Abstract
Hypoxia is a common feature of tumors and an important contributor to malignancy and treatment resistance. The ability of tumor cells to survive hypoxic stress is mediated in part by hypoxia-inducible factor (HIF)-dependent transcriptional responses. More severe hypoxia activates endoplasmatic reticulum stress responses, including the double-stranded RNA-activated protein kinase (PKR)-like endoplasmic reticulum kinase (PERK)/eukaryotic initiation factor 2α (eIF2α)-dependent arm of the unfolded protein response (UPR). Although several studies implicate important roles for HIF and UPR in adaption to hypoxia, their importance for hypoxic cells responsible for therapy resistance in tumors is unknown. By using isogenic models, we find that HIF and eIF2α signaling contribute to the survival of hypoxic cells in vitro and in vivo. However, the eIF2α-dependent arm of the UPR is uniquely required for the survival of a subset of hypoxic cells that determine tumor radioresistance. We demonstrate that eIF2α signaling induces uptake of cysteine, glutathione synthesis, and protection against reactive oxygen species produced during periods of cycling hypoxia. Together these data imply that eIF2α signaling is a critical contributor to the tolerance of therapy-resistant cells that arise as a consequence of transient changes in oxygenation in solid tumors and thus a therapeutic target in curative treatments for solid cancers.
Keywords: growth delay, irradiation, acute hypoxia
Solid tumor microenvironments are characterized by extreme heterogeneities in oxygenation that arise as a result of poorly developed vascular networks. Gradients in oxygen are frequently found surrounding perfused vessels, ranging from normal values (∼5%) near the blood vessel to complete anoxia adjacent to necrosis. This gradient of hypoxia is generally referred to as “chronic” or “diffusion-limited” hypoxia and results from cellular oxygen consumption. Hypoxia can also arise in a temporal manner as a consequence of transient changes in oxygen delivery caused by changes in vessel perfusion (1). This “acute” or “perfusion-limited” hypoxia (2) is often cyclic in nature and can account for a large proportion of hypoxic cells at any given time (3). The steady-state proportion of hypoxic cells in tumors is influenced by the tolerance of individual tumor cells to these different types of hypoxia and varies remarkably among tumors with otherwise similar clinical features (4). For example, in head and neck cancer, oxygen electrode measurements of the hypoxic fraction range from 0% to 97% (5). These differences are important, because the fraction of viable hypoxic cells is a major determinant of outcome, as hypoxic cells are highly resistant to chemotherapy and radiation therapy (5, 6). Reducing cellular tolerance to hypoxia is therefore a strategy to reduce the proportion of hypoxic cells in tumors to improve current cancer therapy.
The mechanisms influencing hypoxia tolerance and therapy resistance in solid tumors are only partially understood. Hypoxia-inducible factor (HIF) transcription factors promote hypoxia tolerance through activation of a large number of genes that influence cellular metabolism and pH regulation (7, 8). In addition, HIFs influence tumor oxygenation directly by promoting angiogenesis, endothelial cell survival, and vasculogenesis (7, 9–13). Stabilization of HIF and activation of its downstream signaling pathways occurs at relatively moderate levels of hypoxia (<2% O2) (14), which is considerably higher than that required to produce radiation resistance (<0.2%) (15).
More severe hypoxia (<0.2% O2) leads to rapid activation of the unfolded protein response (UPR) (14). This is an evolutionarily conserved pathway that responds to endoplasmatic reticulum (ER) stress by the coordinate action of three ER stress sensors present within the ER membrane, PERK (EIF2AK3), inositol requiring kinase 1 (IRE1/ERN1), and activating transcription factor 6 (ATF6) (16). These are activated through a common mechanism involving sequestration of BIP (HSPA5) by misfolded proteins from the luminal domains of the sensors. The kinase PERK phosphorylates the serine 51 residue of eukaryotic initiation factor 2α (eIF2α) to inhibit mRNA translation. In addition to an overall inhibition in protein synthesis, eIF2α phosphorylation redirects translation toward a subset of transcripts, including the transcription factor ATF4 (17, 18). eIF2α phosphorylation and inhibition of mRNA translation is transient because ATF4 induces a second transcription factor, C/EBP homologous protein (CHOP/DDIT3), which in turn regulates expression of growth arrest and DNA damage gene 34 (GADD34/PP1R15A), which dephosphorylates eIF2α and completes a negative feedback loop to restore protein synthesis (19). Hypoxic activation of PERK is of functional importance because PERK-KO mouse embryonic fibroblasts (MEFs) or MEFs with a knock-in eIF2α mutant allele containing a serine-to-alanine mutation at position 51 (S51A) (20), show increased cell death during hypoxia and produce slow-growing tumors with reduced regions of viable hypoxia (21). Furthermore, PERK signaling is essential for the survival of hypoxic cells by preserving the cells’ capacity to maintain high rates of autophagy (22). Abrogation of PERK-mediated signaling results in loss of autophagic capacity and sensitizes cells to hypoxia-induced cell death.
