Abstract
The ontogeny of linguistic functions in the human brain remains elusive. Although some auditory capacities are described before term, whether and how such immature cortical circuits might process speech are unknown. Here we used functional optical imaging to evaluate the cerebral responses to syllables at the earliest age at which cortical responses to external stimuli can be recorded in humans (28- to 32-wk gestational age). At this age, the cortical organization in layers is not completed. Many neurons are still located in the subplate and in the process of migrating to their final location. Nevertheless, we observed several points of similarity with the adult linguistic network. First, whereas syllables elicited larger right than left responses, the posterior temporal region escaped this general pattern, showing faster and more sustained responses over the left than over the right hemisphere. Second, discrimination responses to a change of phoneme (ba vs. ga) and a change of human voice (male vs. female) were already present and involved inferior frontal areas, even in the youngest infants (29-wk gestational age). Third, whereas both types of changes elicited responses in the right frontal region, the left frontal region only reacted to a change of phoneme. These results demonstrate a sophisticated organization of perisylvian areas at the very onset of cortical circuitry, 3 mo before term. They emphasize the influence of innate factors on regions involved in linguistic processing and social communication in humans.
Keywords: hemodynamic response, premature human brain, language, hemispheric lateralization, near infrared spectroscopy
Shortly after birth, human infants already exhibit a variety of sophisticated linguistic capacities, from discriminating syllables and human languages (1) to remembering short stories (2). These capacities rely on a set of perisylvian brain areas similar to the one described in adults, involving temporal but also frontal areas (3), with significant asymmetries favoring the left hemisphere at the level of the planum temporale (4, 5). Because audition is already functional during the last months of pregnancy (6), it is still debated whether evolution has endowed humans with a genetically determined cortical organization particularly suitable to process speech or whether fast learning quickly specializes the auditory network toward speech processing during this initial period (7). In the present work, to inform this debate, we examined the functional organization of the perisylvian areas at the onset of cortical circuitry in preterm infants.
Neuronal migration is still on its way during the last trimester of human gestation. The majority of the neurons still lie in the subplate, and the six-layered lamination of the cortex becomes fully visible only after 32-wk gestational age (wGA) (8). The first contacts of the thalamo-cortical fibers establish with subplate neurons (9). The first synapses appear in the cortical plate around 26 wGA, with a massive relocation of the afferent fibers from the subplate to the cortical plate from 28 to 32 wGA. A dual innervation of pyramidal cells by both subplate and thalamic axons is thus present (10), creating a transient circuitry for incoming inputs, very specific to this period of development. This period is also marked by a fast emergence of short-range connectivity in addition to the long-range association pathways already observed (11). Auditory event-related responses to tones have been recorded from 25 wGA onward (12). Although the cortical origin of these early event related potentials (ERPs) is debatable, a clear cortical response to external sounds has been detected with functional MRI (fMRI) at 33 wGA in fetuses (13).
To probe the functional efficacy of the early cortical circuitry in processing speech stimuli, we examined a linguistic (ba vs. ga) and a nonlinguistic (male vs. female voice) discrimination in 12 sleeping 30 wGA preterm infants (28–32 wGA; Table S1). Beyond their linguistic/nonlinguistic valency, these two contrasts differ on acoustical properties that can affect their discriminability and their processing by the left and right hemisphere. A place of articulation contrast, such as ba/ga, is one of the most challenging phonetic discriminations, easily lost in degraded hearing conditions (14, 15). Its perception requires a fine-grained temporal analysis and mainly relies on a left-lateralized network both in adults (16, 17) and in infants (18, 19). By contrast, the perception of a change in voice sex is based on spectral differences carried by the whole syllable and better processed by the right temporal regions (20–23). The comparison of cortical responses to these two contrasts should clarify whether a sophisticated phonetic discrimination and a simpler acoustic discrimination occur at the same moment in development and whether the delay observed in the anatomical gyration of the left hemisphere has functional consequences for speech processing. Indeed, most sulci, particularly the superior temporal sulcus (STS), appear 1 or 2 wk later on the left than on the right hemisphere (24, 25). This might lead to differences in their respective capacities to encode and discriminate auditory stimuli.
