Abstract
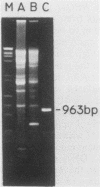
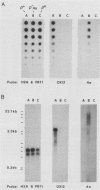
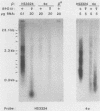
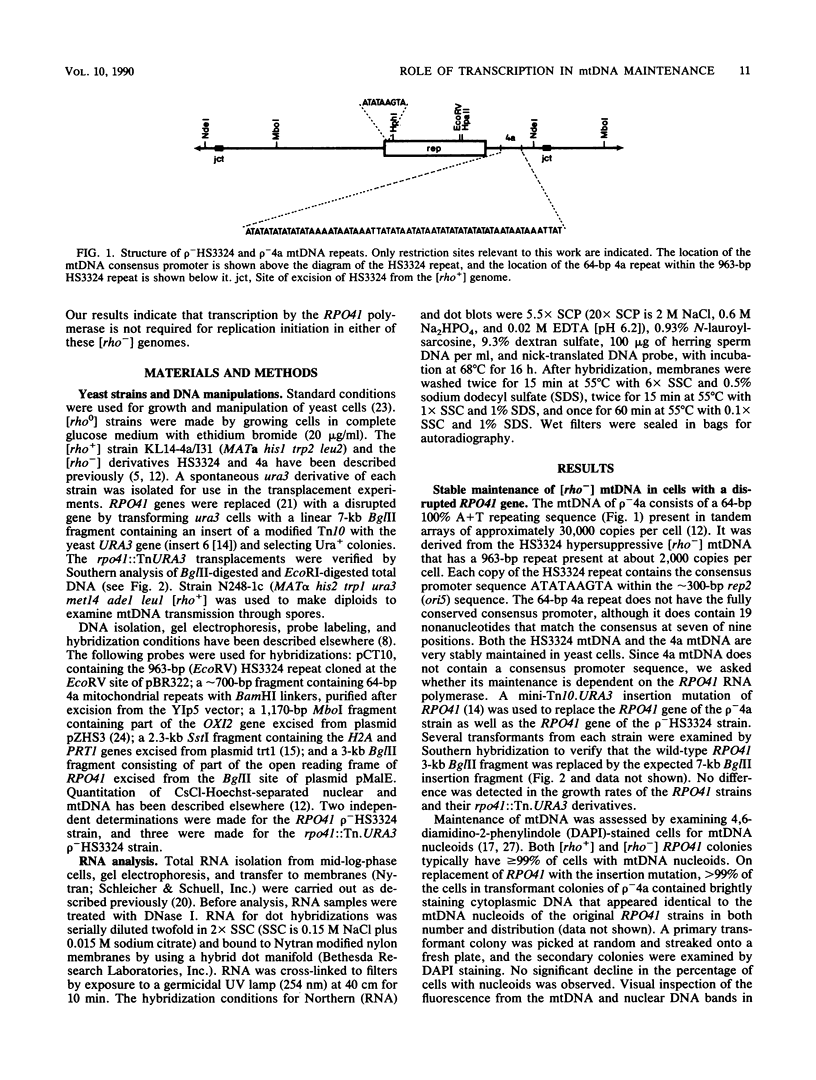
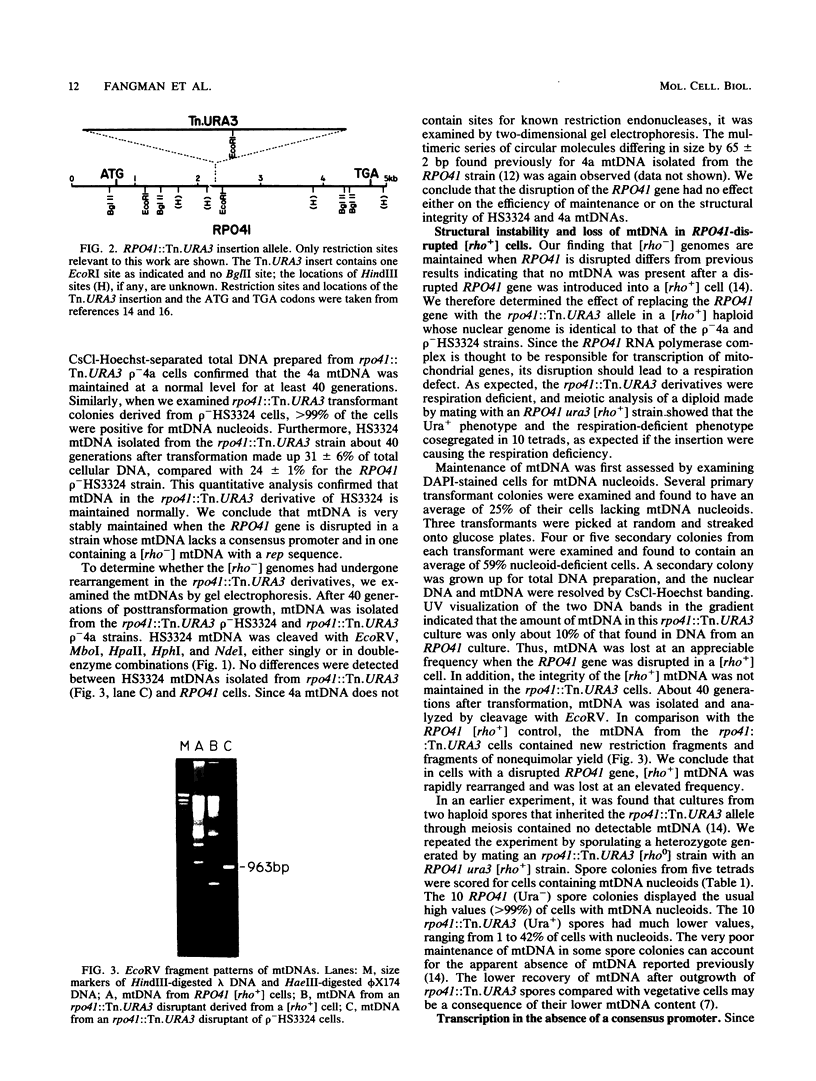
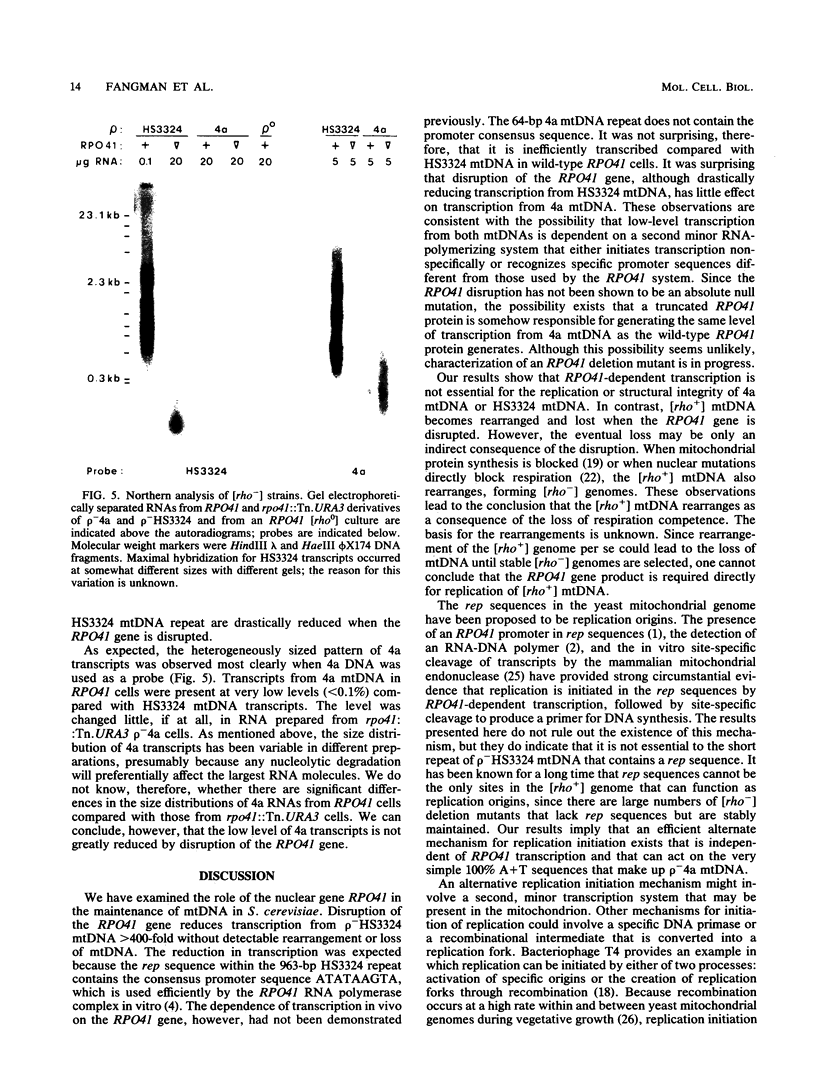
A subset of promoters in the mitochondrial DNA (mtDNA) of the yeast Saccharomyces cerevisiae has been proposed to participate in replication initiation, giving rise to a primer through site-specific cleavage of an RNA transcript. To test whether transcription is essential for mtDNA maintenance, we examined two simple mtDNA deletion ([rho-]) genomes in yeast cells. One genome (HS3324) contains a consensus promoter (ATATAAGTA) for the mitochondrial RNA polymerase encoded by the nuclear gene RPO41, and the other genome (4a) does not. As anticipated, in RPO41 cells transcripts from the HS3324 genome were more abundant than were transcripts from the 4a genome. When the RPO41 gene was disrupted, both [rho-] genomes were efficiently maintained. The level of transcripts from HS3324 mtDNA was decreased greater than 400-fold in cells carrying the RPO41 disrupted gene; however, the low-level transcripts from 4a mtDNA were undiminished. These results indicate that replication of [rho-] genomes can be initiated in the absence of wild-type levels of the RPO41-encoded RNA polymerase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldacci G., Bernardi G. Replication origins are associated with transcription initiation sequences in the mitochondrial genome of yeast. EMBO J. 1982;1(8):987–994. doi: 10.1002/j.1460-2075.1982.tb01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldacci G., Chérif-Zahar B., Bernardi G. The initiation of DNA replication in the mitochondrial genome of yeast. EMBO J. 1984 Sep;3(9):2115–2120. doi: 10.1002/j.1460-2075.1984.tb02099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas T. K., Ticho B., Getz G. S. In vitro characterization of the yeast mitochondrial promoter using single-base substitution mutants. J Biol Chem. 1987 Oct 5;262(28):13690–13696. [PubMed] [Google Scholar]
- Blanc H., Dujon B. Replicator regions of the yeast mitochondrial DNA responsible for suppressiveness. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3942–3946. doi: 10.1073/pnas.77.7.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc H. Two modules from the hypersuppressive rho- mitochondrial DNA are required for plasmid replication in yeast. Gene. 1984 Oct;30(1-3):47–61. doi: 10.1016/0378-1119(84)90104-5. [DOI] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. Preferential inclusion of extrachromosomal genetic elements in yeast meiotic spores. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5380–5384. doi: 10.1073/pnas.77.9.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer B. J., Fangman W. L. The localization of replication origins on ARS plasmids in S. cerevisiae. Cell. 1987 Nov 6;51(3):463–471. doi: 10.1016/0092-8674(87)90642-8. [DOI] [PubMed] [Google Scholar]
- Fangman W. L., Henly J. W., Churchill G., Brewer B. J. Stable maintenance of a 35-base-pair yeast mitochondrial genome. Mol Cell Biol. 1989 May;9(5):1917–1921. doi: 10.1128/mcb.9.