Although PERK/eIF2α and HIF pathways have been implicated by several studies in hypoxia tolerance, they show clear differences in oxygen levels required for their activation. It is unknown to what degree these two pathways contribute differentially to long-term survival of hypoxic cells in tumors responsible for treatment failure. We have investigated this question by using inducible and isogenic tumor models, and our data reveal an essential role for PERK/eIF2α signaling that promotes the survival of radiation-resistant hypoxic cells by stimulating glutathione (GSH) synthesis and reducing reactive oxygen species (ROS) produced during cyclic acute hypoxia.
Results
eIF2α Signaling Is Required for Hypoxic Cell Survival.
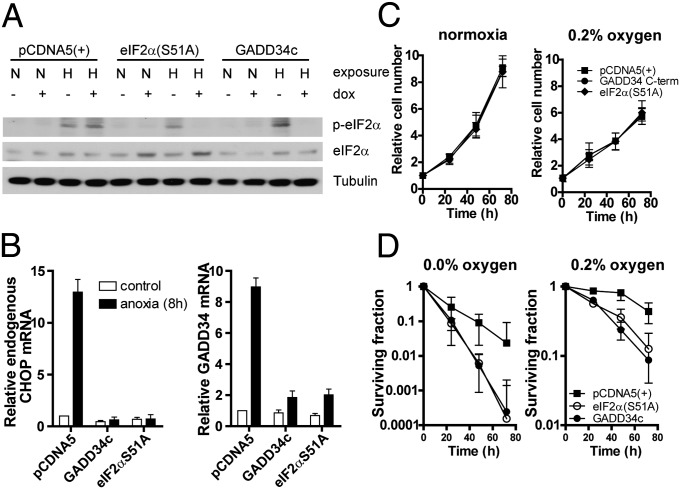
To investigate the role of HIF and UPR/PERK/eIF2α signaling on growth and hypoxia tolerance, we constructed isogenic sets of U373 and HCT116 cells expressing inducible interfering transgenes at a single genomic location (23, 24). eIF2α signaling inhibition is achieved through expression of the C-terminal region of hamster GADD34 (GADD34c; Fig. S1A) or a dominant-negative eIF2α allele (eIF2αS51A; Fig. 1A). Both approaches effectively prevent phosphorylation of eIF2α and expression of the downstream UPR target genes CHOP and GADD34 in U373 lines after exposure to hypoxia (Fig. 1 A and B) (25). Similar to previous results, knockdown experiments demonstrated that eIF2α phosphorylation during hypoxia in these cells is dependent on PERK (20, 21, 23, 26), although these cells also show some dependency on general control nonrepressed 2 (GCN2) (Fig. S2). As shown previously (23), inhibition of eIF2α signaling by either approach had no influence on cell proliferation under normal conditions or during moderate hypoxia (0.2% O2; Fig. 1C and Fig. S1C). Severe hypoxia (<0.02% O2) caused a decrease in cell number in eIF2α signaling-deficient cells, suggestive of increased cell death. We therefore determined hypoxia tolerance by measuring long-term clonogenic survival following exposures to moderate (0.2% O2) or severe (<0.02% O2) hypoxia. Interestingly, inhibition of eIF2α signaling markedly reduced overall survival in U373 (Fig. 1D) and HCT116 (Fig. S1B) cell lines following exposure to severe and moderate hypoxia. Overexpressing GADD34c or eIF2α(S51A) had no effect on colony formation under ambient oxygen conditions and does not alter HIF-1α induction or HIF-dependent gene expression (Fig. S3) (27).
Fig. 1.
PERK/UPR is required for hypoxic cell survival. (A) p-eIF2α immunoblot of isogenic, doxycycline (dox)-inducible U373 cells with control [pCDNA5(+)], and PERK/UPR interfering genes (GADD34c and eIF2αS51A) after hypoxia (O2 < 0.02) exposure. (B) Quantitative PCR for CHOP and endogenous GADD34 after 8 h hypoxia (O2 < 0.02%) exposure of doxycycline-pretreated (16 h) U373 cell lines. (C) Growth curve of U373 cells under normal (Left) or 0.2% oxygen (Right). (D) Clonogenic survival of control and PERK/UPR-deficient cells under severe (O2 < 0.02%) and moderate (0.2% O2) hypoxia.
eIF2α Signaling Promotes Hypoxia Tolerance in Vivo but Not Overall Tumor Growth.
