Abstract
Motor fatigue induced by physical activity is an everyday experience characterized by a decreased capacity to generate motor force. Factors in both muscles and the central nervous system are involved. The central component of fatigue modulates the ability of motoneurons to activate muscle adequately independently of the muscle physiology. Indirect evidence indicates that central fatigue is caused by serotonin (5-HT), but the cellular mechanisms are unknown. In a slice preparation from the spinal cord of the adult turtle, we found that prolonged stimulation of the raphe-spinal pathway—as during motor exercise—activated 5-HT1A receptors that decreased motoneuronal excitability. Electrophysiological tests combined with pharmacology showed that focal activation of 5-HT1A receptors at the axon initial segment (AIS), but not on other motoneuronal compartments, inhibited the action potential initiation by modulating a Na+ current. Immunohistochemical staining against 5-HT revealed a high-density innervation of 5-HT terminals on the somatodendritic membrane and a complete absence on the AIS. This observation raised the hypothesis that a 5-HT spillover activates receptors at this latter compartment. We tested it by measuring the level of extracellular 5-HT with cyclic voltammetry and found that prolonged stimulations of the raphe-spinal pathway increased the level of 5-HT to a concentration sufficient to activate 5-HT1A receptors. Together our results demonstrate that prolonged release of 5-HT during motor activity spills over from its release sites to the AIS of motoneurons. Here, activated 5-HT1A receptors inhibit firing and, thereby, muscle contraction. Hence, this is a cellular mechanism for central fatigue.
Keywords: movement, spike genesis, input-output gain, movement control
Prolonged physical activity leads to motor fatigue (1, 2). The force produced by muscles decreases in part because of the lack of glycogen (3) in the muscle and/or failures at the neuromuscular junctions (4). In addition to this well-described muscle fatigue, motor fatigue also involves an element originating in the CNS (5–8). This “central fatigue” is characterized by a decreased ability to contract the muscle fibers adequately during a motor activity and is observed independently of the muscle fatigue (6). It is often studied during maximal voluntary contractions (5, 6, 9). Nevertheless, it is also present during weak physical contraction (1, 2, 8, 10–12). Central fatigue secures rotation of motor units (13) and prevents hyperactivity of muscles (6). Elements of central fatigue involve the cortex (6), and the spinal cord at the level of motoneurons (MNs) (5).
The cellular mechanisms responsible for a decrease of the activity of MNs remain unknown. However, evidence suggests that central fatigue correlates with increased levels of serotonin (5-HT) in the CNS. Human subjects that perform an intense motor task are faster exhausted after intake of a 5-HT1A receptor agonist (14) or a selective serotonin reuptake inhibitor (15). In animals, injection of the 5-HT precursor tryptophan in the blood or directly in the CNS accelerates the exhaustion occurring during motor activity (16, 17). Moreover, the time to exhaustion is decreased by serotonin receptor agonists and increased by serotonin antagonists (18). It was also demonstrated that the activity of the raphe-spinal neurons correlates with the motor activity, suggesting that 5-HT is released as a function of motor output (19, 20). Finally, spinal MNs are densely innervated by serotonergic synaptic terminals (21, 22). These results are nonetheless puzzling because of studies that demonstrate that 5-HT promotes the activity of MNs by inhibiting leak conductances (23, 24), Ca2+-activated K+ conductances (25), and by facilitating persistent inward currents (26–32). How can 5-HT boost the activity of MNs and, thereby, muscle contraction and at the same time induce central fatigue? We have undertaken this study to resolve this paradox. We confirm that the excitability of MNs is increased by low levels of serotonin release. However, during high levels of release, 5-HT spills over to reach extrasynaptic receptor sites in the axon initial segment and inhibits the generation of action potentials (APs). This inbuilt arrangement prevents the hyperactivity of MNs, promotes motor unit rotation, and reduces detrimental muscle activity.
Results
Release of Endogenous Serotonin Has Multiple Effects.
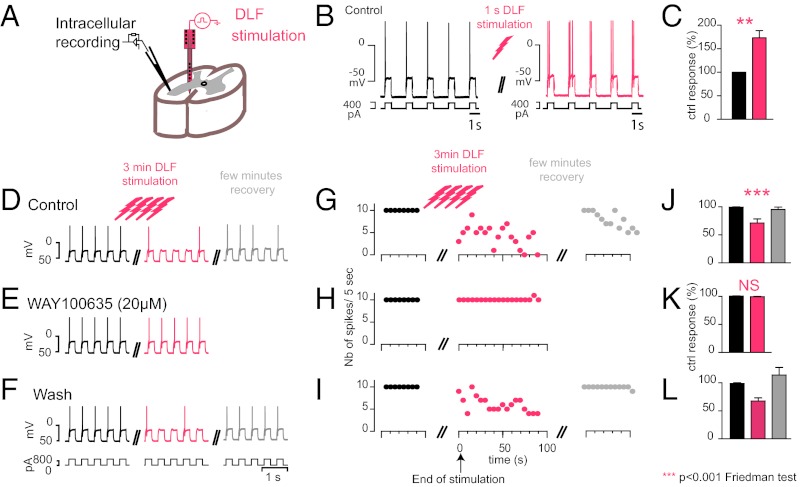
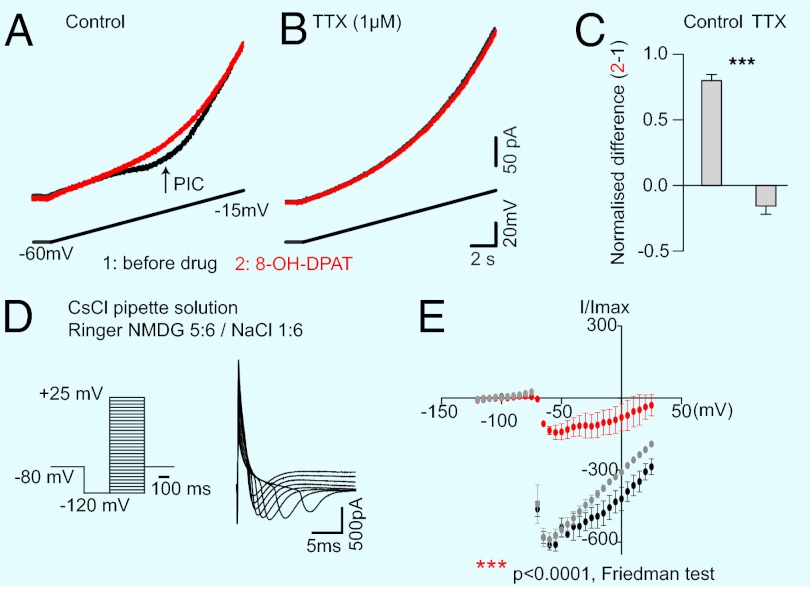
