Abstract
Objective
To study the relationship between self-reported childhood maltreatment and cerebral gray matter in adolescents without psychiatric diagnoses.
Design
Associations between childhood maltreatment (measured by a childhood trauma self-report questionnaire for physical, emotional and sexual abuse, and physical and emotional neglect) and regional gray matter were examined.
Setting
University hospital.
Participants
42 adolescents without psychiatric disorders.
Outcome Measures
Correlations between childhood trauma questionnaire scores and regional gray matter volume were assessed in voxel-based analyses of structural magnetic resonance scans. Relationships between gray matter volume and childhood maltreatment subtypes and gender where explored.
Results
Total childhood trauma questionnaire scores correlated negatively (p<0.005) with gray matter volumes in prefrontal cortex, striatum, amygdala, sensory association cortices and cerebellum. Physical abuse, physical neglect and emotional neglect were associated with rostral prefrontal reductions. Additionally, decreases in dorsolateral and orbitofrontal cortices, insula, and ventral striatum were associated with physical abuse, in cerebellum with physical neglect, and in dorsolateral, orbitofrontal and subgenual prefrontal cortices, striatum, amygdala, hippocampus and cerebellum with emotional neglect. These latter emotion regulation regions were also associated with childhood trauma questionnaire scores in females, while caudate reductions, which may relate to impulse dyscontrol, were seen in males.
Conclusions
Childhood maltreatment was associated with corticostriatal-limbic gray matter reductions in adolescents. These findings suggest that even if adolescents reporting childhood maltreatment exposure do not present with symptoms that meet full criteria for psychiatric disorders, they may have corticostriatal-limbic changes that place them at risk for behavioral difficulties. Vulnerabilities may be moderated by gender and maltreatment subtype.
INTRODUCTION
An estimated 3.7 million children are assessed for child maltreatment (CM) each year in the United States; this number likely underestimates the number of children experiencing maltreatment since many do not come to professional attention.1 Increasing evidence suggests these children may endure long-lasting neural consequences of CM that place them at risk for behavioral and psychiatric sequelae. Converging evidence supports adverse effects of early life stress on morphological development of corticostriatal-limbic structures.2–4 Magnetic resonance imaging (MRI) studies show decreased corticostriatal-limbic gray matter (GM) volume in children and adults reporting exposure to CM.5–14 GM changes in the intervening adolescent epoch can also be inferred. However, few studies focus on the neurobiological effects of CM in adolescents.
Corticostriatal-limbic GM volume decreases are well established in adults reporting CM. The hippocampus has been the region most studied in adults reporting exposure to CM, consistently demonstrating volume decreases.5,8,9,10,13,15 Volume reductions in the prefrontal cortex (PFC), striatum and amygdala have also been demonstrated in studies of adults reporting CM.8–12, 16 Furthermore, preclinical CM models implicate more widespread corticostriatal-limbic involvement,3,16,17 suggesting a whole brain approach may be especially helpful in revealing distributed CM effects. Neuroimaging studies of CM have largely assessed adults with psychiatric disorders, especially post-traumatic stress disorder (PTSD) and borderline personality disorder, limiting ability to ascertain whether brain changes are related to CM and/or the disorders. These studies also often focused on CM broadly, limiting ability to determine effects of CM subtypes.
There are fewer imaging studies of children exposed to maltreatment. PFC volume decreases found in pediatric samples with PTSD secondary to CM suggest some PFC changes observed in adults with CM may originate in childhood.18 However, some regional differences between pediatric and adult manifestations of CM are also suggested. For example, studies of children reporting CM do not show the hippocampal volume decreases prominent in adults. It has been suggested that this may be a result of delayed expression of effects of CM in this brain region.14,19 Similar to adult studies, studies in children have been performed largely in samples with PTSD, and have not investigated effects of abuse or neglect subtypes.
Associations between CM and GM volume in adolescents are inferred, but few studies focus on adolescents. Adolescents have been included in some pediatric studies, though often analyzed together with prepubertal children. One recent study of adolescents reporting more general early-life adversities, ranging from physical neglect and emotional abuse to witnessing domestic violence or experiencing a life threatening injury, did show hippocampus volume decreases.20 This suggests that some differences found in adults, such as those in hippocampus, may be expressed by adolescence. To the best of our knowledge, there has not been a prior study that focused on adolescents reporting CM that used a whole brain approach to study distributed brain effects.
Similarities and differences in the sequelae of different types of abuse and neglect are not known, but could indicate differing vulnerabilities and need for differing detection and intervention approaches.21 Although physical and sexual abuse have been associated with increased depression risk,22,23 studies increasingly suggest emotional maltreatment may have strong effects on the development of negative self-associations and depression.24–28 Furthermore, hippocampal and striatal alterations in adults have been associated with reported childhood emotional neglect,9,29 suggesting that emotional neglect may have long-lasting effects on corticostriatal-limbic regions subserving emotional regulation. Sex difference may also modify effects of CM on corticostriatal-limbic morphology. The sexually dimorphic development of stress-sensitive corticostriatal-limbic regions30–33 have been suggested to contribute to sex differences in psychiatric disorders, such as in regions subserving emotions and increased depression risk in females and regions subserving impulse control and increased risk for substance abuse in males.34–39
In this morphometric structural MRI study, we used a whole-brain approach to study the regional distribution of GM volume differences associated with self-reported CM in adolescents without psychiatric diagnoses. We hypothesized CM severity would be inversely associated with volume in distributed corticostriatal-limbic GM regions, including PFC, striatum, amygdala and hippocampus. Furthermore, we hypothesized that different maltreatment subtypes would be associated with varying regional patterns of GM reductions, with emotional maltreatment associated with reductions in corticostriatal-limbic regions subserving emotional regulation. We also performed exploratory analyses by gender. We anticipated that GM volume reductions in corticostriatal-limbic brain regions subserving emotional regulation would be associated with CM exposure in females, while GM volume reductions in regions subserving impulse control would be associated with CM in males.