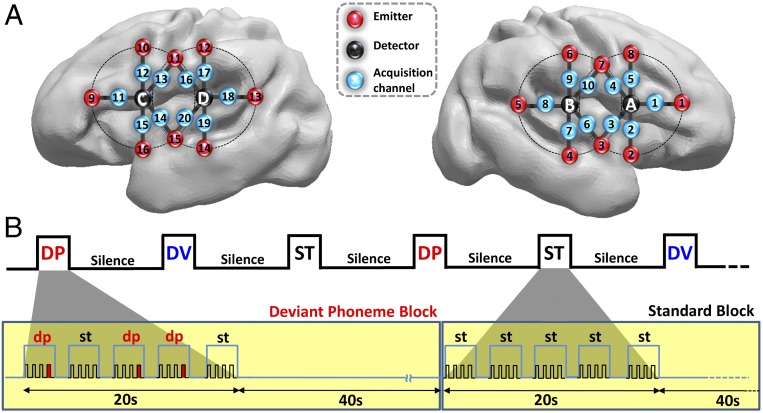
Because it is ethically problematic to move preterm infants to a brain-imaging unit, we used functional near-infrared spectroscopy (fNIRS) to test them at bedside. Oxy- (HbO) and deoxy-hemoglobin (Hb) absorb light specifically at different wavelengths. It is thus possible to measure changes in their concentration in the vessels surrounding a neurally active region and to infer the neural response to external stimuli in the cortex coarsely located between a light emitter and a detector placed on the scalp (26). Here, we used 10 measurement channels placed above the perisylvian areas of each hemisphere and a block design (20 s of stimulation followed by 40 s of silence) to present syllables organized in three types of blocks (Fig. 1). In standard blocks (ST), all syllables were identical. In deviant blocks, 15% were different, either beginning with another consonant in deviant phoneme blocks (DP) or changing in voice sex in deviant voice blocks (DV). Repetition of the same stimulus classically produces a decrease in neural activity, whereas a sudden change causes a recovery of the neural activity in regions coding the parameter that changed (18, 27). This recovery, or mismatch response, affects the amplitude and temporal characteristics (i.e., slope and latency to the peak) of the hemodynamic response in adults (28–30) and infants (3, 22). We thus expected a weaker amplitude and slower response in standard blocks relative to deviants blocks if preterms perceive the change of stimulus (22). We tested amplitude differences between experimental conditions first with cluster-based statistics considering each time bin and channel and second with an ANOVA on the area under the curve (AUC) to reveal the channels with the most robust and sustained differences. Then we evaluated the response dynamics in each condition by determining its significant onset relative to baseline in each channel and its global slope in each hemisphere. Because these analyses suggested a transient response for the deviant voice condition not captured by the previous analyses, we reexamined the amplitude differences while focusing on the first 6 s of stimulation (i.e., during the rising edge of the hemodynamic response).
Fig. 1.
(A) Estimated projection of the optodes (channels 1–10: right hemisphere; channels 11–20: left hemisphere) on the brain of a 30 wGA preterm infant (Fig. S1). (B) Experimental paradigm, deviant phoneme (DP), deviant voice (DV), and standard (ST) blocks (Materials and Methods).
Results
Hemodynamic Responses to Speech in Preterms’ Perisylvian Regions.
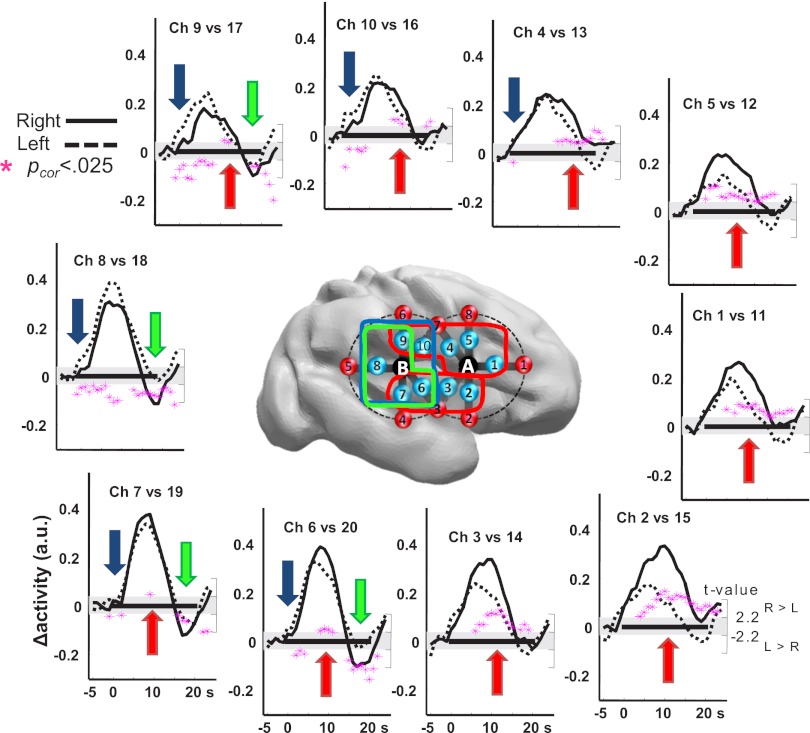
The neuro-vascular coupling already described in premature infants (31) was sufficiently mature to track brain responses to our stimulation paradigm in bilateral perisylvian channels. HbO increased and Hb decreased shortly after auditory stimulation, with a time course similar to full-term infants and adults (3, 32), peaking ∼5–7 s after the stimulus onset (Movie S1; Fig. S2). We focused our analyses on the HbO signal, whose signal-to-noise ratio is better than Hb, and used cluster-based statistics to probe asymmetries in the responses to all conditions merged together. The responses were mostly right lateralized as established by a larger right than left amplitude in a suprasylvian and in a temporal cluster of channels (ch): ch 1, 4, 5, 9, and 10 (5–23 s; Pcluster-cor = 0.0002) and ch 2, 3, 6, and 7 (6–25 s; Pcluster-cor = 0.0005). However, a left-hemispheric lateralization was observed over the posterior temporal and supramarginal regions: on these channels, the response started earlier [ch 4 and 6–10 (1–11 s); Pcluster-cor = 0.005], and the final undershoot was less pronounced [ch 6–9 (15–25 s); Pcluster-cor = 0.002] on the left than on the right hemisphere (Fig. 2).