We tested the effects of release of endogenous 5-HT on a thick slice (1.5 mm) preparation from the spinal cord of the adult turtle spinal cord. We monitored the excitability of MNs by injecting intracellular depolarizing current pulses before and after stimulation of the raphe-spinal pathway located in the dorsolateral funiculus (DLF; Fig. 1A) (22), in the presence of blockers for fast glutamate, GABA, and glycine synaptic transmission (SI Materials and Methods). In agreement with previous studies (26, 31, 33), we found that a brief stimulation of the DLF (1 s at 10–40 Hz) increased the excitability of MNs because the number of APs generated by depolarizing current pulses was significantly increased (Fig. 1B; from 1.00 to 1.73 ± 0.15; Wilcoxon test; n = 15 pairs). This effect is caused by the activation of somatodendritic 5-HT2 receptors, which facilitate a persistent inward current (PIC) mediated by L-type Ca2+ channels (31, 33). Because the rate of discharge of raphe neurons is correlated with motor activity (19), we tested the effect of a prolonged DLF stimulation, mimicking the release of 5-HT occurring during long efforts. In that case, the facilitation of MN activity was substituted by a decrease in excitability (Fig. 1 C, F, and L; n = 6). The switch from excitatory effects and inhibitory effect occurred after 30 s (Fig. S1). The inhibitory effect was abolished by the selective 5-HT1A receptor antagonist N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide (WAY-100635) (20 µM) (34) in a reversible manner (Fig. 1 D–L; n = 6). These data show that besides the facilitatory effect induced by 5-HT2 receptors, 5-HT has an inhibitory effect that involves 5-HT1A receptors. Serotonin has a higher affinity for 5-HT1A receptors than for 5-HT2 receptors (35). This fact, combined with the observation that the inhibitory effect requires a stronger synaptic stimulation, suggests a differential location of the two types of receptors. To confront this hypothesis, we activated 5-HT1A receptors focally.
Fig. 1.
Dual modulation of MNs firing induced by stimulation of the raphe spinal pathway. (A) Experimental setup. (B) Intracellular recording of a MN in control (black) and after a stimulation of the DLF during 1 s at 40 Hz (pink). (C) The excitability, estimated by counting the number of APs evoked by depolarizing current pulses injected in the soma, was increased (mean firing frequency after stimulation normalized to control 1.73 ± 0.15, P = 0.0027, two-tailed Wilcoxon test), whereas the membrane potential remained unchanged. (D–F) A 3-min stimulation (40 Hz) induced a significant and reversible inhibition of the motoneuronal firing (F; P = 0.0001, two-tailed Friedman test, n = 3 slices). (G–I) This effect was blocked in the presence of WAY-100635 (20 µM) (I; NS, nonsignificant, P = 0.1389; two-tailed Wilcoxon test, n = 3 slices) in a reversible manner (J–L; n = 2 slices).
Motoneurons Firing is Inhibited by 5-HT1A Receptors.
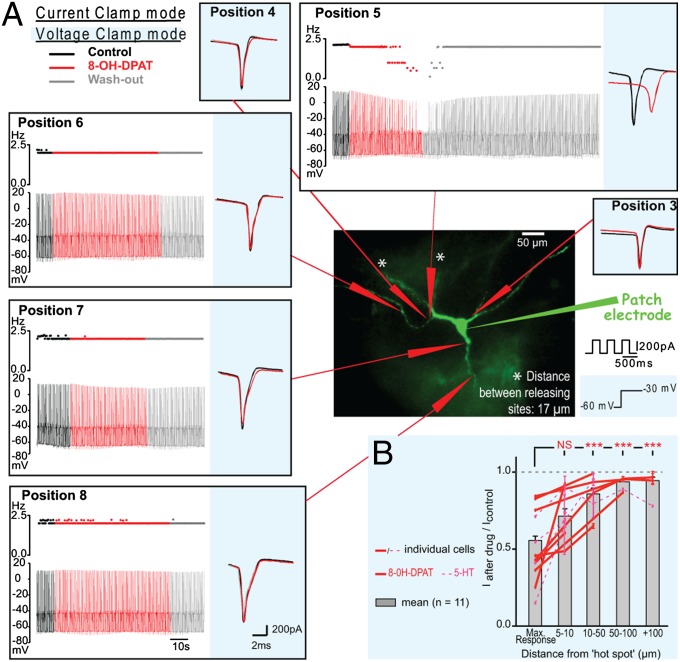
We recorded MNs with the whole-cell patch clamp technique in thin spinal cord slices (300 µm thick). A fluorophore present in the pipette solution (Alexa 488) was used to visualize the dendritic arborization under an epifluorescent microscope (Fig. 2A) during the recording. We tested the effect of a focal activation of 5-HT1A receptors with a pipette filled with the 5-HT1A/7 receptor agonist (±)-2-dipropylamino-8-hydroxy-1,2,3,4-tetrahydronaphthalene hydrobromide (8-OH-DPAT) that was released by means of microiontophoresis. We examined the focal effect of 8-OH-DPAT (40 mM) on APs evoked by trains of brief depolarizing pulses (Fig. 2A, white background) in the presence of antagonists for ionotropic glutamate/GABA/glycine receptors and of [R]-3-[2-(2-[4-methylpiperidin-1-yl]ethyl)pyrrolidine-1-sulfonyl]phenol (SB269970) (10 µM), a selective antagonist for 5-HT7 receptors. We found that when the agonist was applied at one particular “hot spot” near the soma, it prevented the genesis of APs (Fig. 2A; n = 4). The failures of APs were illustrated by the decrease in firing frequency (Fig. 2A, position 5) (similar results were observed when a repetitive firing was evoked by a prolonged depolarizing current pulse, n = 3; Fig. S2). The APs that were not abolished were reduced in amplitude in the four cells tested (Fig. 2 A and B; P < 0.0001, Wilcoxon test). The effect was powerful because MNs remained silent for several seconds after the end of drug application (Fig. 2A). However, it was possible to rescue the firing by increasing the intensity of the stimulation (Fig. S3A), showing that the ability to generate spikes was only reduced.
Fig. 2.