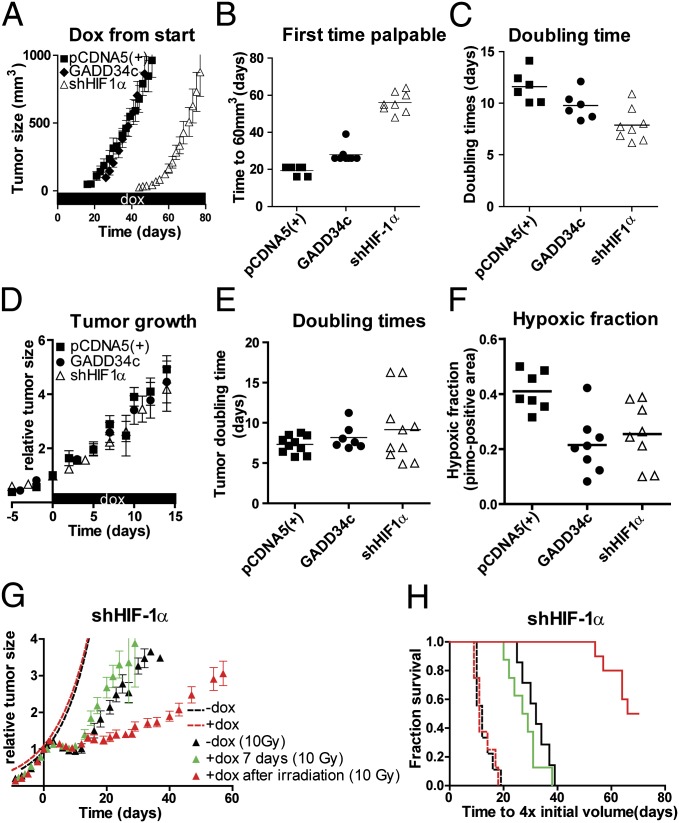
To assess the importance of eIF2α signaling on hypoxia tolerance and growth in a more relevant context, we exploited the ability to inducibly inhibit this pathway in established tumor xenografts. Groups of mice were injected with the PCDNA5 control or GADD34c or eIF2αS51A inducible isogenic lines and allowed to form xenografts of ∼150 mm3. As in cell culture, doxycycline administration resulted in effective functional disruption of eIF2α signaling in vivo, as evidenced by reduced phospho-eIF2α (p-eIF2α) staining (Fig. S4C). In addition, we observed a decrease in CHOP and endogenous GADD34 mRNA expression in tumors from the doxycycline-treated GADD34c and eIF2αS51A models [P < 0.01 and P < 0.05 in GADD34c and eIF2α(S51A) for CHOP and GADD34, respectively; Fig. S4C]. However, disruption of eIF2α signaling after tumor establishment had no significant effect on overall tumor growth (Fig. S4 A and B). This contrasts with the effects of disrupting eIF2α signaling before tumor initiation, which significantly slows tumor establishment (see Fig. 4B) as also shown previously (21).
Fig. 4.
PERK/UPR and HIF deficiency delays tumor formation. (A) Tumor growth of s.c. implanted isogenic HCT116 cells targeting HIF-1α (i.e., shHIF-1α), GADD34c, and control cells [pCDNA5(+)] preexposed (24 h) to doxycycline (dox) were injected into doxycycline receiving nude mice [mean ± SEM, pCDNA5(+), n = 6; GADD34c, n = 8; shHIF-1α, n = 8]. (B) Palpability (>60 mm3) of tumors. (C) Doubling time of individual tumors. (D) After establishment (150 mm3), mice received doxycycline in their drinking water [mean ± SEM; pCDNA5(+), n = 10; GADD34c, n = 7; shHIF-1α, n = 10]. (E) Doubling time of the individual tumors. (F) Tumors were harvested after 7 d doxycycline, and hypoxia was assessed by pimonidazole immunohistochemistry of the viable tumor tissue. (G) Mice with established tumors (150 mm3) received doxycycline in drinking water. Growth (dotted lines) and regrowth after irradiation (t = 0) was followed over time in animals that received no doxycycline (−dox, n = 7) or doxycycline from day −4 to day +3 [+dox 7 d (10 Gy), n = 8] or doxycycline after irradiation [+dox after irradiation (10 Gy), n = 10]. (H) Kaplan–Meier survival for tumors in Fig. 4 D and G to with the endpoint of reaching four times the initial volume.
To assess the effect of eIF2α inhibition on hypoxia tolerance in vivo, we analyzed the microenvironments of established U373 tumors following administration of doxycycline for 7 d. Strikingly, an almost twofold reduction in the fraction of viable hypoxic tissue was observed in the GADD34c and eIF2αS51A tumors (P < 0.05; Fig. 2 A and C). This reduction was associated with a significant increase in necrosis in the GADD34c and eIF2αS51A tumors, respectively (P < 0.05; Fig. 2 B and D). Other microenvironmental features, including vessel density and overall cell proliferation, remained unchanged (Fig. S4D).
Fig. 2.
PERK/UPR inhibition reduces the tumor hypoxic fraction. (A) Pimonidazole (pimo) immunohistochemistry (pimonidazole positive viable fraction, green; blood vessels, red; Hoechst dye, blue) and (B) H&E on U373 isogenic tumors. (C) Quantification of pimonidazole immunohistochemistry of the viable tumor tissue and (D) necrotic fraction.
eIF2α Signaling Promotes Survival of Therapy-Resistant Cells.