Fig. 2.
Functional asymmetry to speech sounds in premature infants. The right and left grand average HbO responses to all conditions are plotted for symmetrical channels, whose locations are presented over a right hemisphere view of a preterm brain. The black rectangle along the x axis indicates the duration of the stimulation block (0–20 s). Pink stars indicate the t-value of the samples (right scale) included in significant clusters (Pcor < 0.025). Values are positive when right > left and negative for the opposite direction. The four significant temporo-spatial clusters are identified by colored arrows on the plots and lines surrounding the channels on the brain. Responses are larger on the right hemisphere (red clusters) but are faster (blue cluster) and last longer (green cluster) in the posterior left regions.
Phoneme and Voice Changes Elicit Different Responses.
We then examined the HbO responses to deviant stimuli. The hemodynamic response in the DP condition was faster, larger, and lasted longer in almost all channels relative to ST (Fig. 3; Movie S1). Cluster-based statistics included all channels into a single cluster within each hemisphere, significant for the entire duration of the analyzed segment (2–24 s; right: Pcluster-cor < 0.0001; left: Pcluster-cor = 0.0005; Fig. 3). The analysis of the AUC confirmed that the discrimination response was significant in left supramarginal ch 17–18, left frontal ch 12, and right posterior temporal ch 7 (Pcor < 0.003; Figs. S3 and S4 for individual responses).
Fig. 3.
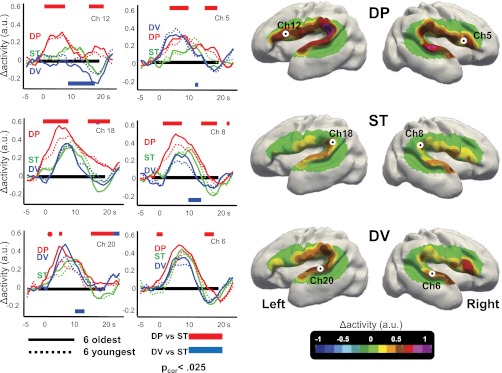
Discriminative responses to auditory stimuli in premature infants. (Right) Surface-based topographic color map of the HbO response at the peak of the hemodynamic response for the three conditions. (Left) HbO time courses for the youngest and oldest subsets of six infants, recorded over left Broca’s area (ch 12), left planum temporale (ch 18), left superior temporal gyrus (ch 20), and their counterlateral right channels (ch 5, ch 8, and ch 6, respectively). The colored rectangles indicate the time windows during which the deviant conditions differ significantly from the standard condition using cluster-based statistics over the whole group. The direction of the effect is given by the location of the bar under or above the x-line. Preterm infants discriminate the change of phoneme, notably in the left inferior frontal region. This effect was stable across our age range (28–32 wGA).
This robust response to a change of phoneme stood in sharp contrast to the response to a change of voice. Deviant-voice blocks elicited a significantly weaker and shorter response than standard blocks [right hemisphere cluster: ch 1–10 (8–17 s); Pcluster-cor = 0.003; left temporal cluster: ch 14, 15, and 20 (7–16 s), Pcluster-cor = 0.023; frontal/central cluster: ch 12, 13, and 16 (10–23 s); Pcluster = 0.017; Fig. 3; Figs. S5–S7], except over the left temporal and supramarginal regions where the sharp decrease of the signal at the end of the ST blocks was not observed in DV blocks [ch 14 and 17–20 (15–25 s); Pcluster-cor = 0.011]. The AUC analysis revealed a significant difference only in one channel (left temporal ch15) corresponding to a weaker response for DV than for ST. Thus, phonemic and voice changes elicited different responses in preterm brains. This difference between deviant blocks was confirmed by the direct comparison of DP vs. DV conditions which identified two significant clusters [all right channels (2–20 s); Pcluster-cor = 0.0002; left suprasylvian ch 11–13 and 16–18 (2–23 s); Pcluster-cor = 0.0027; Fig. S5] and by the AUC analysis showing significant differences for ch 7, 9, 15, 17, and 18 (Pscor < 0.003; Fig. S3).