Location-sensitive inhibition of MNs by 5-HT1A receptors. (A) I-clamp (white background) and V-clamp (blue background) recordings from a MN during application of 8-OH-DPAT (40 mM) at different spots on the membrane. The excitability was either tested by depolarizing current pulses (I-clamp; each pulse induced two spikes in control; black) or voltage pulses from −60 mV to −20 mV (V-clamp; each step induced a transient inward current). The release at various positions had no effect except at position 5, where there was a decrease in the excitability. The effects were reproduced at the end of the experiment by releasing the drug at position 5 once more. (B) Normalized amplitude of the reduction of the transient inward current as a function of the distance between the releasing site and the hot spot. Data points for individual cells are linked (8-OH-DPAT: red lines, n = 7; 5-HT: dashed pink lines, n = 4). The evoked inward currents were significantly reduced at the hot spot (normalized mean current amplitude: 0.56 ± 0.03) and 5–10 µm away (0.71 ± 0.05). The effect was significantly stronger when the drug was applied at the hot spot than when it was applied further than 10 µm away (0.91 ± 0.02, P < 0.0001, one-way ANOVA, n = 71 releasing sites, ***P < 0.0001, Tukey’s post hoc test).
The dramatic effect on the spike initiation was observed only when the agonist was puffed at one hot spot in the vicinity of the soma. In contrast, 8-OH-DPAT did not affect the firing at any of the many other membrane spots tested, although it facilitated a small outward current (Fig. S3B). None of the AP parameters were affected when the drug was applied at other sites (Fig. 2A). Similar results were obtained when 5-HT was ejected from a pipette by pressure (Fig. 2B; n = 4; see Fig. S4), ruling out the possibility of an artifact induced by microiontophoresis. For each MN, the analysis revealed a distinct hot spot at which the activation of serotonergic receptors had a strong effect (Fig. 2B; mean amplitude ratio: 0.56 ± 0.03, n = 12 MNs). When the drug was released on the membrane 5–10 µm away from this hot spot, the effect became weaker (mean ratio: 0.71 ± 0.05) and it was significantly reduced when applied further away from the hot spot (mean ratio: 0.91 ± 0.02; see Fig. 2A for illustration; no difference in ratio were revealed by a one-way ANOVA between values at 10–50 vs. 50–100 vs. >100 µm, P = 0.18, n = 38). We verified that the drug had no effect on remote receptors by applying it 10 µm away from the membrane: Minimal effect was observed, even during prolonged releases of 8-OH-DPAT (Figs. S5 and S6).
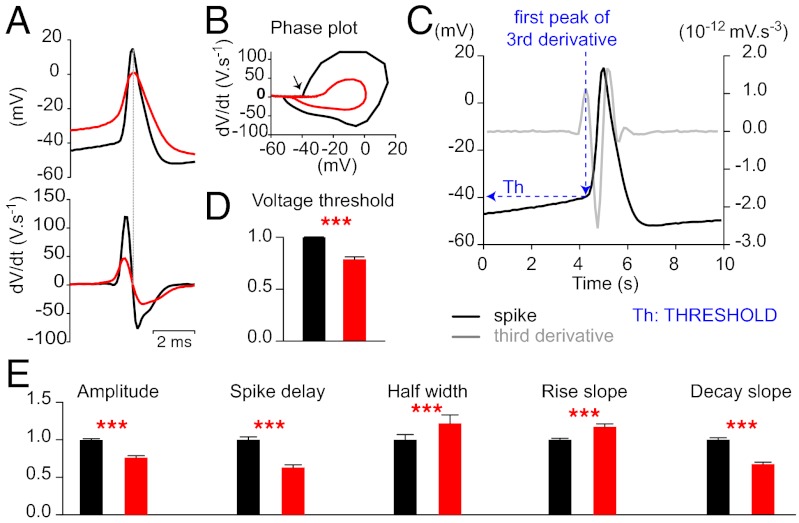
To analyze the modulation further, we measured the spike threshold (Fig. 3C) characterized as the sudden acceleration of rate-of-change of voltage in a phase plot of the first derivative of the membrane potential as a function of the voltage (arrow on Fig. 3B). The application of 8-OH-DPAT (40mM) increased the spike threshold significantly (P < 0.0001; Wilcoxon test; n = 40 pairs) without changing the resting membrane potential (−62.8 ± 0.56 mV in control; −62.6 ± 0.50 mV after drug application; P = 0.0686; Wilcoxon test, n = 80). Moreover 8-OH-DPAT delayed the AP onset (Fig. 3E, P < 0.0001, Wilcoxon test, n = 47 pairs) and increased the spike half-width (P < 0.0001, Wilcoxon test, n = 38 pairs). It also decreased both the rates of rise and fall of the AP (Fig. 3E; P < 0.0001, Wilcoxon test, n = 39 and 56, respectively). Altogether, these results indicate a dramatic inhibitory effect of 8-OH-DPAT on the AP itself.
Fig. 3.
The 5-HT1A receptors at the hotspot inhibit APs. (A Upper) Overlap of a spike before (black) and after drug at the hot spot (red). The traces were adjusted so that the spikes overlapped. The induction of the spike after drug was delayed. (A Lower) First derivative of the traces. (B) Phase plot of the first derivative as a function of the membrane potential. (C) The spike threshold was measured according to the method described in Henze and Buzsaki (50). The third derivative of the spike was calculated by using the software GraphPad Prism version 5. The membrane potential that corresponded to the first positive peak of this derivative was used as the value of threshold. The threshold was used as the baseline value for the measurements of the AP amplitude. (D) The spike threshold (arrow on B) was significantly increased by 8-OH-DPAT (40 mM) for each cell (P ≤ 0.0005, paired t test) and for all of the MNs taken together (control: 1.00 ± 0.003 mV, after drug: 0.79 ± 0.02 mV; ***P < 0.0001, Wilcoxon test, n = 40). (E) The amplitude of the spikes (control: 1.00 ± 0.03 mV; after drug; 0.68 ± 0.02 mV) and the rising slope (control: 1.00 ± 0.04 V⋅s−1, after drug: 0.63 ± 0.04 V⋅s−1) were decreased by the drug (P < 0.0001, Wilcoxon test, n = 40). The latency (control: 1.00 ± 0.02 ms, drug: 1.17 ± 0.04 ms; n = 84) and half-width of APs (control: 1.00 ± 0.07 ms, drug: 1.22 ± 0.11 ms, n = 47) were increased by 8-OH-DPAT (P < 0.0001, Wilcoxon test).