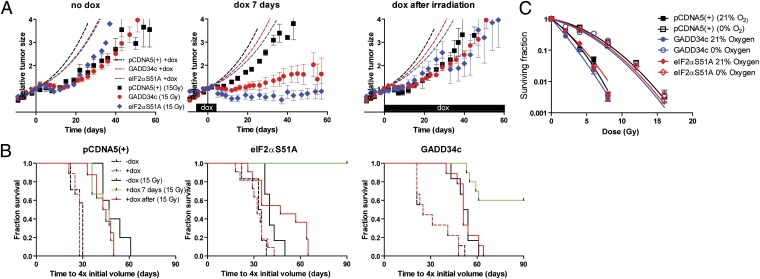
Hypoxic cells are a major limiting factor in the efficacy of radiation therapy, as they require nearly three times as much radiation as nonhypoxic cells for equivalent cell kill. We thus determined the radiation response of established U373 tumors following eIF2α inhibition. Tumors were treated with or without doxycycline and then irradiated with a single dose of 15 Gy. This dose kills the vast majority of nonhypoxic cells (>99.999%), but not hypoxic cells (∼0.5% survive; Fig. 3C). As a consequence, the tumor growth delay closely reflects the number of long-term viable hypoxic cells present at the time of irradiation. Treatment with 15 Gy resulted in a significant growth delay in all groups (Fig. 3A). In the absence of doxycycline, no differences in radiation sensitivity were observed between tumor types (Fig. 3A, Left). However, transient eIF2α inhibition before irradiation in the GADD34c and eIF2αS51A models increased radiation response, with many tumors failing to regrow within 90 d of follow-up (Fig. 3A, Middle). Consistent with a direct effect on the viability of radiation-resistant hypoxic cells, this sensitization required inhibition of eIF2α before irradiation; no synergy was observed when eIF2α was inhibited after radiation treatment (Fig. 3A, Right).
Fig. 3.
PERK/UPR targeting before irradiation sensitizes tumors to irradiation. Established U373 isogenic xenografts were irradiated (t = 0) when indicated (15 Gy) at ∼150 mm3. (A) (Left) No doxycycline (dox) was administered to the animals [mean ± SEM; pCDNA5(+), n = 5; GADD34c, n = 6; eIF2αS51A, n = 6). Regular growth of the tumors without irradiation (dotted lines). (Center) Doxycycline was administered for 7 d [t = −4 to t = 3, irradiation at t = 0; mean ± SEM; pCDNA5(+), n = 6; GADD34c, n = 10; eIF2αS51A, n = 6]. (Right) Doxycycline administration after irradiation [t = 0; mean ± SEM; pCDNA5(+), n = 8; GADD34c, n = 9; eIF2αS51A, n = 11]. (B) Kaplan–Meier survival for tumors in Fig. 3A and Fig. S4A to reach four times the initial volume after regular growth (dotted lines) or irradiation without (black line) or with doxycycline administration (doxycycline from t = −4 to t = 3, irradiation at t = 0, green line; doxycycline after irradiation, red line). (C) Clonogenic survival cells after irradiation under normal and hypoxic (O2 < 0.02%) conditions.
To illustrate the differences in radiation sensitivity of the individual tumors, the data are replotted in Fig. 3B as Kaplan–Meier plots. Assessed in this way, radiation treatment can be observed to extend the median survival of mice with PCDNA5 control tumors from 30 to 47 d irrespective of doxycycline treatment (P < 0.05; Fig. 3B, Left). In contrast, both PERK/UPR-deficient models show a dramatic increase in survival when irradiated, when eIF2α signaling is targeted before irradiation. In these doxycycline-treated groups, the eIF2αS51A and GADD34c unirradiated mice have a median survival similar to PCDNA5 controls (∼30 d), whereas 100% (P < 0.01; Fig. 3B, Middle) and 60% (P < 0.05; Fig. 3B, Right) of mice survive for >90 d, respectively, when irradiated.
Regardless of eIF2α status, all cell lines showed a similar radiation response in vitro, and, as expected, hypoxic cells required ∼2.5 to threefold more dose for equivalent toxicity (Fig. 3C). These data indicate that the decrease in hypoxic fraction before irradiation is the primary cause of improved response to radiation in vivo.
Comparison of HIF-1 and eIF2α Signaling Pathways on Hypoxia Tolerance and Therapy Resistance.
To compare the relative importance of eIF2α with that of the HIF pathway, we generated isogenic HCT116 cell lines with regulated expression of GADD34c, eIF2αS51A, and an shRNA directed against HIF-1α (shHIF-1α). We were unable to generate a similar functional U373 shHIF-1α line as a result of higher endogenous expression levels of HIF-1α. Doxycycline exposure in the shHIF-1α HCT116 line prevented accumulation of HIF-1α protein under hypoxic conditions (Fig. S5A) and reduced HIF transcriptional targets, including carbonic anhydrase 9 (CA9), vascular endothelial growth factor (VEGF), and glucose transporter 1 (Glut1) (Fig. S5B). Knockdown of HIF-1α also reduced hypoxia tolerance as assessed by clonogenic survival in response to severe (<0.02% O2) and moderate (0.2% O2) hypoxia, and these effects were similar to that observed following eIF2α inhibition in this cell line (Fig. S5D and Fig. S1B). Interestingly, HIF-1α knockdown also reduced proliferation under moderate hypoxia (0.2% O2; Fig. S5C), in contrast to eIF2α inhibition (Fig. S1C).