Dynamics of the Hemodynamic Response.
Stimulus repetition generally causes a decrease in neural activity, whereas change elicits a signal recovery (3, 22, 28–30, 33). As deviant blocks always began with a deviant trial, we expected a boost in neural activity due to the novel stimulus and therefore a higher metabolic demand from the earliest seconds of stimulation. This hypothesis was confirmed by a steeper slope for DV and DP relative to ST over the right hemisphere (P = 0.002; Fig. 4C), but only for DP relative to ST (P = 0.005) over the left. Over the right hemisphere, the first significant increase of HbO over the baseline level was ∼2.5/3.8 s for the DP/DV vs. 6.9 s for the ST condition, but ∼1.7 (DP) vs. 7.9/8.3 s for DV/ST over the left hemisphere (Fig. 4 A and B).
Fig. 4.
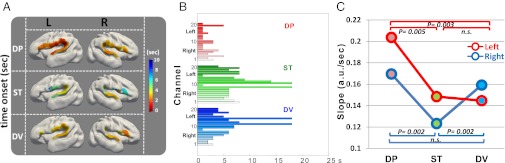
Dynamics of the HbO response. Topographic map (A) and bar plot (B) showing the latency of the first significant change in HbO relative to baseline in the different conditions. (C) Slope of the HbO response computed across all channels for each condition and hemisphere. The responses to both deviant conditions are faster than ST over the right hemisphere. Over the left hemisphere, only the DP condition is significantly different from the ST condition.
These analyses thus showed similar initial dynamics for both deviant conditions over the right hemisphere, suggesting a transient positive response to novelty even in the DV condition. This response might have been missed by our previous amplitude analyses that integrated over the whole block. We therefore reanalyzed the amplitude of the activation during the first 6 s of stimulation. We observed a larger response for DP vs. ST in channels similar to the above AUC analysis (left ch 12–13 and 16–18 and right ch 2 and 7; P < 0.002), confirming the early modulation of the activation by the perception of a phonemic change. For DV, a significant response was observed only in the right frontal ch 5 (DV vs. ST, Pcor = 0.0002; DP vs. ST, Pcor = 0.009; DP vs. DV, not significant). The right frontal region thus appeared sensitive to both types of changes, whereas its homologous left region only reacted to a change of phoneme (ch 12: DV vs. ST, not significant; DP vs. ST, Pcor = 0.0005; DP vs. DV, Pcor = 0.005; Fig. 3). This pattern yielded a significant hemisphere (ch 5 and 12) × condition (DP and DV) interaction (Pcor = 0.004).
Discrimination Responses Are Present from 29 wGA Onward.
Finally, we examined the stability of the responses in our age range from 28 to 32 wGA (Fig. 3; Fig. S7; Table S2). There was no significant correlation between age and the differences between conditions, either on the first 6 s of stimulation or over the entire block and no difference in the discrimination responses between the six youngest (mean age at test, 29w4d GA) and the six oldest infants (31w6d GA) using cluster-based statistics. Furthemore, most previous analyses were replicated in the youngest group. These babies reacted to a change of phoneme [e.g., analysis of the first 6 s DP vs. ST: main effect of condition: F(1,5) = 14.6, P = 0.012; condition × channel: F(9,45) = 6.57, P < 0.0001] and used the left inferior frontal region to detect the change of phoneme [ch 12: DP vs. ST, F(1,5) = 10.8, P = 0.022]. The response to a change of voice was also congruent with the general results. The condition × channel interaction was significant [F(9,45)= 3.72, P = 0.001], although not the main effect of condition [F(1,5) < 1]. The right inferior frontal region reacted to a change of voice [ch 5: DV vs. ST, F(1,5) = 8.15, P = 0.036], yielding a significant hemisphere × condition interaction [ch 5 vs. 12 × DP vs. DV: F(1,5) = 7.9, P = 0.037]. As expected, the same results were observed in the oldest group (Table S2).
Discussion
Our results demonstrate that the human brain, at the very onset of the establishment of a cortical circuit for auditory perception, already discriminates subtle differences in speech syllables. At this point in brain development, the majority of the neurons have not yet reached their final location, and their connectivity is still developing. Nevertheless, the early discrimination network is not limited to auditory areas but already involves inferior frontal regions.