In voltage clamp mode, we examined the effect of 8-OH-DPAT on the transient inward current resulting from an unclamped AP evoked by a voltage step to −20 mV from a holding of −60 mV (Fig. 2A, blue background). In agreement with the observations made in current clamp, we found that when 40 mM 8-OH-DPAT was applied at the hot spot, the transient inward current was delayed, its kinetic was slowed, its amplitude was reduced (Fig. S6) and, in 4 cells of 14, the voltage threshold for evoking the transient current was increased (Fig. S7). Here again, the effect was restricted to the hot spot. When the drug was applied a few micrometers away from the hot spot, it did not have any measurable effect (position 4 on Fig. 2A). The effect of 8-OH-DPAT was abolished when the selective 5-HT1A receptor antagonist (20 µM) was bath applied (Fig. S8; n = 2).
Serotonin Inhibits AP Genesis.
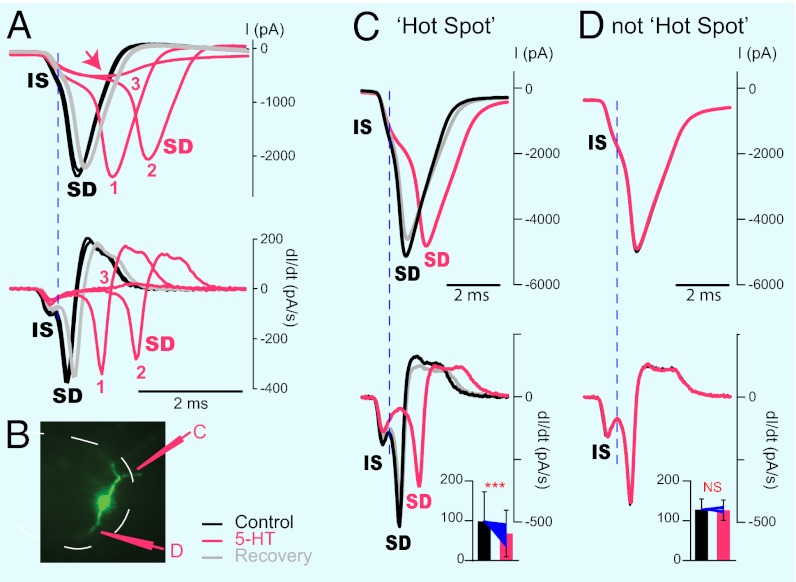
We then checked whether the activation of 5-HT1A receptors at the hot spot had an effect on the spike initiation. When antidromic APs are triggered in MNs by stimulation of motor nerves, it is sometimes possible to distinguish a double inflection on the rising phase of the spike. The early phase corresponds to the spike generated at the axon initial segment (AIS) (initial spike; IS), whereas the late phase, which can fail in an all or none manner, is caused by the back-propagating spike occurring in the somato-dendritic (SD) compartment (36–38). In current clamp mode, spikes evoked by a depolarizing pulse applied in the soma did not show any obvious IS component, probably because of the very fast activation of the SD spike occurring in a depolarized soma. For this reason, we evoked unclamped APs in voltage clamp mode by applying depolarizing voltage steps with an amplitude adjusted a few millivolts above the threshold. Under these conditions, we could clearly distinguish two components on the initial part of the inward current (n = 15) (Fig. 4 A, C, and D). The differentiated current trace revealed an early and a delayed peaks that were presumably corresponding to the IS and SD spikes (Fig. 4 A and C, lower traces). To substantiate this interpretation, we puffed enough 5-HT (15 mM) at the hot spot to induce failures of the AP (Fig. 4A). The differentiated current trace clearly showed that the initial component of the spike decreased gradually without changing its latency, whereas the SD component occurred later until it disappeared in an all or none manner (Fig. 4A, trial 3). This observation confirmed that the first component corresponds to the IS and the second to the SD spike. For all of the MNs tested, we found that 5-HT or 8-OH-DPAT applied on the hot spot inhibited the IS spike (Fig. 4C; P < 0.0001; Wilcoxon test; n = 55 spikes). When the puff/iontophoresis electrode was moved to other points of the membrane, it had no effect on the IS component of the spike (Fig. 4D) (P > 0.05; Wilcoxon test, n = 19 spikes). These observations confirmed that, at the hot spot, 5-HT inhibits the initiation of the AP.
Fig. 4.
The current inhibited by 5-HT (15 mM) is responsible for the genesis of APs. (A Upper) Voltage clamp recording of a MN before (black), during (pink: 1, 2, 3), and after puff ejection of 5-HT at the hot spot. (A Lower) First derivative of the current trace. Two peaks were clearly present. Serotonin induced a reduction of the amplitude of the initial peak without changing its latency, whereas it delayed the second peak and eventually annihilated it (3). The peaks correspond to the IS and the SD spike. (B) Picture illustrating the locations of the 5-HT puff electrode. (C Upper) Voltage clamp recording of another MN before, during, and after puff ejection of 5-HT at the hot spot. (C Lower) First derivative of the current trace. The IS spike was inhibited by 5-HT in a reversible manner. Histogram, mean effect of 5-HT or 8-OH-DPAT on the IS peak of the differentiated current trace. Both 5-HT and 8-OH-DPAT significantly inhibited the IS spike (***P < 0.0001, Wilcoxon test, n = 55 spikes). Blue lines, individual measurements normalized to the mean value in control conditions. (D) Recording of the same MN when 5-HT was puffed at another point of the membrane. There, 5-HT had no effect on the IS spike. Histogram, mean effect of 5-HT or 8-OH-DPAT when released at other points of the membrane. No significant difference (P > 0.05; Wilcoxon test; n = 19 spikes).
The 5-HT1A Receptors at the Hot Spot Inhibit a Na+ Conductance.
We then tested whether 5-HT1A receptors inhibited a Na+ conductance. MNs are the largest cells from the central nervous system. For this reason, it is virtually impossible to clamp APs. Fortunately, the Na+ channels responsible for AP genesis can also produce a PIC (39). We therefore tested the effect of 8-OH-DPAT (40 mM) on slow depolarizing voltage ramps. In control conditions, the current trace was characterized by a typical negative inflection corresponding to a PIC (Fig. 5A). The release of 8-OH-DPAT at the hot spot inhibited the PIC (red trace in Fig. 5A; n = 6; mean decrease: 44.51% ± 3.67% of the total Na PIC; P = 0.0005; Wilcoxon test, n = 4 cells; in presence of 10 µM SB269970, n = 5). This PIC remained in the presence of the L-type Ca2+ channel antagonist nifedipine (10 µM, n = 2, Fig. 5A) but disappeared in the presence of tetrodotoxin (TTX, 1 µM; Fig. 5B; n = 4). Under this condition, 8-OH-DPAT had no more effect on the current (n = 4), demonstrating that 5-HT1A receptors at the hot spot inhibit a Na+ current.
