [Google Scholar]
- 8.Nagashima H, Giannakis C, Ashman RJ, Nottle MB. Sex differentiation and germ cell production in chimeric pigs produced by inner cell mass injection into blastocysts. Biol Reprod. 2004;70(3):702–707. doi: 10.1095/biolreprod.103.022681. [DOI] [PubMed] [Google Scholar]
- 9.Jackson PGG, Cockcroft PD. Handbook of Pig Medicine. Philadelphia: Elsevier; 2007. pp. 128–142. [Google Scholar]
- 10.McLaren A. Sex chimaerism and germ cell distribution in a series of chimaeric mice. J Embryol Exp Morphol. 1975;33(1):205–216. [PubMed] [Google Scholar]
- 11.Mystkowska ET, Tarkowski AK. Observations on CBA-p-CBA-T6T6 mouse chimeras. J Embryol Exp Morphol. 1968;20(1):33–52. [PubMed] [Google Scholar]
- 12.Müller SM, et al. Gene targeting of VEGF-A in thymus epithelium disrupts thymus blood vessel architecture. Proc Natl Acad Sci USA. 2005;102(30):10587–10592. doi: 10.1073/pnas.0502752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445(7130):886–891. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- 14.Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. 2006;11(4):519–533. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Tachibana M, et al. Generation of chimeric rhesus monkeys. Cell. 2012;148(1-2):285–295. doi: 10.1016/j.cell.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawamata M, Nishimori K. Mice deficient in Dmrt7 show infertility with spermatogenic arrest at pachytene stage. FEBS Lett. 2006;580(27):6442–6446. doi: 10.1016/j.febslet.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 17.Mesnil M, Yamasaki H. Bystander effect in herpes simplex virus-thymidine kinase/ganciclovir cancer gene therapy: Role of gap-junctional intercellular communication. Cancer Res. 2000;60(15):3989–3999. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.