METHODS
Participants included 42 adolescents (ages 12–17years, mean 15.33±1.37years, 50% female, 19 Caucasian, 19 African American, 4 more than one race) without DSM-IV Axis I diagnoses confirmed by the revised Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version 2.0 administered to participants and their parents/guardians.40 Subjects were recruited from a sample of children identified at birth to be at high risk for CM and followed longitudinally (by LCM). Participants were also recruited from the greater New Haven Connecticut community, allowing for a sample of adolescents reporting a spectrum of CM severity. Subjects were without history of neurological illness, head trauma with loss of consciousness or major medical disorder. Written informed consent was obtained from parents/guardians, and assent from minors, in accordance with the institutional review board of the Yale School of Medicine.
Children completed the Childhood Trauma Questionnaire (CTQ),41,42 a self-report questionnaire on experience of five subtypes of maltreatment in childhood: physical abuse, physical neglect, emotional abuse, emotional neglect and sexual abuse. Physical abuse is defined as bodily assaults on a child by an older person that pose a risk of or result in injury (e.g., “People in my family hit me so hard that it left me with bruises or marks”). Physical neglect is defined as failure of caregivers to provide a child's basic physical needs including food, shelter, safety, supervision and health (e.g. “I didn't have enough to eat”). Emotional abuse is defined as verbal assaults on a child's sense of worth or well-being, or any humiliating, demeaning, or threatening behavior directed toward a child by an older person (e.g., “People in my family said hurtful or insulting things to me”). Emotional neglect is defined as the failure of caretakers to provide basic psychological and emotional needs, such as love, encouragement, belonging and support (e.g. “People in my family felt close to each other”). Sexual abuse is defined as sexual contact or conduct between a child and an older person (e.g. “Someone tried to make me do sexual things or try sexual things”). Participants rate items on the CTQ using a 5-point scale ranging from “never true” to “very often true.” Each CTQ subscale has five items so that subscale scores range from five (no maltreatment) to twenty-five (severe maltreatment). The five CM subtypes scores are summed for a Total CTQ score. All but six participants reported some form of CM, defined as a score of five or more on any of the five CM subtype scores. Physical abuse was reported by sixteen subjects, physical neglect by eighteen, emotional abuse by twenty-three, emotional neglect by thirty-four, and sexual abuse by six.
T-tests were performed to examine possible gender differences in age, CTQ total and subscale scores using Statistical Package for the Social Sciences for Windows, Version 11.1.43
High-resolution structural MRI scans were obtained on a 3T Trio Scanner (Siemens, Erlangen, Germany) using a three-dimensional Magnetization Prepared Rapid Acquisition Gradient Echo T1-weighted sequence (TR=1500ms, TE=2.83ms, FOV=256 × 256 mm2, matrix=256 × 256, 1.0mm sagittal slices without gap, 160 slices, NEX=2). Images were processed with the Statistical and Parametric Mapping 5 (SPM5) (http://www.fil.ion.ucl.ac.uk/spm) using our previous protocol.44 Briefly, the SPM5 segmentation function was implemented for bias correction, segmentation and spatial normalization. The modulated GM images were spatially smoothed using an 8-mm full width at half maximum Gaussian kernel.
Whole-brain linear regression analysis was performed in SPM5, covarying for age, to investigate the relationship between total CTQ scores and GM volume. Additional regression analyses of CTQ subscales and GM volume were performed only for subjects who reported the maltreatment subtype. Separate, exploratory regressions of CTQ total scores were also performed for female participants and for male participants. Results were considered significant at p<0.005 (uncorrected) and clusters >50voxels.
RESULTS
Females and males did not differ significantly in age, CTQ total or CTQ subscale scores. Total CTQ scores showed a significant inverse correlation with GM volume in bilateral dorsolateral prefrontal cortex (DLPFC) [Brodmann's Area (BA) 46/9], bilateral rostral prefrontal cortex (RPFC) (BA10), left subgenual PFC (sgPFC) (BA25), bilateral striatum and right amygdala, as well as left parietal (BA40/7) and right temporoparietal (BA22/40) association cortices, bilateral temporal cortex (BA20/21), right fusiform gyrus (BA20/37), bilateral occipital association cortex (BA18/19), bilateral cerebellum, and regions of hypothalamus and midbrain (Figure 1).
Figure 1. Gray Matter Decreases Associated with Childhood Maltreatment.