Fig. 5.
The 5-HT1A receptors at the hotspot inhibit a sodium current. (A) Recording of a MN in voltage clamp mode. A depolarizing ramp (lower trace) evoked a persistent inward current (PIC, arrow) in control (black, trace 1) that was inhibited by 8-OH-DPAT (40 mM) released at the hotspot (red trace 2). (B) When TTX (1 µM) was bath applied, the PIC was not present anymore and 8-OH-DPAT had no effect anymore. (C) Normalized difference (2-1) of current before (1) and after 8-OH-DPAT (2) in control (0.80 ± 0.23) and in 1 µM TTX (0.16 ± 0.31, Wilcoxon test, ***P < 0.0001). (D Left) Experimental protocol. (D Right) Examples of inward currents evoked in control at different voltage steps. (E) IV curve of the mean amplitude of currents evoked in the same cell as in D. The agonist reduced the amplitude of the currents (P < 0.0001, Friedman test, n = 3 cells; the decrease was also significant for each cell considered individually; P < 0.0001, Friedman test, n = 180).
To analyze the modulation further, we isolated the Na+ current evoked by voltage steps (Fig. 5D). We optimized the space-clamp by using a CsCl patch solution and replacing five-sixth of the NaCl in the Ringer by N-methyl-D-glucamine. The 8-OH-DPAT (40 mM) applied at the hot spot decreased the amplitude of the transient inward current (Fig. 5E; P < 0.0001, Friedman test, n = 3; same result obtained for each three cells recorded). More importantly, it diminished the normalized amount of charges crossing the membrane from 0.973 ± 0.011 in control to 0.64 ± 0.08 after 8-OH-DPAT (0.95 ± 0.04 after recovery, P = 0.001, repeated-measures ANOVA followed by Tukey post hoc test, n = 18; control vs. drug q = 7.004 and P < 0.01, control vs. recovery q = 0.6873 and P > 0.05, drug vs. recovery q = 6.316 and P < 0.01), demonstrating that the overall effect was inhibitory.
Serotonin Inhibits AP Genesis at the Axon Initial Segment of MNs.
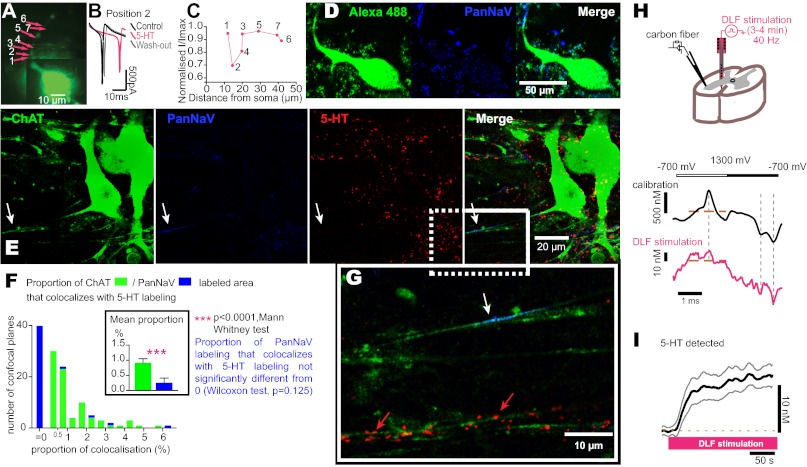
To determine the nature of the hot spot, we filled the recorded MNs (Fig. 6A) with biocytin and visualized them after fixation (Fig. 6D), by a binding to streptavidin–fluorophore. Immunohistochemical labeling of the voltage-sensitive Na+ channels with a specific antibody (Pan Na+ Channels, PanNaV, 1/250) revealed that the hot spot was the AIS because this was the only region with a high density of Na+ channels (Fig. 6 A–D, n = 4).
Fig. 6.
The 5-HT1A receptors that inhibit APs are located at the AIS of MNs. (A) Picture of a MN taken during the recording. The arrows indicate different spots where 5-HT (15 mM) was released. (B) 5-HT inhibited the inward current evoked by voltage steps (from −60 to −20 mV). (C) Normalized effect on the current amplitude plotted against the distance of the releasing site from the soma. The numbers correspond to the releasing sites in A. (D) Postrecording immunohistochemical labeling of the same MN as in A. The merge revealed that the hot spot was located on the AIS. (E) Single confocal plane of a triple immunohistochemical labeling of two other MNs with antibodies directed against ChAT, PanNaV, and 5-HT. PanNaV labeling stained the AIS of the MN on the left (arrow). 5-HT revealed the presence of serotonergic boutons. The merge showed no 5-HT boutons at the AIS. (G) Magnified fluorescent picture corresponding to the rectangle in the merge picture in E. The white arrow points at the AIS. Red arrows show 5-HT bouton-like punctuate staining on a dendrite. (F) Histogram of the proportion of image area labeled by anti-ChAT (green) or anti-PanNaV (blue) that is colabeled with anti-5HT; n = 3 animals, 10 slices, 83 pictures for 14 AIS observed. The analysis of the PanNaV labeling was restricted to motoneuronal AIS. (F Inset) Mean proportion of 5-HT stained areas on ChAT labeled MNs (0.92 ± 0.13), and on PanNaV labeled AIS (0.25 ± 0.16) are significantly different (P < 0.0001, two-tailed Mann–Whitney test). The mean proprotion of PanNaV labeling that colocalized with 5-HT labeling was not significantly different from zero. (P = 0.125, Wilcoxon test). (H) Experimental procedure. (H Lower) Cyclic voltammograms recorded with a calibrating solution of 500 nM 5-HT (black) and in the ventral horn of a slice during a prolonged stimulation of the DLF. The top line shows the oxidation (empty rectangle) and reduction (solid rectangle) phases of the voltammogram. The vertical dashed lines show the three characteristic peak currents for 5-HT oxidation (∼600 mV) and reduction (∼0 mV and −600 mV, vs. Ag/AgCl). The horizontal brown lines indicate the baseline from which detected concentrations are measured. (I) Mean peak 5-HT signals as identified in H during a prolonged stimulation of the DLF (n = 8 recording sites in three animals).