The importance of inhibiting eIF2α was then compared with inhibiting HIF-1α during or after xenograft establishment in mice. Continuous administration of doxycycline starting at tumor implantation showed that initiation of xenografts from eIF2α signaling deficient lines was slightly delayed (P < 0.01; Fig. 4 A and B), but, when they had been established, grew at rates comparable to the PCDNA5 controls (Fig. 4C). A much longer delay was observed for initiation of HIF-1α–deficient tumors (P < 0.001); however, when they had been established, they grew slightly faster than control or UPR-deficient tumors (doubling time, 6.9 d vs. 7.5 d and 7.6 d for the two eIF2α-deficient models; P < 0.05; Fig. 4C). Inhibition of eIF2α signaling or HIF-1α after xenograft establishment had no effect on tumor growth or doubling time of the tumors (Fig. 4 D and E). Thus, HIF and, to some extent, eIF2α signaling are important for initial engraftment and tumor initiation, but are not required for tumor growth after establishment.
Similar to results in the U373 tumors, 7 d of doxycycline treatment resulted in a decrease in the hypoxic fraction (P < 0.05; Fig. 4F) in the eIF2α-signaling deficient models (from 41% to 23%). Inhibition of HIF-1α in the established tumors also resulted in a similar decrease in the hypoxic fraction from 41% to 25% (P < 0.05; Fig. 4F).
Finally, we compared the therapeutic relevance of the decrease in hypoxic fraction following inhibition of HIF-1α or eIF2α in these isogenic models. Surprisingly, unlike the effects of inhibiting eIF2α, the reduction in hypoxia following knockdown of HIF-1α did not increase the sensitivity of these tumors to irradiation (Fig. 4 G and H). Thus, although HIF-1α supports the survival of a population of hypoxic cells, this population appears distinct from that which contributes to radiation resistance. Similar to the U373 isogenic cells, no difference in the radiation sensitivity of the shHIF-1α– or eIF2α-deficient lines was observed in vitro.
In contrast, HIF-1α silencing after irradiation caused a large delay in rate of regrowth (P < 0.05; Fig. 4 G and H). This effect was not observed in either of the GADD34c or eIF2αS51A tumors (Fig. 3 A and B and Fig. S5 E and F).
eIF2α-Signaling Deficient, but Not HIF-Deficient, Cells Are Sensitized to Cyclic Hypoxia.
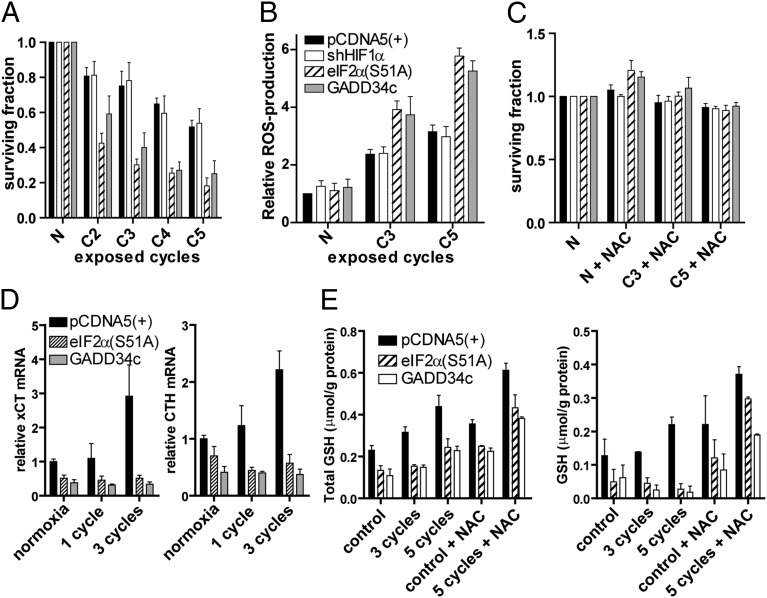
As inhibition of HIF-1α and eIF2α signaling resulted in a similar decrease in tumor hypoxia (i.e., pimonidazole-positive cells) but had different consequences on radiation response, we hypothesized that these pathways may promote survival of cells that become hypoxic through different mechanisms (i.e., acute or chronic hypoxia) (28). To assess the sensitivity of cells to acute hypoxia, we measured clonogenic survival after repeated cycles of severe hypoxia (1 h, O2 < 0.02%) followed by reoxygenation (1 h). Whereas the HIF-deficient cells displayed a comparable sensitivity as the control cells, both eIF2α signaling-deficient lines were markedly sensitized to cycling hypoxia (Fig. 5A). Reoxygenation following hypoxia can cause generation of ROS, which contribute to the toxicity of cycling hypoxia (29). Indeed, the eIF2α signaling-deficient cells had approximately twofold higher levels of ROS during cycling hypoxia (Fig. 5B). Importantly, elevated ROS in these cells are specific to cyclic hypoxic stress as ROS levels were similar in control and eIF2α signaling-deficient cells under aerobic conditions and following irradiation (Fig. S6A). Furthermore, no differences were observed in the toxicity generated by exposure to ROS inducing agents (Fig. S6B).