Earlier research already established that the left inferior frontal region, one of the key regions of the adult linguistic network, is already active during speech perception in postterm infants (3, 34). However, it was suggested that its involvement might be secondary to infant’s first vocal productions (35, 36). The present results refute this interpretation: preterm infants at this age do not vocalize, and due to their clinical and immature condition, the present participants were not orally fed during their first days of life and were often intubated, restricting their orosensory experience. The early involvement of frontal regions in the speech network might rather represent the functional counterpart of the genetic homogeneity of the “speech regions” revealed by transcriptome analyses during midfetal life. Gene expression in the inferior frontal regions is more similar to other temporal and parietal speech areas than to other frontal areas (37). Leroy et al. (34) observed a correlation in the maturation of the inferior frontal region (area 44), the posterior temporal region, and the arcuate fasciculus during the first weeks of life. These results suggest that the superior temporal, inferior parietal, and inferior frontal regions, although distributed across three lobes, belong to a single functional unit.
A phoneme-sensitive cortical network was functional even in our younger infants and remained stable during the investigated time range (Fig. 3). Although we cannot certify that during the 3 d separating birth from the testing day, the environment did not trigger a very rapid development of cortical circuitry, one should remember that these infants were in an extremely precarious condition in an incubator in an intensive care unit and thus had limited contact with the human vocal environment. Peña et al. (38, 39) further demonstrated that preterms do not benefit from their additional exposure to speech, because the first stages of language acquisition are determined by postterm maturational age rather than by the duration of extrauterine life. As is the case for other complex ecological capacities in immature animals, the sophisticated organization of human perisylvian cortex thus appears driven by genetic factors more than by environmental exposure. In rats, for example, a rudimentary neural system for spatial orientation is observed in pups before their first exploration of the environment (40). Newly hatched and thus visually naïve chickens prefer stimuli with a natural biological motion (41). Similarly, phonetic discrimination appears to be part of the early endowment of a human brain. Further work should examine whether this discrimination capacity is shared with other immature mammals or specific to the human lineage.
By contrast with the classical hemodynamic response induced by a novel phoneme, the response to voice deviants was observable only as a fast response, notably over the right hemisphere, which rapidly faded significantly below the standard level after 7 s. This result was unexpected. The voice difference is based on larger acoustic differences than the phonetic contrast, and it was previously shown that preterms discriminate a change in tone frequency (6) and thus encode auditory spectral properties useful for voice perception. One possibility is that we missed the most anterior part of the right superior temporal region, a site of voice discrimination in adults (20) and infants (22, 23), as well as insula and subcortical structures inaccessible to our technique. Neural inhibition or vascular steal from these regions might explain the secondary decrease of the HbO signal significantly below the ST response. Further studies with more extensive recording locations and other brain-imaging techniques are necessary to understand whether these hemodynamic differences might reflect a different efficiency of preterm infants in processing voices and phonemes. In any case, the significant differences we recorded here between both deviant conditions highlight the functional regionalization already present in the preterm infant’s cortex. In that respect, the difference in frontal activation is particularly striking. The left frontal region reacted solely to a phoneme change whereas the controlateral right frontal region reacted to both changes, suggesting that it acts as a generic novelty detector as previously observed in infants (4) and adults (42).
This asymmetry in frontal activation provides additional evidence for an early hemispheric differentiation in humans. Many sulci appear 1 or 2 wk earlier on the right than on the left side (24, 43). Furthermore, cerebral blood flow at rest is larger (44) and the EEG power is higher (45) in the right hemisphere relative to the left before term. These results are congruent with the largest amplitude of the right responses recorded here over most of the channels (Fig. 2). In this context, the reverse asymmetry over the posterior region is noteworthy but congruent with the same left hemisphere bias repeatedly reported at the level of the planum temporale in infants for speech stimuli (3–5, 22) but not for music (22, 46). This functional asymmetry cannot be explained by a different distance between our sensors and the brain: we measured no left–right asymmetries in distance at these posterior sites (SI Text). The nature of the left-hemispheric functional advantage for speech processing in the posterior temporal region needs to be further explored, but the left–right difference in response speed observed here might suggest faster processing in the left auditory associative areas, allowing a better temporal sampling of auditory input and thus particularly adapted to the fast formant transitions present in speech (4, 16).
To conclude, our results reveal an early organization of the immature human brain into functions useful for deciphering the speech signal. Seventy-six percent of human genes are expressed in the fetal brain from 18 to 23 wGA, and 44% of these are differentially regulated (37). This genetic complexity might endow the human brain with the early functional competences observed here. Although our research demonstrates that brain networks sensitive to phonemes and voices are present at the very onset of cortical organization in humans, it does not challenge the fact that experience is also crucial for their fine tuning and for learning the specific properties of the native language.