We assessed the innervation of the AIS by 5-HT boutons. In contrast to the dense 5-HT puncta near somatodendritic compartments (Fig. 6 E and G), we found that the AIS was devoid of 5-HT bouton-like punctate stainings. This observation suggested that 5-HT1A receptors that modulate the Na+ current at the AIS are activated only if 5-HT spills out to reach sites beyond its boutons. To explore the question of a 5-HT spillover in the ventral horn of the spinal cord (i.e., where MN somata and their AIS are localized; ref. 21), we placed a carbon-fiber electrode in the lateral part of the ventral horn of a slice and detected extracellular 5-HT by cyclic voltammetry (Fig. 6H). We mimicked a physiological discharge of raphe neurons by stimulating the DLF at a frequency of 40 Hz (same protocol as in Fig. 1D). After a few seconds of stimulation, the concentration of 5-HT increased from an undetectable control level to 12.3 ± 0.2 nM (Fig. 6I; mean time to half-maximal concentration: 23.7 s; n = 8), demonstrating a spillover to the extrasynaptic space in the MN pool.
Discussion
Our data show that 5-HT released during low activity in the raphe-spinal pathway promotes the excitability of MNs. However, our data suggest that during prolonged activity, 5-HT spillover reaches the AIS where it activates 5-HT1A receptors, which inhibit the generation of APs in MNs and, in turn, muscle contraction (Fig. S9). These results provide a cellular mechanism for central fatigue.
The fatigue is caused by a failure of spike generation in MNs induced by stimulation of the DLF. Three arguments suggest that the stimulation induced a synaptic release of 5-HT. (i) Anatomical studies demonstrated that the raphe-spinal pathway is located in the DLF (22). (ii) The inhibition of firing induced by DLF stimulation was reversibly blocked by the selective 5-HT1A receptor antagonist WAY-100635 (40) (Fig. 1 G–I). (iii) The stimulation induced an increase of the extracellular concentration of 5-HT detected by cyclic voltammetry (Fig. 6I). Importantly, the concentration of 5-HT detected (approximately 12 nM) is well within the concentration range (10−9 M) required for the activation of 5-HT1A receptors (35, 41). The involvement of 5-HT1A receptors is confirmed by the direct modulation induced by 8-OH-DPAT in the presence of the 5-HT7 receptor antagonist SB269970 (Fig. 2).
Our finding is that the modulation activated by 5-HT1A receptors is restricted to a hot spot of membrane identified as the AIS. Our interpretation is supported by several observations. (i) The hotspot is always located on one neurite emerging from the soma of the MN. (ii) The release of 5-HT or 5-HT1A receptor agonist inhibits spike initiation (Fig. 4) by selectively modulating a current mediated by Na+ channels (Fig. 5). (iii) The hotspot is characterized by a high density of Na+ channels (Fig. 6). It was recently reported that exogenous application of dopamine at the AIS regulates the firing of brainstem interneurons by inhibiting low threshold voltage-gated calcium channels (42, 43). These observations suggest that the AIS is a plastic structure that can be adjusted to the environment within seconds. Here, we demonstrate directly that such modulation can be induced by endogenous transmitter, in the absence of any pharmacological manipulation (Figs. 1 and 6 H and I). In other cells, 5-HT1A receptors were reported to inhibit a Na+ current (44, 45) via the cAMP-PKA system (44). This intracellular pathway, which might be specific for the AIS, is consequently the most likely candidate.
It is also remarkable that the modulation appears to be due to spillover of 5-HT. This interpretation is supported by three observations. (i) We have shown that the effect induced by microiontophoresis/puff is focal (position 4 on Fig. 2A and Figs. S4 and S5). (ii) Immunohistochemical staining revealed no serotonergic boutons at the AIS, in contrast to the dense innervation at the soma and dendrites (Fig. 6 E–G). (iii) We demonstrated directly that the electrical stimulation of the raphe-spinal pathway increased the extracellular concentration of 5-HT in the MN pools (Fig. 6 H and I).
Under physiological conditions, APs in a MN induce contraction in the muscle fibers innervated. Inhibition of firing will therefore decrease the force generated, similar to what occurs during central fatigue (6). The mechanism we uncovered shares many aspects of the phenomenology of central fatigue. Several studies have shown that an increase in the central concentration of 5-HT leads to fatigue (46, 47). Our results are also consistent with pharmacological studies performed in humans. It was demonstrated that oral intake of the 5-HT1A receptor agonist buspirone or the selective serotonin reuptake inhibitor paroxetine trigger faster exhaustion during motor tasks (14, 15). Moreover, during central fatigue, the gain of MNs is reduced (5, 8). However, we do not rule out that extraspinal mechanisms (1, 2) also contribute to these observations in humans.
Serotonin modulates MNs in several ways. Most in vitro studies performed with bath applied drugs report that 5-HT promotes the excitability of MNs by modulating resting conductances (23), the medium after hyperpolarization following APs (25) or persistent inward currents mediated by Ca2+ (28, 31, 33) or Na+ channels (27). Most of these modulations are caused by the activation of 5-HT2 receptors located in the somatodendritic compartments. It is essential that the modulation of intrinsic properties remains within a limited range, otherwise MNs would become hyperexcitable and symptoms such as spastic muscle contraction would occur. This phenomenon is indeed what happens after spinal cord injuries, when, in the absence of endogenous 5-HT, 5-HT2 receptors become autoactive (48). The mechanism we uncovered in this study may provide a way to prevent hyperexcitability of MNs under physiological conditions. Because the AIS is the site for generation of APs in MNs (49), it acts as gatekeeper. By inhibiting the generation of APs, 5-HT bypasses facilitatory up-stream modulation in the somatodendritic region.
Serotonin activates both facilitatory 5-HT2 receptors on somatodendritic compartments and inhibitory 5-HT1A receptors at the AIS. The lack of serotonergic innervation on this latter compartment provides an inbuilt mechanism that boosts MN excitability at low levels of release and automatically reduces output by spillover at high levels of activity.
Materials and Methods
For detailed methods, see SI Materials and Methods.