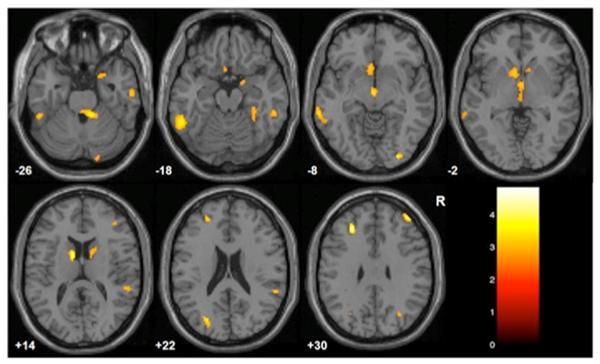
The images display regions where gray matter volume was inversely correlated with Childhood Trauma Questionnaire total scores, p<0.005 (uncorrected), cluster ≥50voxels. The color bar displays T-values, Montreal Neurological Institute z-plane coordinates (millimeters); R=right.
CTQ subscales scores showed inverse associations with GM volumes for self-reported: physical abuse with left DLPFC (BA46/9), left RPFC (BA10), right orbitofrontal cortex (OFC) (BA47), and right ventral striatum, right insula and right temporal association cortex (BA20/21) (Figure 2a), physical neglect with left RPFC (BA10), right parietal association cortex (BA40/39) and bilateral cerebellum (Figure 2b), emotional neglect with bilateral DLPFC (BA46/9), bilateral RPFC (BA10), bilateral dorsal anterior cingulate cortex (BA24/32), right superior frontal gyrus (BA8), right OFC (BA47), bilateral sgPFC (BA25), bilateral striatum, bilateral amygdala and hippocampus, left parietal association cortex (BA40), right temporal association cortex (BA20/38), left occipital association cortex (BA18/19), bilateral cerebellum, and regions of the midbrain and hypothalamus (Figure 2c). No significant results were found for emotional and sexual abuse subscales.
Figure 2. Gray Matter Decreases Associated with Childhood Maltreatment Subtypes.
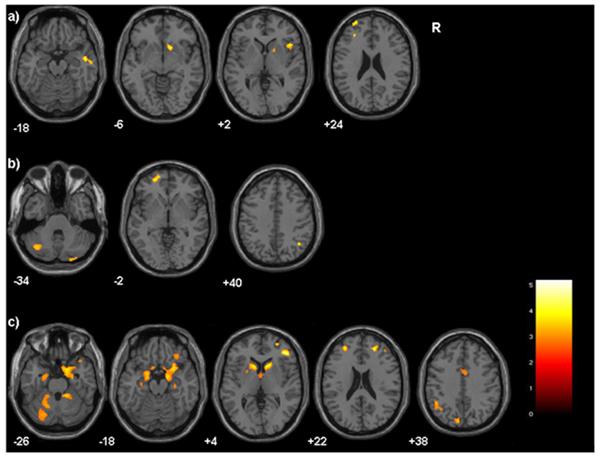
Axial-oblique images display regions where gray matter volume was inversely correlated with Childhood Trauma Questionnaire subscale scores, p<0.005(uncorrected), cluster ≥50voxels for: a)physical abuse b)physical neglect c)emotional neglect. The color bar displays T-values, Montreal Neurological Institute z-plane coordinates (millimeters); R=right.
In females, CTQ total scores were inversely correlated with volume in right RPFC (BA10), bilateral OFC (BA11/47), bilateral sgPFC (BA25/32), bilateral amygdala and hippocampus, right insula, bilateral temporal association cortex (BA20/21/38), bilateral fusiform gyrus (BA20), right temporo-occipital association cortex (BA37/19), left occipital association cortex (BA18/19), and bilateral cerebellum (Figure 3a). In males, CTQ total scores were inversely correlated with volume in bilateral caudate, bilateral temporoparietal cortex (BA37/40) and left temporo-occipital association cortex (BA37/19) (Figure 3b). There was a trend towards an inverse association with left RPFC (BA10); the cluster size was beneath the study threshold at 44 voxels.
Figure 3. Gray Matter Decreases Associated with Childhood Maltreatment in Females and Males.
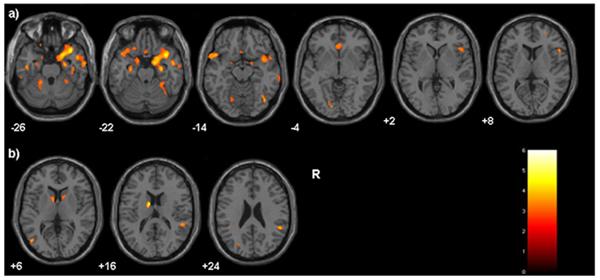
T1 axial-oblique images display regions where gray matter volume was inversely correlated with Childhood Trauma Questionnaire scores in a) females and b) males, p<0.005 (uncorrected), cluster≥50. The color bar displays T-values, Montreal Neurological Institute z-plane coordinates (millimeters); R=right.
COMMENT
Our results indicate that self-reported CM is associated with reductions in GM volume in a distributed corticostriatal-limbic system, including the DLPFC, RPFC, sgPFC, striatum, amygdala and hippocampus, as well as parietal, temporal and occipital association cortices and cerebellum. These findings were present in a sample of adolescents without psychiatric disorders. Results of exploratory analyses, while preliminary, support prominent reductions in RPFC volume common across physical abuse, physical neglect and emotional neglect CM subtypes, as well as patterns of additional regional volume decreases in the CM subtypes. Findings in females were in regions associated with emotional regulation, whereas in males with regions subserving impulse control.