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faugeron-Fonty G., Le Van Kim C., de Zamaroczy M., Goursot R., Bernardi G. A comparative study of the ori sequences from the mitochondrial genomes of twenty wild-type yeast strains. Gene. 1984 Dec;32(3):459–473. doi: 10.1016/0378-1119(84)90020-9. [DOI] [PubMed] [Google Scholar]
- Greenleaf A. L., Kelly J. L., Lehman I. R. Yeast RPO41 gene product is required for transcription and maintenance of the mitochondrial genome. Proc Natl Acad Sci U S A. 1986 May;83(10):3391–3394. doi: 10.1073/pnas.83.10.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford L. M., Osley M. A., Ludwig T. R., 2nd, McLaughlin C. S. Cell-cycle regulation of yeast histone mRNA. Cell. 1981 May;24(2):367–375. doi: 10.1016/0092-8674(81)90326-3. [DOI] [PubMed] [Google Scholar]
- Masters B. S., Stohl L. L., Clayton D. A. Yeast mitochondrial RNA polymerase is homologous to those encoded by bacteriophages T3 and T7. Cell. 1987 Oct 9;51(1):89–99. doi: 10.1016/0092-8674(87)90013-4. [DOI] [PubMed] [Google Scholar]
- Miyakawa I., Sando N., Kawano S., Nakamura S., Kuroiwa T. Isolation of morphologically intact mitochondrial nucleoids from the yeast, Saccharomyces cerevisiae. J Cell Sci. 1987 Nov;88(Pt 4):431–439. doi: 10.1242/jcs.88.4.431. [DOI] [PubMed] [Google Scholar]
- Mosig G. The essential role of recombination in phage T4 growth. Annu Rev Genet. 1987;21:347–371. doi: 10.1146/annurev.ge.21.120187.002023. [DOI] [PubMed] [Google Scholar]