Adult turtles (Chrysemys scripta elegans) were anesthetized by propolipid (0.3 mL/100 g) or ketamine (100 mg/kg) and pentobarbital (50 mg/kg) and decapitated. All procedures complied with the Danish legislation and the guidelines of the University of Oxford for the voltammetry experiments. All slices were prepared from the lumbar enlargement of the spinal cord and kept in cold Ringer. Patch clamp recordings were performed while visualizing the neurons under an upright fluorescent microscope. The patch pipette solution (122 mM K-gluconate, 2.5 mM MgCl2, 5.6 mM Mg-gloconate, 5 mM K-Hepes, 5 mM HEPES, 5 mM Na2ATP, 1 mM EGTA, and 2.5 mM biocytine; K-OH to adjust the pH to 7.4) contained a fluorescent dye to stain the dendritic arborization during whole-cell recordings. Drugs were applied via bath, microiontophoresis, or pressure ejection. Neuronal electrical activity was recorded with a Multiclamp 700B amplifier. The dorsolateral funiculus was stimulated extracellularly with a multiple-channel electrode inserted into the slice. At the end of an experiment, slices were fixated with 1% paraformaldehyde for 30 min and then stained with primary antibodies directed against ChAT (Choline Acetyltransferase, 1/50) and PanNaV (1:250) during 6 d and later secondary antibodies coupled to fluorescent dies. The fluorescent labeling was visualized under a confocal microscope. Evoked extracellular 5-HT signals were detected by a carbon-fiber electrode placed in the lateral part of the spinal cord slices by using fast-scan cyclic voltammetry. Data are presented as mean ± SEM. Nonparametric tests were used when a Gaussian distribution could not be approximated.
Supplementary Material
Acknowledgments
We thank Hanne B. Rasmussen for her help on adapting the histo-immunolabeling protocol to the turtle and Jørn Hounsgaard, Jens Mitdgaard, Raul Russo, Jakob B. Sørensen, and Matthew Tresch for their comments on a previous version of the manuscript. The project was funded by Danish Medical Research Council Grant 09-123456, Lundbeckfonden (www.lundbeckfonden.com), Owensenske Fond, Agnes and Poul Friis Fond, Association Française contre les Myopathies (www.afm-telethon.fr), and Antidoping Danmark.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216150110/-/DCSupplemental.
References
- 1.Smith JL, Martin PG, Gandevia SC, Taylor JL. Sustained contraction at very low forces produces prominent supraspinal fatigue in human elbow flexor muscles. J Appl Physiol. 2007;103(2):560–568. doi: 10.1152/japplphysiol.00220.2007. [DOI] [PubMed] [Google Scholar]
- 2.Taylor JL, Gandevia SC. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol. 2008;104(2):542–550. doi: 10.1152/japplphysiol.01053.2007. [DOI] [PubMed] [Google Scholar]
- 3.Bergström J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967;71(2):140–150. doi: 10.1111/j.1748-1716.1967.tb03720.x. [DOI] [PubMed] [Google Scholar]
- 4.Stephens JA, Taylor A. Fatigue of maintained voluntary muscle contraction in man. J Physiol. 1972;220(1):1–18. doi: 10.1113/jphysiol.1972.sp009691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler JE, Taylor JL, Gandevia SC. Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci. 2003;23(32):10224–10230. doi: 10.1523/JNEUROSCI.23-32-10224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 7.Reid C. The mechanism of voluntary muscular fatigue. BMJ. 1927;2(3481):545–546. doi: 10.1136/bmj.2.3481.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson KV, Edwards SC, Van Tongeren C, Bawa P. Properties of human motor units after prolonged activity at a constant firing rate. Exp Brain Res. 2004;154(4):479–487. doi: 10.1007/s00221-003-1678-z. [DOI] [PubMed] [Google Scholar]
- 9.Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: Evidence for suboptimal output from the motor cortex. J Physiol. 1996;490(Pt 2):529–536. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zijdewind I, Zwarts MJ, Kernell D. Influence of a voluntary fatigue test on the contralateral homologous muscle in humans? Neurosci Lett. 1998;253(1):41–44. doi: 10.1016/s0304-3940(98)00609-0. [DOI] [PubMed] [Google Scholar]
- 11.Eichelberger TD, Bilodeau M. Central fatigue of the first dorsal interosseous muscle during low-force and high-force sustained submaximal contractions. Clin Physiol Funct Imaging. 2007;27(5):298–304. doi: 10.1111/j.1475-097X.2007.00751.x. [DOI] [PubMed] [Google Scholar]
- 12.Søgaard K, Gandevia SC, Todd G, Petersen NT, Taylor JL. The effect of sustained low-intensity contractions on supraspinal fatigue in human elbow flexor muscles. J Physiol. 2006;573(Pt 2):511–523. doi: 10.1113/jphysiol.2005.103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bawa P, Pang MY, Olesen KA, Calancie B. Rotation of motoneurons during prolonged isometric contractions in humans. J Neurophysiol. 2006;96(3):1135–1140. doi: 10.1152/jn.01063.2005. [DOI] [PubMed] [Google Scholar]
- 14.Marvin G, et al. The effects of buspirone on perceived exertion and time to fatigue in man. Exp Physiol. 1997;82(6):1057–1060. doi: 10.1113/expphysiol.1997.sp004080. [DOI] [PubMed] [Google Scholar]
- 15.Wilson WM, Maughan RJ. Evidence for a possible role of 5-hydroxytryptamine in the genesis of fatigue in man: Administration of paroxetine, a 5-HT re-uptake inhibitor, reduces the capacity to perform prolonged exercise. Exp Physiol. 1992;77(6):921–924. doi: 10.1113/expphysiol.1992.sp003660. [DOI] [PubMed] [Google Scholar]
- 16.Farris JW, Hinchcliff KW, McKeever KH, Lamb DR, Thompson DL. Effect of tryptophan and of glucose on exercise capacity of horses. J Appl Physiol. 1998;85(3):807–816. doi: 10.1152/jappl.1998.85.3.807. [DOI] [PubMed] [Google Scholar]
- 17.Soares DD, Lima NR, Coimbra CC, Marubayashi U. Evidence that tryptophan reduces mechanical efficiency and running performance in rats. Pharmacol Biochem Behav. 2003;74(2):357–362. doi: 10.1016/s0091-3057(02)01003-1. [DOI] [PubMed] [Google Scholar]
- 18.Bailey SP, Davis JM, Ahlborn EN. Serotonergic agonists and antagonists affect endurance performance in the rat. Int J Sports Med. 1993;14(6):330–333. doi: 10.1055/s-2007-1021187. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology. 1999;21(2, Suppl):9S–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- 20.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15(7 Pt 2):5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez FJ, et al. Distribution of 5-hydroxytryptamine-immunoreactive boutons on alpha-motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol. 1998;393(1):69–83. [PubMed] [Google Scholar]