Rodent and non-human primate models of CM show morphological alterations in PFC, striatum, amygdala and hippocampus.45–54 The mechanisms that underlie these changes are not clear; acute effects of stress on corticostriatal-limbic morphology demonstrated in the basic models include epigenetic effects on the hypothalamic-pituitary-adrenal (HPA) axis, dysregulated functioning of neurotransmitter and intracellular signaling pathways, as well as reductions in neurotrophic factors and neurogenesis.3,50–54 These morphological changes are associated with behavioral changes, including increased impulsive, anxious and depressive behaviors.49,55–57 Effects sustained through adolescence have been postulated to result from secondary dendritic spine remodeling and alterations in neurodevelopmental trajectories.2,58
Associations between self-reported CM and volume reductions in RPFC and DLPFC were prominent findings in this study. The PFC is one of the brain regions most vulnerable to stress in animal models, showing stress-related decreases in dendritic length, branching and spine densities.3, 48,50.59 The RPFC is associated with behavioral control functions including directing attention, decision-making, response inhibition and emotional regulation.60–63 DLPFC functions overlap RPFC functions, and include working memory, cognitive reappraisal of affective experience and behavioral planning.64–68 Reductions in these PFC regions, coupled with our findings in striatum, with which the PFC shares strong connections,69 suggest CM is associated with morphological alterations in a neural system that subserves behavioral, cognitive and emotional control, functions that are frequently disrupted in those reporting CM.70
Reports of physical abuse were also associated with reductions in the insula. The insula is central to interoceptive functions that monitor bodily and emotional states, and has been implicated in the experience of bodily ownership and agency, as well as empathic perception of these states in others.71–75 We speculate that the association observed between physical abuse and the insula could contribute to alterations in perceptions of bodily ownership and personal agency, as well as dissociative symptoms observed in persons who have been exposed to childhood physical abuse.76–78
Volume decreases in sensory association regions, including temporoparietal and occipital areas, were noted across analyses. Consistent with studies showing alterations in emotional face perception in adults,79 adolescents,80 and children81,82 exposed to CM, we found decreases in the fusiform gyrus, a region associated with face perception.83 Our results in parietal association regions are of interest given the association of attentional biases in perception with CM history in both adults and children.84,85 Findings in these sensory association regions are consistent with parietal alterations found in adults reporting CM exposure and borderline personality disorder86 and parietal and occipital alterations in PTSD,87 suggesting CM may alter perceptual integration through adulthood via GM changes.
Reported physical and emotional neglect were associated with volume decreases in cerebellum. The cerebellum has reciprocal connections with other CM-associated regions, including PFC, amygdala and hippocampus88,89 and contains high concentrations of glucocorticoid receptors.90 Rodent models of neglect suggest alterations in glucocorticoid receptor expression and cell degeneration in the cerebellum.91 Previous reports show reductions in cerebellar volume in neglected children92 and children with PTSD secondary to maltreatment.93 Cerebellar response has been associated with traumatic reminders in PTSD,94 recollection of emotional autobiographical information and fear conditioning.95 Together, these findings suggest further studies of the cerebellum in affective and anxious symptoms associated with CM are warranted.
Emotional neglect was additionally associated with volume reductions in a neural system subserving emotional regulation, including OFC, sgPFC, striatum, amygdala and hippocampus,96,97 in which abnormalities have been shown in mood disorders.98–100 Our findings are consistent with rodent models of postnatal neglect that show decreases in brain-derived neurotrophic factor and neurogenesis in these corticostriatal-limbic regions.47,52,57,101 These findings suggest that early emotional neglect may alter the development of this emotional regulation system, conferring increased risk for the development of mood disorders.
Our preliminary analyses within the female group suggest the association between CM and the neural system that subserves emotional regulation may be potent in female adolescents. Within this group, inverse associations were found between total CTQ scores and GM volume in the RPFC, OFC, sgPFC, insula, temporal cortex, amygdala, hippocampus and cerebellum. Pubertal hormones have organizing effects on the development of this system, and its development has been shown to be sexually dimorphic.31,32,102 Animal models suggest that estrogen may mediate stress sensitivity in PFC103 and hippocampus.104 In contrast, in males, there was a trend towards reductions in RPFC and reductions were significant in caudate; these regions are key components in the neural circuitry that underlies impulse control.69,70 Development of this circuitry is also sexually dimorphic;31,105 estrogen has been demonstrated to have a neuroprotective effect in the striatum, suggesting reduced vulnerability in this region in females.106 We speculate that the different regional patterns of gray matter decreases associated with self-reported CM in females and males may mediate their different vulnerabilities to disorders of mood and impulse control in adolescence.22,35,39,107–109
Limitations of this study include small sample sizes, particularly for those reporting sexual abuse. While findings in emotional regulation regions in association with emotional maltreatment were consistent with hypotheses, emotional neglect was the most frequently reported subtype; findings seen only within this subgroup may have resulted from greater power to detect differences. Timing and duration of CM exposure may influence GM differences.110 CTQ ratings are limited by the use of retrospective self-reports and do not assess ages at which maltreatment occurred, making exploration of possible differential effects of the timing of maltreatment on brain development difficult. As the regional distribution of the volume decreases with CM are similar to those found in psychiatric and behavioral difficulties, we interpreted the volume decreases to represent vulnerability factors. However, especially as the adolescents studied did not meet criteria for disorders despite adversity, it is also possible that the gray matter decreases reflect resiliency. Longitudinal studies could help clarify interaction of CM and development, and whether findings observed represent risk or resiliency factors.