- Myers A. M., Pape L. K., Tzagoloff A. Mitochondrial protein synthesis is required for maintenance of intact mitochondrial genomes in Saccharomyces cerevisiae. EMBO J. 1985 Aug;4(8):2087–2092. doi: 10.1002/j.1460-2075.1985.tb03896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M., Sclafani R. A., Fangman W. L., Rosamond J. Molecular characterization of cell cycle gene CDC7 from Saccharomyces cerevisiae. Mol Cell Biol. 1986 May;6(5):1590–1598. doi: 10.1128/mcb.6.5.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Thalenfeld B. E., Hill J., Tzagoloff A. Assembly of the mitochondrial membrane system. Characterization of the oxi2 transcript and localization of its promoter in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1983 Jan 10;258(1):610–615. [PubMed] [Google Scholar]
- Williamson D. H., Fennell D. J. Apparent dispersive replication of yeast mitochondrial DNA as revealed by density labelling experiments. Mol Gen Genet. 1974;131(3):193–207. doi: 10.1007/BF00267959. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Fennell D. J. The use of fluorescent DNA-binding agent for detecting and separating yeast mitochondrial DNA. Methods Cell Biol. 1975;12:335–351. doi: 10.1016/s0091-679x(08)60963-2. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Faugeron-Fonty G., Baldacci G., Goursot R., Bernardi G. The ori sequences of the mitochondrial genome of a wild-type yeast strain: number, location, orientation and structure. Gene. 1984 Dec;32(3):439–457. doi: 10.1016/0378-1119(84)90019-2. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Marotta R., Faugeron-Fonty G., Goursot R., Mangin M., Baldacci G., Bernardi G. The origins of replication of the yeast mitochondrial genome and the phenomenon of suppressivity. Nature. 1981 Jul 2;292(5818):75–78. doi: 10.1038/292075a0. [DOI] [PubMed] [Google Scholar]