- 22.Kiehn O, Rostrup E, Møller M. Monoaminergic systems in the brainstem and spinal cord of the turtle Pseudemys scripta elegans as revealed by antibodies against serotonin and tyrosine hydroxylase. J Comp Neurol. 1992;325(4):527–547. doi: 10.1002/cne.903250406. [DOI] [PubMed] [Google Scholar]
- 23.Perrier JF, Alaburda A, Hounsgaard J. 5-HT1A receptors increase excitability of spinal motoneurons by inhibiting a TASK-1-like K+ current in the adult turtle. J Physiol. 2003;548(Pt 2):485–492. doi: 10.1113/jphysiol.2002.037952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang MY, Dun NJ. 5-Hydroxytryptamine responses in neonate rat motoneurones in vitro. J Physiol. 1990;430:87–103. doi: 10.1113/jphysiol.1990.sp018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grunnet M, Jespersen T, Perrier JF. 5-HT1A receptors modulate small-conductance Ca2+-activated K+ channels. J Neurosci Res. 2004;78(6):845–854. doi: 10.1002/jnr.20318. [DOI] [PubMed] [Google Scholar]
- 26.Delgado-Lezama R, Perrier JF, Nedergaard S, Svirskis G, Hounsgaard J. Metabotropic synaptic regulation of intrinsic response properties of turtle spinal motoneurones. J Physiol. 1997;504(Pt 1):97–102. doi: 10.1111/j.1469-7793.1997.097bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harvey PJ, Li X, Li Y, Bennett DJ. 5-HT2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol. 2006;96(3):1158–1170. doi: 10.1152/jn.01088.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hounsgaard J, Mintz I. Calcium conductance and firing properties of spinal motoneurones in the turtle. J Physiol. 1988;398:591–603. doi: 10.1113/jphysiol.1988.sp017059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrier JF, Alaburda A, Hounsgaard J. Spinal plasticity mediated by postsynaptic L-type Ca2+ channels. Brain Res Brain Res Rev. 2002;40(1-3):223–229. doi: 10.1016/s0165-0173(02)00204-7. [DOI] [PubMed] [Google Scholar]
- 31.Perrier JF, Delgado-Lezama R. Synaptic release of serotonin induced by stimulation of the raphe nucleus promotes plateau potentials in spinal motoneurons of the adult turtle. J Neurosci. 2005;25(35):7993–7999. doi: 10.1523/JNEUROSCI.1957-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perrier JF, Tresch MC. Recruitment of motor neuronal persistent inward currents shapes withdrawal reflexes in the frog. J Physiol. 2005;562(Pt 2):507–520. doi: 10.1113/jphysiol.2004.072769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrier JF, Hounsgaard J. 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol. 2003;89(2):954–959. doi: 10.1152/jn.00753.2002. [DOI] [PubMed] [Google Scholar]
- 34.Markstein R, et al. Pharmacological characterisation of 5-HT receptors positively coupled to adenylyl cyclase in the rat hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1999;359(6):454–459. doi: 10.1007/pl00005375. [DOI] [PubMed] [Google Scholar]
- 35.Bradley PB, et al. Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology. 1986;25(6):563–576. doi: 10.1016/0028-3908(86)90207-8. [DOI] [PubMed] [Google Scholar]
- 36.Araki T, Otani T. Response of single motoneurons to direct stimulation in toad’s spinal cord. J Neurophysiol. 1955;18(5):472–485. doi: 10.1152/jn.1955.18.5.472. [DOI] [PubMed] [Google Scholar]
- 37.Brock LG, Coombs JS, Eccles JC. Intracellular recording from antidromically activated motoneurones. J Physiol. 1953;122(3):429–461. doi: 10.1113/jphysiol.1953.sp005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fatt P. Sequence of events in synaptic activation of a motoneurone. J Neurophysiol. 1957;20(1):61–80. doi: 10.1152/jn.1957.20.1.61. [DOI] [PubMed] [Google Scholar]
- 39.Hu W, et al. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat Neurosci. 2009;12(8):996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- 40.Forster EA, et al. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. Eur J Pharmacol. 1995;281(1):81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- 41.Azmitia EC. Modern views on an ancient chemical: Serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull. 2001;56(5):413–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- 42.Bender KJ, Ford CP, Trussell LO. Dopaminergic modulation of axon initial segment calcium channels regulates action potential initiation. Neuron. 2010;68(3):500–511. doi: 10.1016/j.neuron.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bender KJ, Uebele VN, Renger JJ, Trussell LO. Control of firing patterns through modulation of axon initial segment T-type calcium channels. J Physiol. 2012;590(Pt 1):109–118. doi: 10.1113/jphysiol.2011.218768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imendra KG, Fujiyama R, Miyamoto T, Okada Y, Sato T. Serotonin inhibits voltage-gated sodium current by cyclic adenosine monophosphate-dependent mechanism in bullfrog taste receptor cells. Neurosci Lett. 2000;294(3):151–154. doi: 10.1016/s0304-3940(00)01567-6. [DOI] [PubMed] [Google Scholar]
- 45.Imendra KG, Miyamoto T, Okada Y, Toda K. Serotonin differentially modulates the electrical properties of different subsets of taste receptor cells in bullfrog. Eur J Neurosci. 2002;16(4):629–640. doi: 10.1046/j.1460-9568.2002.02107.x. [DOI] [PubMed] [Google Scholar]
- 46.Meeusen R, Watson P, Hasegawa H, Roelands B, Piacentini MF. Central fatigue: The serotonin hypothesis and beyond. Sports Med. 2006;36(10):881–909. doi: 10.2165/00007256-200636100-00006. [DOI] [PubMed] [Google Scholar]
- 47.Davis JM, Alderson NL, Welsh RS. Serotonin and central nervous system fatigue: Nutritional considerations. Am J Clin Nutr. 2000;72(2, Suppl):573S–578S. doi: 10.1093/ajcn/72.2.573S. [DOI] [PubMed] [Google Scholar]
- 48.Rank MM, Li X, Bennett DJ, Gorassini MA. Role of endogenous release of norepinephrine in muscle spasms after chronic spinal cord injury. J Neurophysiol. 2007;97(5):3166–3180. doi: 10.1152/jn.01168.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coombs JS, Curtis DR, Eccles JC. The generation of impulses in motoneurones. J Physiol. 1957;139(2):232–249. doi: 10.1113/jphysiol.1957.sp005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henze DA, Buzsáki G. Action potential threshold of hippocampal pyramidal cells in vivo is increased by recent spiking activity. Neuroscience. 2001;105(1):121–130. doi: 10.1016/s0306-4522(01)00167-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.