Materials and Methods
Participants.
Twelve healthy preterm neonates (mean GA at birth, 30.3 ± 1.6 wGA) were tested asleep (recording age, 3.4 ± 1.2 d, corresponding to 30.7 ± 1.5 wGA). All infants had normal auditory and neurological clinical evaluations and were considered at low risk for brain damage (see details in Table S1). Parents were informed of the study and provided their written informed consent. The study was approved by the local ethics committee (Comité Pour la Protection des Personnes Nord-Ouest II).
Experimental Paradigm and Data Recording.
Four digitized syllables /ba/ and /ga/, naturally produced by a French male (bam, gam) and a French female speaker (baf, gaf), were matched for intonation, intensity, total duration (285 ms), prevoicing, and voiced formant transition duration (40 and 45 ms, respectively). These stimuli, used in a previous ERP experiment, induce discrimination responses in 4-mo-old full-term infants (47).
The syllables were presented in series of four (stimulus onset synchrony = 600 ms) to form three types of trials (standard, deviant voice, and deviant phoneme trials). In standard trials, the same syllable was repeated four times (e.g., gam gam gam gam). In deviant trials, the last syllable changed relatively to the first three either along the voice dimension (e.g., gam gam gam gaf), or along the phonetic dimension (e.g., gam gam gam bam). Three types of block were constituted and presented randomly, separated by 40 s of silence for a total duration of 108 min (Fig. 1). Each block (duration 20 s) comprised five trials separated by an intertrial interval of 1,600 ms. In standard blocks (ST), all trials were standard trials. Deviant blocks always began with a deviant trial, and then two standard and two deviant trials were randomly intermixed. Deviant trials were along the voice dimension in DV blocks and along the phonetic dimension in DP blocks. In each block, the repeated syllable was kept constant and was randomly chosen among the four possible syllables (bam, gam, baf, and gaf) to present each syllable the same number of times in each condition and infant.
The infants were tested asleep at night to avoid the day light and the intense day activity of a neonatal care unit. Stimuli were presented at a comfortable hearing level (∼70 dB) via speakers placed at the infant’s feet, and brain responses were measured with a multichannel frequency domain–based optical imaging system (Imagent; ISS). A special probe made from soft and flexible foam was smoothly secured relatively to anatomical markers to cover the left and right perisylvian areas, providing 10 recording points on each side (see SI Text for more details on the recording system).
Data Processing and Statistical Analyses.
After converting signal intensities in Hb/HbO concentrations, artifacted blocks were rejected (z-score > 4 in any channel and time sample). The remaining data were band-pass filtered (0.03–0.5 Hz) and segmented relative to the onset of each block (−5 to +25 s). After a linear detrend and a baseline correction were applied, the segments were averaged by condition and channel in each subject (average number of blocks per condition: 19, 19, and 21 for ST, DV, and DP, respectively).
Statistical analyses were performed on the amplitude of the HbO signal (see SI Text for complete details on the analyses). Experimental conditions (left vs. right responses, ST vs. DP, ST vs. DV, DV vs. DP) were compared by using two-tailed t tests on the considered variable. A total of 4,096 (212 subjects) random permutations of the original conditions were performed to establish the permutation P value for each comparison. To control the risk of false positive due to multiple comparisons, we either used cluster-based statistics as implemented in the Fieldtrip Matlab toolbox (48), or we corrected the permutation P value by the number of measures done in the considered analysis following the Holm’s procedure.
Our statistical approach was as follows. First, we used cluster-based statistics on the 30 time bins × 20 channels to automatically identify, without prior hypotheses, spatial and temporal clusters showing a significant difference between experimental conditions. Second, to circumscribe our effects to their main spatial location, we reduced the HbO response in each channel and condition to the AUC. Third, to study the dynamics of the response, we evaluated the latency of the first of two samples showing a significant amplitude increase relative to baseline in each condition and channel. We completed this analysis by an analysis of the slope of the HbO signal averaged across all channels of each hemisphere. Because this analysis revealed significant differences between standard and deviant blocks, we next focused on the signal averaged on the first 6 s of the blocks. Finally, to evaluate a potential effect of maturation during our age range, we separated our group in two subsets of six infants, younger and older than 31 wGA at birth (mean age at test, 29w4d vs. 31w6d GA), and looked for differences between these two subgroups in the DP vs. ST and DV vs. ST comparisons using cluster-based analyses. To question the precocity of these discrimination responses, we also performed the same analyses as above, restricted to the younger group.