We identified brain alterations in a sample of adolescents who did not meet criteria for psychiatric diagnoses, suggesting that in the absence of known psychiatric disorders CM may alter corticostriatal-limbic GM. The functions of these regions suggest that GM changes may contribute to a spectrum of behavioral difficulties experienced by adolescents exposed to CM, including alterations in self and interpersonal perceptions, as well as in impulse, cognitive and emotional control.27,111–113 The CTQ scale used to assess CM was rated by the adolescents themselves. Though there are alternate methods of determining CM histories, such as reports by caregivers and/or established in the clinical and child welfare settings, self-reports have been shown previously to be reliable and may be sensitive to CM that may not reach clinical awareness.41,42,114,115 Moreover, it has been theorized that an individual's perception of the neglect and/or maltreatment may be especially relevant in the psychological and neuropsychological development of the child.116
Adolescence is a particularly vulnerable time for the development of mood, anxiety and addictive disorders and CM has been linked to increased vulnerability to these disorders.25,69,117–119 This study suggests that corticostriatal-limbic brain changes may mediate increased risk for these disorders in association with self-reported CM. Together, these findings highlight the critical need for improved understanding of effects of both childhood abuse and neglect in adolescents and possible differences in their effects on brain development. Though adolescents with CM history may have symptoms and behaviors that may not yet reach full criteria for psychiatric diagnoses, detection and early intervention may help to improve functioning and reduce risk for the development of mood, addictive and other psychiatric disorders.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute of Mental Health R01MH69747 (HPB), R01MH070902 (HPB), K01MH086621 (FW), National Institute of Health (NIH) Roadmap for Medical Research Common Fund grant UL1-DE19586 (RS) and NIH Roadmap for Medical Research/Common Fund (RS), National Institute of Drug Abuse RL1DA024856 (HPB, LCM), K05DA020091 (LCM) and PL1-DA24859 (RS), Women's Health Research at Yale (HPB, CMM), National Alliance for Research in Schizophrenia and Depression (HPB, FW), Attias Family Foundation (HPB), and Klingenstein Foundation (FW). The funding organizations did not have roles in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Dr. Blumberg had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
We thank Susan Quatrano, Philip Markovich, Kathryn Armstrong, Sarah Nicholls, Matthew Freiburger, and Matthew Hirschtritt for their efforts with the research subjects, Cheryl Lacadie, Karen Martin, Terry Hickey and Hedy Sarofin for technical expertise, and the research subjects for their participation.
REFERENCES
- 1.U.S. Department of Health and Human Services, Administration for Children and Families, Children's Bureau Child Maltreatment: Reports from the States to the National Child Abuse and Neglect Data System. 2010 http://www.acf.hhs.gov/programs/cb/stats_research/index.htm#can.
- 2.Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- 3.Monroy E, Hernandez-Torres E, Flores G. Maternal separation disrupts dendritic morphology of neurons in prefronal cortex, hippocampus, and nucleus accumbens in male rat offspring. J Chem Neuroanat. 2010 doi: 10.1016/j.jchemneu.2010.05.005. In Press. [DOI] [PubMed] [Google Scholar]
- 4.Bremner JD, Vermetten E. Stress and development. behavioral and biological consequences. Dev Psychopathol. 2001;13:473–489. doi: 10.1017/s0954579401003042. [DOI] [PubMed] [Google Scholar]
- 5.Bremner JD, Vythilingam M, Vermetten E, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 6.Carrion VG, Weems CF, Watson C, Eliez S, Menon V, Reiss AL. Converging evidence for abnormalities of the prefrontal cortex and evaluation of midsagittal structures in pediatric posttraumatic stress disorder: an MRI study. Psychiatry Res. 2009;172:226–234. doi: 10.1016/j.pscychresns.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2001;50:305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- 8.Driessen M, Herrmann J, Stahl K, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 9.Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzhal EM. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J Psychiatr Res. 2010 doi: 10.1016/j.jpsychires.2010.01.006. In Press. [DOI] [PubMed] [Google Scholar]
- 10.Schmahl CG, Vermetten E, Elzinga BM, Douglas Bremner J. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. 2003;122:193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 11.Soloff P, Nutche J, Goradia D, Diwadkar V. Structural brain abnormalities in borderline personality disorder: a voxel-based morphometry study. Psychiatry Res. 2008;164:223–236. doi: 10.1016/j.pscychresns.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomoda A, Suzuki H, Rabi K, Sheu YS, Polcari A, Teicher MH. Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. NeuroImage. 2009;47(Suppl2):T66–T71. doi: 10.1016/j.neuroimage.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vythilingam M, Heim C, Newport J, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus. 2008;18:729–736. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- 15.Bremner JD, Randall P, Vermetten E, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen RA, Grieve S, Hoth KF, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59:975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Strathearn L, Mayes LC. Cocaine addiction in mothers: potential effects on maternal care and infant development. Ann N Y Acad Sci. 2010;1187:172–183. doi: 10.1111/j.1749-6632.2009.05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Bellis MD, Keshavan MS, Shifflett H, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- 19.De Bellis MD, Hooper SR, Wooley DP, Shenk CE. Demographic, maltreatment, and neurobiological correlates of PTSD symptoms in children and adolescents. J Pediatr Psychol. 2010;35:570–577. doi: 10.1093/jpepsy/jsp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, Hammen CL. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry. 2010;67:357–364. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manly JT, Kim JE, Rogosch FA, Cicchetti D. Dimensions of child maltreatment and children's adjustment: contributions of developmental timing and subtype. Dev Psychopathol. 2001;13:759–782. [PubMed] [Google Scholar]