Fig. 5.
PERK/UPR activation, but not HIF signaling, is required for survival after cycling hypoxia. (A) Clonogenic survival of isogenic HCT116 cells, after targeting HIF or eIF2α signaling, exposed to normoxia or two, three, four, or five cycles of 1 h hypoxia (O2 < 0.02%) followed by 1 h of reoxygenation (mean ± SEM; n = 3). (B) DHR flow cytometry after normoxia or exposure to three or five cycles. DHR was added during the final reoxygenation period (mean ± SEM; n = 3). (C) Clonogenic survival of isogenic HCT116 cells preloaded with the ROS scavenger NAC (n = 3; mean ± SEM). (D) cCT and CTH mRNA expression levels as determined by quantitative PCR (E). Total GSH and free GSH were determined in control and eIF2α signaling-deficient cells (n = 3; mean ± SEM).
PERK activation during the UPR has been shown to result in phosphorylation and activation of nuclear factor (erythroid-derived 2)-like 2 (NRF2), a master regulatory of the antioxidant response. Although this appears to occur in an eIF2α-independent manner (30), we tested if differences in NRF2 activation could explain differences in ROS levels and toxicity of cyclic hypoxia in our models. However, we observed no differences in nuclear translocation of an NRF2 reporter, or activation of NRF2 endogenous transcriptional targets between control and eIF2α signaling-deficient cells (Fig. S7 A and B). Furthermore, knockdown of Kelch like-ECH-associated protein 1 (KEAP1), and thus activation of NRF2, was unable to rescue the sensitivity of eIF2α signaling-deficient cells to cyclic hypoxia (Fig. S7 C and D).
To test the importance of elevated ROS on hypoxia tolerance, we treated cells with the ROS scavenger, N-acetylcysteine (NAC) during cyclic hypoxia. NAC treatment prevented ROS generation and toxicity in all isogenic lines (Fig. 5C). These data indicate that eIF2α signaling plays an important role in mitigating the production or effects of ROS during cycling hypoxia.
eIF2α signaling has previously been implicated in mediating resistance to oxidative stress (31) by increasing intracellular cysteine and GSH synthesis. Increased cysteine levels are mediated through eIF2α- and ATF4-dependent expression of cystine/glutamate transporter xCT (SLC7A11) (32), a subunit specific to the cystine/glutamate antiporter system Xc−, as well as cystathione γ-lyase (CTH), which produces intracellular cysteine from serine- and homocysteine-derived cystathione (33). We found that, in control cells, xCT and CTH are induced during cyclic hypoxia (Fig. 5D), whereas, in eIF2α signaling-deficient cells, basal expression is decreased and the cyclic hypoxia-induced increase is entirely prevented. Similar results were obtained for γ-glutamylcysteine synthetase (Fig. S8A), the first and rate-limiting enzyme for GSH synthesis. The nonspecific subunit of Xc−, SLC3A2, and GSH synthetase are not dependent on eIF2α signaling. Consistent with these defects, we found that eIF2α signaling-deficient cells have decreased levels of total glutathione (GSH and oxidized GSH; GSSG; Fig. 5E, Left). Furthermore, when exposed to cycling hypoxia, these cells are insufficient to maintain a stable pool of “free” GSH to react with oxidizing agents (Fig. 5E, Right). In contrast, cells with intact eIF2α signaling, which induce cysteine transporters and GSH biogenesis enzymes, had increased free GSH after exposure to cyclic hypoxia. Preincubation with NAC increased GSH synthesis in all cell lines and rescued free GSH levels in eIF2α signaling-deficient cells during cyclic hypoxia. Similar results were obtained in U373 cells (Fig. S8B). These data demonstrate that the increased ROS and cell death observed in the eIF2α signaling-deficient cells reflects a defect in responding to cyclic hypoxia by increasing intracellular cysteine and GSH synthesis.
Discussion
Hypoxia is a common but heterogeneous feature of solid tumors and a major limiting factor in successful cancer treatment (7). Although this has been known for some time, the basis for the wide variation in the levels of hypoxia and their influence on treatment outcome among patients’ tumors is still poorly understood. The proportion of viable cells at various different oxygen concentrations within individual tumors is influenced by deficiencies in oxygen delivery (inadequate vessel development and/or function) and by differences in cellular tolerance to hypoxia. The ability of tumor cells to survive moderate or severe levels of hypoxia is strongly influenced by adaptive mechanisms including HIF and eIF2α pathway. Here, by using inducible and isogenic models, we show that, even though both of these pathways influence hypoxic levels in tumors, the eIF2α pathway is uniquely important for the survival of a subset of hypoxic cells in established tumors that are radiation-resistant and can contribute to regrowth of the tumor following treatment.