Supplementary Material
Acknowledgments
We thank Petra Huppi for providing preterm MRI data. This work was supported by the Picardie regional council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212220110/-/DCSupplemental.
References
- 1.Mehler J, et al. A precursor of language acquisition in young infants. Cognition. 1988;29(2):143–178. doi: 10.1016/0010-0277(88)90035-2. [DOI] [PubMed] [Google Scholar]
- 2.DeCasper AJ, Spence MJ. Prenatal maternal speech influences newborn's perception of speech sounds. Infant Behav Dev. 1986;9(2):133–150. [Google Scholar]
- 3.Dehaene-Lambertz G, et al. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proc Natl Acad Sci USA. 2006;103(38):14240–14245. doi: 10.1073/pnas.0606302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298(5600):2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- 5.Peña M, et al. Sounds and silence: An optical topography study of language recognition at birth. Proc Natl Acad Sci USA. 2003;100(20):11702–11705. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Draganova R, et al. Sound frequency change detection in fetuses and newborns, a magnetoencephalographic study. Neuroimage. 2005;28(2):354–361. doi: 10.1016/j.neuroimage.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Elman JL, et al. Rethinking Innateness: A Connectionist Perspective on Development. Cambridge, MA: MIT Press; 1996. [Google Scholar]
- 8.Kostović I, Judas M, Petanjek Z, Simić G. Ontogenesis of goal-directed behavior: Anatomo-functional considerations. Int J Psychophysiol. 1995;19(2):85–102. doi: 10.1016/0167-8760(94)00081-o. [DOI] [PubMed] [Google Scholar]
- 9.Moore AR, Zhou WL, Jakovcevski I, Zecevic N, Antic SD. Spontaneous electrical activity in the human fetal cortex in vitro. J Neurosci. 2011;31(7):2391–2398. doi: 10.1523/JNEUROSCI.3886-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi E, Folkerth RD, Galaburda AM, Grant PE. Emerging cerebral connectivity in the human fetal brain: An MR tractography study. Cereb Cortex. 2012;22(2):455–464. doi: 10.1093/cercor/bhr126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rotteveel JJ, de Graaf R, Stegeman DF, Colon EJ, Visco YM. The maturation of the central auditory conduction in preterm infants until three months post term. V. The auditory cortical response (ACR) Hear Res. 1987;27(1):95–110. doi: 10.1016/0378-5955(87)90029-3. [DOI] [PubMed] [Google Scholar]
- 13.Jardri R, et al. Fetal cortical activation to sound at 33 weeks of gestation: A functional MRI study. Neuroimage. 2008;42(1):10–18. doi: 10.1016/j.neuroimage.2008.04.247. [DOI] [PubMed] [Google Scholar]
- 14.Miller GA, Nicely PE. An analysis of perceptual confusions among some English consonants. J Acoust Soc Am. 1955;27(2):338–352. [Google Scholar]
- 15.Kraus N, et al. Auditory neurophysiologic responses and discrimination deficits in children with learning problems. Science. 1996;273(5277):971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- 16.Zatorre RJ, Belin P. Spectral and temporal processing in human auditory cortex. Cereb Cortex. 2001;11(10):946–953. doi: 10.1093/cercor/11.10.946. [DOI] [PubMed] [Google Scholar]
- 17.Dehaene-Lambertz G, et al. Neural correlates of switching from auditory to speech perception. Neuroimage. 2005;24(1):21–33. doi: 10.1016/j.neuroimage.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 18.Dehaene-Lambertz G, Dehaene S. Speed and cerebral correlates of syllable discrimination in infants. Nature. 1994;370(6487):292–295. doi: 10.1038/370292a0. [DOI] [PubMed] [Google Scholar]
- 19.Dehaene-Lambertz G, Baillet S. A phonological representation in the infant brain. Neuroreport. 1998;9(8):1885–1888. doi: 10.1097/00001756-199806010-00040. [DOI] [PubMed] [Google Scholar]
- 20.Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403(6767):309–312. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- 21.Bristow D, et al. Hearing faces: How the infant brain matches the face it sees with the speech it hears. J Cogn Neurosci. 2009;21(5):905–921. doi: 10.1162/jocn.2009.21076. [DOI] [PubMed] [Google Scholar]
- 22.Dehaene-Lambertz G, et al. Language or music, mother or Mozart? Structural and environmental influences on infants’ language networks. Brain Lang. 2010;114(2):53–65. doi: 10.1016/j.bandl.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Blasi A, et al. Early specialization for voice and emotion processing in the infant brain. Curr Biol. 2011;21(14):1220–1224. doi: 10.1016/j.cub.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. 1977;1(1):86–93. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- 25.Dubois J, et al. Primary cortical folding in the human newborn: An early marker of later functional development. Brain. 2008;131(Pt 8):2028–2041. doi: 10.1093/brain/awn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aslin RN. Questioning the questions that have been asked about the infant brain using near-infrared spectroscopy. Cogn Neuropsychol. 2012;29(1-2):7–33. doi: 10.1080/02643294.2012.654773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulanovsky N, Las L, Nelken I. Processing of low-probability sounds by cortical neurons. Nat Neurosci. 2003;6(4):391–398. doi: 10.1038/nn1032. [DOI] [PubMed] [Google Scholar]