- 22.Weiss EL, Longhurst JG, Mazure CM. Childhood sexual abuse as a risk factor for depression in women: psychosocial and neurobiological correlates. Am J Psychiatry. 1999;156:816–828. doi: 10.1176/ajp.156.6.816. [DOI] [PubMed] [Google Scholar]
- 23.Wexler BE, Lyons L, Lyons H, Mazure CM. Physical and sexual abuse during childhood and development of psychiatric illnesses during adulthood. J Nerv Ment Dis. 1997;185:522–524. doi: 10.1097/00005053-199708000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents. Results from the adverse childhood experiences study. Am J Psychiatry. 2003;160:1453–1460. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- 25.Spinhoven P, Elzinga BM, Hovens JG, et al. The specificity of childhood adversities and negative life events across the life span to anxiety and depressive disorders. J Affect Disord. 2010 doi: 10.1016/j.jad.2010.02.132. In Press. [DOI] [PubMed] [Google Scholar]
- 26.Teicher MH, Samson JA, Polcari A, McGreenery CE. Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. Am J Psychiatry. 2006;163:993–1000. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- 27.van Harmelen A, de Jong PJ, Glashouwer KA, Spinhoven P, Penninx BW, Elzinga BM. Child abuse and negative explicit and automatic self-associations: The cognitive scars of emotional maltreatment. Behav Res Ther. 2010;48:486–494. doi: 10.1016/j.brat.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Maciejewski PK, Mazure CM. Fear of criticism and rejection mediates an association between childhood emotional abuse and adult onset of major depression. Cognitive Therapy and Research. 2006;30:105–122. [Google Scholar]
- 29.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- 31.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McEwen BS. Permanence of brain sex differences and structural plasticity of the adult brain. Proc Natl Acad Sci U S A. 1999;96:7128–7130. doi: 10.1073/pnas.96.13.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neufang S, Specht K, Hausmann M, et al. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 2009;19:464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- 34.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 35.Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994;115:424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- 36.Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychol Med. 1999;29:1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- 37.Nagoshi CT, Wilson JR, Rodriguez LA. Impulsivity, sensation seeking, and behavioral and emotional responses to alcohol. Alcohol Clin Exp Res. 1991;15:661–667. doi: 10.1111/j.1530-0277.1991.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 38.Nolen-Hoeksema S, Hilt L. Possible contributors to the gender differences in alcohol use and problems. J Gen Psychology. 2006;133:357–374. doi: 10.3200/GENP.133.4.357-374. [DOI] [PubMed] [Google Scholar]
- 39.Chaplin TM, Hong K, Bergquist K, Sinha R. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol Clin Exp Res. 2008;32:1242–1250. doi: 10.1111/j.1530-0277.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein DP, Stein JA, Newcomb MD, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 42.Scher CD, Stein MB, Asmundson GJ, McCreary DR, Forde DR. The childhood trauma questionnaire in a community sample: psychometric properties and normative data. J Trauma Stress. 2001;14:843–857. doi: 10.1023/A:1013058625719. [DOI] [PubMed] [Google Scholar]
- 43.SPSS Inc. Chicago, IL: 2001. www.spss.com. [Google Scholar]
- 44.Kalmar JH, Wang F, Chepenik LG, et al. Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:636–642. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hains AB, Arnsten AF. Molecular mechanisms of stress-induced prefrontal cortical impairment: implications for mental illness. Learning & Memory. 2008;15:551–564. doi: 10.1101/lm.921708. [DOI] [PubMed] [Google Scholar]
- 46.Kikusui T, Ichikawa S, Mori Y. Maternal deprivation by early weaning increases corticosterone and decreases hippocampal BDNF and neurogenesis in mice. Psychoneuroendocrinology. 2009;34:762–772. doi: 10.1016/j.psyneuen.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- 48.Radley JJ, Rocher AB, Miller M, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 49.Sciolino NR, Bortolato M, Eisenstein SA, et al. Social isolation and chronic handling alter endocannabinoid signaling and behavioral reactivity to context in adult rats. Neuroscience. 2010;168:371–386. doi: 10.1016/j.neuroscience.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- 52.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neuroscience. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 53.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwandt ML, Lindell SG, Sjoberg RL, et al. Gene-environment interactions and response to social intrusion in male and female rhesus macaques. Biol Psychiatry. 2010;67:323–330. doi: 10.1016/j.biopsych.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenblum LA, Forger C, Noland S, Trost RC, Coplan JD. Response of adolescent bonnet macaques to an acute fear stimulus as a function of early rearing conditions. Dev Psychobiol. 2001;39:40–45. doi: 10.1002/dev.1026. [DOI] [PubMed] [Google Scholar]