It is clear that the hypoxic fraction of cells within tumors contains subpopulations with different biological phenotypes and unique contributions to therapy resistance. The overall hypoxic fraction, as measured by pimonidazole, was equally decreased in tumors following inducible inhibition of eIF2α or HIF-1 pathways in established tumors. However, the decrease that occurred following transient inhibition of HIF did not result in an improved tumor response to radiation therapy. The consequence of HIF inhibition on steady-state levels of moderate or severely hypoxic cells in tumors is complex and difficult to predict. It is likely that HIF signaling directly supports the survival of some cells in response to chronic hypoxia exposure, as loss of HIF reduced survival and proliferation of these same cells to long exposures of moderate or severe hypoxia. However, HIF also inhibits (i) mitochondrial oxygen consumption by inducing PDK1 and (ii) tumor oxygen delivery by inducing angiogenesis. Thus, demand and supply of oxygen are altered following HIF inhibition in vivo. Our data indicate that transient HIF inhibition does not substantially alter the fraction of radiation-resistant cells. In contrast, inhibition of HIF in the same model after radiation treatment resulted in a marked delay of tumor growth. These results are consistent with previously reported effects of the HIF small-molecule inhibitor YC-1, which, when given before radiation therapy, inhibits radiation response, whereas treatment afterward enhances response (34). Several other recent publications have confirmed in the context of radiation treatment outcome that HIF signaling is primarily important for driving recovery of tumor vasculature in the hypoxic microenvironment following therapy-induced vessel damage (12, 13).
In contrast, our data demonstrate a critical role for eIF2α signaling in promoting survival of a therapy-relevant fraction of hypoxic cells. Inducible inhibition of eIF2α signaling reduced the pimonidazole-positive fraction, increased areas of necrosis, and resulted in a striking improvement in tumor response to treatment. Unlike the situation for chronic hypoxic exposures, the eIF2α pathway was also uniquely important for the survival of cells exposed to repeated short cycles of severe hypoxia in vitro. ROS levels increase somewhat during hypoxia through still-unclear mechanisms, but are particularly elevated following reoxygenation (29). ROS generated during repeated exposures to hypoxia appear to be the source of toxicity in the eIF2α-signaling deficient cells in vitro, as toxicity was rescued by treatment with NAC. Furthermore, our data reveal that elevated ROS and the resulting toxicity stem from an underlying defect in the ability of eIF2α signaling-defective cells to induce enzymes required for cysteine uptake and GSH biosynthesis pathways. Together, these data demonstrate that PERK signaling functions to increase GSH biosynthesis and prepare cells for detoxification of ROS produced by cyclic hypoxia. The importance of this defense mechanism is demonstrated by the resulting loss of hypoxia and increased radiation response of tumors in which eIF2α signaling is inducibly inhibited in vivo.
Although detoxification of ROS appears to be the primary mechanism responsible for the sensitivity of eIF2α signaling-deficient cells to cycling hypoxia, other features of eIF2α regulation and signaling may be relevant. Transient vessel occlusion results in rapid and extreme hypoxia (i.e., anoxia) in a large number of tumor cells. eIF2α signaling is well suited to respond to this stress, because eIF2α phosphorylation and inhibition of protein synthesis occur rapidly during severe hypoxia (Fig. S3) (35). In addition, eIF2α/ATF4 signaling during hypoxia supports increased flux through the autophagy pathway, aiding the clearance of ROS-producing damaged mitochondria (22, 36).
Our results reveal a potential opportunity for development of agents that target the PERK signaling arm of the UPR. The UPR has been considered a target primarily for secretory tumors such as myeloma, which experience constitutive ER stress associated with secretion of immunoglobulins. Our data suggest that PERK inhibition will selectively sensitize cycling hypoxic cells due to its requirement to protect against ROS-induced cell death. Additional work is needed to evaluate the potential of newly developed PERK inhibitors (37) in combination with radiotherapy and chemotherapy.
Materials and Methods
U373-MG and HCT116 cells were engineered for acceptance of a single shuttle vector (inducibly) expressing the interfering gene (flp-in T-rex system, Invitrogen) after doxycyline exposure. NMRI-nu (nu/nu) mice were used for in vivo assessment of growth and radiation sensitivity. A comprehensive description is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors also thank Natasja Lieuwes and Kim Savelkouls for their technical assistance. This work was supported by Dutch Science Organization ZonMW VENI Grant 916.76.158 (to K.M.R.), Dutch Cancer Society KWF Grants UM 2010-4714 (to K.M.R. and P.L.), UM 2012-5506 (to K.M.R.), and UM 2008-4068 (to B.G.W.), the Ontario Ministry of Health and Long Term Care, Terry Fox New Frontiers Research Program Grant PPG09-020005 (to M.K. and B.G.W.), Ontario Institute for Cancer Research and Terry Fox Research Institute Selective Therapies Program (B.G.W.), Canadian Institute for Health Research Grant 201592 (to M.K. and B.G.W.), the Terry Fox Foundation/Research Institute (new investigator award to M.K.), and European Union Seventh Framework Programme/METOXIA Project 222741 (to P.L., M.K., and B.G.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210633110/-/DCSupplemental.