- 28.Kruggel F, von Cramon DY. Temporal properties of the hemodynamic response in functional MRI. Hum Brain Mapp. 1999;8(4):259–271. doi: 10.1002/(SICI)1097-0193(1999)8:4<259::AID-HBM9>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dehaene-Lambertz G, et al. Functional segregation of cortical language areas by sentence repetition. Hum Brain Mapp. 2006;27(5):360–371. doi: 10.1002/hbm.20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sigman M, Jobert A, Lebihan D, Dehaene S. Parsing a sequence of brain activations at psychological times using fMRI. Neuroimage. 2007;35(2):655–668. doi: 10.1016/j.neuroimage.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 31.Roche-Labarbe N, Wallois F, Ponchel E, Kongolo G, Grebe R. Coupled oxygenation oscillation measured by NIRS and intermittent cerebral activation on EEG in premature infants. Neuroimage. 2007;36(3):718–727. doi: 10.1016/j.neuroimage.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Telkemeyer S, et al. Sensitivity of newborn auditory cortex to the temporal structure of sounds. J Neurosci. 2009;29(47):14726–14733. doi: 10.1523/JNEUROSCI.1246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thierry G, Ibarrola D, Démonet JF, Cardebat D. Demand on verbal working memory delays haemodynamic response in the inferior prefrontal cortex. Hum Brain Mapp. 2003;19(1):37–46. doi: 10.1002/hbm.10101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leroy F, et al. Early maturation of the linguistic dorsal pathway in human infants. J Neurosci. 2011;31(4):1500–1506. doi: 10.1523/JNEUROSCI.4141-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kujala A, et al. Speech-sound discrimination in neonates as measured with MEG. Neuroreport. 2004;15(13):2089–2092. doi: 10.1097/00001756-200409150-00018. [DOI] [PubMed] [Google Scholar]
- 36.Perani D, et al. Neural language networks at birth. Proc Natl Acad Sci USA. 2011;108(38):16056–16061. doi: 10.1073/pnas.1102991108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson MB, et al. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62(4):494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peña M, Pittaluga E, Mehler J. Language acquisition in premature and full-term infants. Proc Natl Acad Sci USA. 2010;107(8):3823–3828. doi: 10.1073/pnas.0914326107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peña M, Werker JF, Dehaene-Lambertz G. Earlier speech exposure does not accelerate speech acquisition. J Neurosci. 2012;32(33):11159–11163. doi: 10.1523/JNEUROSCI.6516-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langston RF, et al. Development of the spatial representation system in the rat. Science. 2010;328(5985):1576–1580. doi: 10.1126/science.1188210. [DOI] [PubMed] [Google Scholar]
- 41.Vallortigara G, Regolin L, Marconato F. Visually inexperienced chicks exhibit spontaneous preference for biological motion patterns. PLoS Biol. 2005;3(7):e208. doi: 10.1371/journal.pbio.0030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazoyer BM, et al. The cortical representation of speech. J Cogn Neurosci. 1993;5(4):467–479. doi: 10.1162/jocn.1993.5.4.467. [DOI] [PubMed] [Google Scholar]
- 43.Dubois J, et al. Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex. 2008;18(6):1444–1454. doi: 10.1093/cercor/bhm180. [DOI] [PubMed] [Google Scholar]
- 44.Lin P-Y, et al. Regional and hemispheric asymmetries of cerebral hemodynamic and oxygen metabolism in newborns. Cereb Cortex. 2013;23(2):339–348. doi: 10.1093/cercor/bhs023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myers MM, et al. Developmental profiles of infant EEG: Overlap with transient cortical circuits. Clin Neurophysiol. 2012;123(8):1502–1511. doi: 10.1016/j.clinph.2011.11.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perani D, et al. Functional specializations for music processing in the human newborn brain. Proc Natl Acad Sci USA. 2010;107(10):4758–4763. doi: 10.1073/pnas.0909074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dehaene-Lambertz G. Cerebral specialization for speech and non-speech stimuli in infants. J Cogn Neurosci. 2000;12(3):449–460. doi: 10.1162/089892900562264. [DOI] [PubMed] [Google Scholar]
- 48.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.