- 57.Kikusui T, Mori Y. Behavioural and neurochemical consequences of early weaning in rodents. J Neuroendocrinol. 2009;21:427–431. doi: 10.1111/j.1365-2826.2009.01837.x. [DOI] [PubMed] [Google Scholar]
- 58.Arnsten AF, Shansky RM. Adolescence. vulnerable period for stress-induced prefrontal cortical function? Introduction to part IV. Ann N Y Acad Sci. 2004;1021:143–147. doi: 10.1196/annals.1308.017. [DOI] [PubMed] [Google Scholar]
- 59.Pascual R, Zamora-Leon SP. Effects of neonatal maternal deprivation and postweaning environmental complexity on dendritic morphology of prefrontal pyramidal neurons in the rat. Acta Neurobiol Exp. 2007;67:471–479. doi: 10.55782/ane-2007-1663. [DOI] [PubMed] [Google Scholar]
- 60.Christoff K, Prabhakaran V, Dorfman J, et al. Rostrolateral prefrontal cortex involvement in relational integration during reasoning. NeuroImage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- 61.Burgess PW, Gilbert SJ, Dumontheil I. Function and localization within rostral prefrontal cortex (area 10) Philos Trans R Soc Lond B Biol Sci. 2007;362:887–899. doi: 10.1098/rstb.2007.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 63.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- 65.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koechlin E, Corrado G, Pietrini P, Grafman J. Dissociating the role of the medial and lateral anterior prefrontal cortex in human planning. Proc Natl Acad Sci U S A. 2000;97:7651–7656. doi: 10.1073/pnas.130177397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mansouri FA, Tanaka K, Buckley MJ. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci. 2009;10:141–152. doi: 10.1038/nrn2538. [DOI] [PubMed] [Google Scholar]
- 68.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 69.Ferry AT, Ongur D, An X, Price JL. Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. J Comp Neurol. 2000;425:447–470. doi: 10.1002/1096-9861(20000925)425:3<447::aid-cne9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 70.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 72.Farrer C, Frith CD. Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency. NeuroImage. 2002;15:596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- 73.Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 75.Corradi-Dell'acqua C, Ueno K, Ogawa A, Cheng K, Rumiati RI, Iriki A. Effects of shifting perspective of the self: an fMRI study. NeuroImage. 2008;40:1902–1911. doi: 10.1016/j.neuroimage.2007.12.062. [DOI] [PubMed] [Google Scholar]
- 76.Briere J, Rickards S. Self-awareness, affect regulation, and relatedness: differential sequels of childhood versus adult victimization experience. J Nerv Ment Dis. 2007;195:497–503. doi: 10.1097/NMD.0b013e31803044e2. [DOI] [PubMed] [Google Scholar]
- 77.van der Kolk BA, Roth S, Pelcovitz D, Sunday S, Spinazzola J. The disorders of extreme stress: The empirical foundation of complex adaptation to trauma. J Trauma Stress. 2005;18:389–399. doi: 10.1002/jts.20047. [DOI] [PubMed] [Google Scholar]
- 78.Toth SL, Cicchetti D, Macfie J, Emde RN. Representations of self and other in the narratives of neglected, physically abused, and sexually abused preschoolers. Dev Psychopathol. 1997;9:781–796. doi: 10.1017/s0954579497001430. [DOI] [PubMed] [Google Scholar]
- 79.Gibb BE, Schofield CA, Coles ME. Reported history of childhood abuse and young adults' information-processing biases for facial displays of emotion. Child Maltreat. 2009;14:148–156. doi: 10.1177/1077559508326358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leist T, Dadds MR. Adolescents' ability to read different emotional faces relates to their history of maltreatment and type of psychopathology. Clin Child Psychol Psychiatry. 2009;14:237–250. 80. doi: 10.1177/1359104508100887. [DOI] [PubMed] [Google Scholar]
- 81.Masten CL, Guyer AE, Hodgdon HB, et al. Recognition of facial emotions among maltreated children with high rates of post-traumatic stress disorder. Child Abuse Negl. 2008;32:139–153. doi: 10.1016/j.chiabu.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pollak SD, Cicchetti D, Hornung K, Reed A. Recognizing emotion in faces: developmental effects of child abuse and neglect. Dev Psychol. 2000;36:679–688. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- 83.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pine DS, Mogg K, Bradley BP, et al. Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. Am J Psychiatry. 2005;162:291–296. doi: 10.1176/appi.ajp.162.2.291. [DOI] [PubMed] [Google Scholar]
- 85.Fani N, Bradley-Davino B, Ressler KJ, McClure-Tone EB. Attention bias in adult survivors of childhood maltreatment with and without posttraumatic stress disorder. Cognitive Therapy Research. 2010 In Press. [Google Scholar]
- 86.Irle E, Lange C, Sachsse U. Reduced size and abnormal asymmetry of parietal cortex in women with borderline personality disorder. Biol Psychiatry. 2005;57:173–182. doi: 10.1016/j.biopsych.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 87.Bremner JD, Vermetten E, Vythilingam M, et al. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol Psychiatry. 2004;55:612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 88.Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Snider RS, Maiti A. Cerebellar contributions to the Papez circuit. J Neurosci Res. 1976;2:133–146. doi: 10.1002/jnr.490020204. [DOI] [PubMed] [Google Scholar]