References
- 1.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8(6):425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magagnin MG, Koritzinsky M, Wouters BG. Patterns of tumor oxygenation and their influence on the cellular hypoxic response and hypoxia-directed therapies. Drug Resist Updat. 2006;9(4-5):185–197. doi: 10.1016/j.drup.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Durand RE, Aquino-Parsons C. Non-constant tumour blood flow—implications for therapy. Acta Oncol. 2001;40(7):862–869. doi: 10.1080/02841860152703508. [DOI] [PubMed] [Google Scholar]
- 4.Wouters BG, van den Beucken T, Magagnin MG, Lambin P, Koumenis C. Targeting hypoxia tolerance in cancer. Drug Resist Updat. 2004;7(1):25–40. doi: 10.1016/j.drup.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Nordsmark M, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77(1):18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Hockel M, et al. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56(19):4509–4515. [PubMed] [Google Scholar]
- 7.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 8.Sørensen BS, et al. Proteins upregulated by mild and severe hypoxia in squamous cell carcinomas in vitro identified by proteomics. Radiother Oncol. 2009;92(3):443–449. doi: 10.1016/j.radonc.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Greijer AE, et al. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1) J Pathol. 2005;206(3):291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- 10.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 11.Seagroves TN, et al. Transcription factor HIF-1 is a necessary mediator of the Pasteur effect in mammalian cells. Mol Cell Biol. 2001;21(10):3436–3444. doi: 10.1128/MCB.21.10.3436-3444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moeller BJ, et al. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005;8(2):99–110. doi: 10.1016/j.ccr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Kioi M, et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120(3):694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koumenis C, Wouters BG. “Translating” tumor hypoxia: Unfolded protein response (UPR)-dependent and UPR-independent pathways. Mol Cancer Res. 2006;4(7):423–436. doi: 10.1158/1541-7786.MCR-06-0150. [DOI] [PubMed] [Google Scholar]
- 15.Denekamp J, editor. Amsterdam: Elsevier; 1989. Physiological hypoxia and its influence on radiotherapy. The biological basis on radiotherapy. [Google Scholar]
- 16.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 17.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA. 2004;101(31):11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167(1):27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima E, et al. The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress: elucidation by GADD34-deficient mice. FASEB J. 2003;17(11):1573–1575. doi: 10.1096/fj.02-1184fje. [DOI] [PubMed] [Google Scholar]
- 20.Koritzinsky M, et al. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 2006;25(5):1114–1125. doi: 10.1038/sj.emboj.7600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bi M, et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24(19):3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rouschop KM, et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120(1):127–141. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koritzinsky M, et al. Phosphorylation of eIF2alpha is required for mRNA translation inhibition and survival during moderate hypoxia. Radiother Oncol. 2007;83(3):353–361. doi: 10.1016/j.radonc.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Rouschop KM, et al. Deregulation of cap-dependent mRNA translation increases tumour radiosensitivity through reduction of the hypoxic fraction. Radiother Oncol. 2011;99(3):385–391. doi: 10.1016/j.radonc.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 25.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153(5):1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koumenis C, et al. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol. 2002;22(21):7405–7416. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Beucken T, et al. Hypoxia-induced expression of carbonic anhydrase 9 is dependent on the unfolded protein response. J Biol Chem. 2009;284(36):24204–24212. doi: 10.1074/jbc.M109.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ljungkvist AS, Bussink J, Kaanders JH, van der Kogel AJ. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiat Res. 2007;167(2):127–145. doi: 10.1667/rr0719.1. [DOI] [PubMed] [Google Scholar]
- 29.Zweier JL, Kuppusamy P, Lutty GA. Measurement of endothelial cell free radical generation: Evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci USA. 1988;85(11):4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cullinan SB, et al. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23(20):7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harding HP, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 32.Lewerenz J, Maher P. Basal levels of eIF2alpha phosphorylation determine cellular antioxidant status by regulating ATF4 and xCT expression. J Biol Chem. 2009;284(2):1106–1115. doi: 10.1074/jbc.M807325200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickhout JG, et al. Integrated stress response modulates cellular redox state via induction of cystathionine γ-lyase: Cross-talk between integrated stress response and thiol metabolism. J Biol Chem. 2012;287(10):7603–7614. doi: 10.1074/jbc.M111.304576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harada H, et al. Treatment regimen determines whether an HIF-1 inhibitor enhances or inhibits the effect of radiation therapy. Br J Cancer. 2009;100(5):747–757. doi: 10.1038/sj.bjc.6604939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koritzinsky M, et al. The hypoxic proteome is influenced by gene-specific changes in mRNA translation. Radiother Oncol. 2005;76(2):177–186. doi: 10.1016/j.radonc.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 36.Rouschop KM, et al. Autophagy is required during cycling hypoxia to lower production of reactive oxygen species. Radiother Oncol. 2009;92(3):411–416. doi: 10.1016/j.radonc.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 37.Atkins C, et al. Characterization of a novel PERK kinase inhibitor with anti-tumor and anti-angiogenic activity. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-12-3109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.