- 90.Lawson A, Ahima RS, Krozowski Z, Harlan RE. Postnatal development of corticosteroid receptor immunoreactivity in the rat cerebellum and brain stem. Neuroendocrinology. 1992;55:695–707. doi: 10.1159/000126189. [DOI] [PubMed] [Google Scholar]
- 91.Wilber AA, Wellman CL. Neonatal maternal separation alters the development of glucocorticoid receptor expression in the interpositus nucleus of the cerebellum. Int J Dev Neurosci. 2009;27:649–654. doi: 10.1016/j.ijdevneu.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 92.Bauer PM, Hanson JL, Pierson RK, Davidson RJ, Pollak SD. Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biol Psychiatry. 2009;66:1100–1106. doi: 10.1016/j.biopsych.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Bellis MD, Kuchibhatla M. Cerebellar volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2006;60:697–703. doi: 10.1016/j.biopsych.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 94.Yang P, Wu MT, Hsu CC, Ker JH. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neurosci Lett. 2004;370:13–18. doi: 10.1016/j.neulet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 95.Sacchetti B, Scelfo B, Strata P. Cerebellum and emotional behavior. Neuroscience. 2009;162:756–62. doi: 10.1016/j.neuroscience.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 96.LeDoux JE. Brain mechanisms of emotion and emotional learning. Curr Opin Neurobiol. 1992;2:191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- 97.Sinha R, Lacadie C, Skudlarski P, Wexler BE. Neural circuits underlying emotional distress in humans. Ann N York Acad Sci. 2004;1032:254–257. doi: 10.1196/annals.1314.032. [DOI] [PubMed] [Google Scholar]
- 98.Blumberg HP, Charney DS, Krystal JH. Frontotemporal neural systems in bipolar disorder. Semin Clin Neuropsychiatry. 2002;7:243–254. doi: 10.1053/scnp.2002.35220. [DOI] [PubMed] [Google Scholar]
- 99.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seminowicz DA, Mayberg HS, McIntosh AR, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. NeuroImage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 101.Macri S, Laviola G, Leussis MP, Andersen SL. Abnormal behavioral and neurotrophic development in the younger sibling receiving less maternal care in a communal nursing paradigm in rats. Psychoneuroendocrinology. 2010;35:392–402. doi: 10.1016/j.psyneuen.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 102.McCormick CM, Mathews IZ. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86:220–233. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 103.Shansky RM, Glavis-Bloom C, Lerman D, et al. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol Psychiatry. 2004;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- 104.Dalla C, Whetstone AS, Hodes GE, Shors TJ. Stressful experience has opposite effects on dendritic spines in the hippocampus of cycling versus masculinized females. Neurosci Lett. 2009;449:52–56. doi: 10.1016/j.neulet.2008.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 1994;4:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- 106.Dluzen DE. Neuroprotective effects of estrogen upon the nigrostriatal dopaminergic system. J Neurocytol. 2000;29:387–399. doi: 10.1023/a:1007117424491. [DOI] [PubMed] [Google Scholar]
- 107.Batten SV, Aslan M, Maciejewski PK, Mazure CM. Childhood maltreatment as a risk factor for adult cardiovascular disease and depression. J Clin Psychiatry. 2004;65:249–254. doi: 10.4088/jcp.v65n0217. [DOI] [PubMed] [Google Scholar]
- 108.Mazure CM, Maciejewski PK. The interplay of stress, gender and cognitive style in depressive onset. Arch Womens Ment Health. 2003;6:5–8. doi: 10.1007/s00737-002-0161-3. [DOI] [PubMed] [Google Scholar]
- 109.Buss C, Lord C, Wadiwalla M, et al. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J Neurosci. 2007;27:2592–2595. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience. 2010;3:1–18. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fergusson DM, Lynskey MT. Physical punishment/maltreatment during childhood and adjustment in young adulthood. Child Abuse Negl. 1997;21:617–630. doi: 10.1016/s0145-2134(97)00021-5. [DOI] [PubMed] [Google Scholar]
- 112.De Bellis M, Hooper SR, Spratt EG, Woolley DP. Neuropsychological findings in childhood neglect and their relationships to pediatric PTSD. J Int Neuropsychol Soc. 2009;15:868–878. doi: 10.1017/S1355617709990464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- 114.Fink LA, Bernstein D, Handelsman L, Foote J, Lovejoy M. Initial reliability and validity of the childhood trauma interview: a new multidimensional measure of childhood interpersonal trauma. Am J Psychiatry. 1995;152:1329–1335. doi: 10.1176/ajp.152.9.1329. [DOI] [PubMed] [Google Scholar]
- 115.Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 116.Browne C, Winkelman C. The effect of childhood trauma on later psychological adjustment. J Interpers Violence. 2007;22:684–697. doi: 10.1177/0886260507300207. [DOI] [PubMed] [Google Scholar]
- 117.Parker G, Hadzi-Pavlovic D, Greenwald S, Weissman M. Low parental care as a risk factor to lifetime depression in a community sample. J Affect Disorders. 1995;33:173–180. doi: 10.1016/0165-0327(94)00086-o. [DOI] [PubMed] [Google Scholar]
- 118.Galler JR, Bryce CP, Waber D, et al. Early childhood malnutrition predicts depressive symptoms at ages 11–17. J Child Psychol Psychiatry. 2010;51:789–798. doi: 10.1111/j.1469-7610.2010.02208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Colvert E, Rutter M, Beckett C, et al. Emotional difficulties in early adolescence following severe early deprivation: findings from the English and Romanian adoptees study. Dev Psycopathol. 2008;20:547–567. doi: 10.1017/S0954579408000278. [DOI] [PubMed] [Google Scholar]


