Abstract
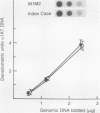
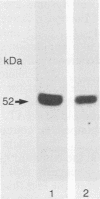
The Mmineral springs alpha 1-antitrypsin (alpha 1AT) allele, causing alpha 1AT deficiency and emphysema, is unique among the alpha 1AT-deficiency alleles in that it was observed in a black family, whereas most mutations causing alpha 1AT deficiency are confined to Caucasian populations of European descent. Immobilized pH gradient analysis of serum demonstrated that alpha 1AT Mmineral springs migrated cathodal to the normal M2 allele. Evaluation of Mmineral springs alpha 1AT as an inhibitor of neutrophil elastase, its natural substrate, demonstrated markedly lower than normal function. Characterization of the alpha 1AT Mmineral springs gene demonstrated that it differed from the common normal M1(Ala213) allele by a single-base substitution causing the amino acid substitution Gly-67 (GGG)----Glu-67 (GAG). Capitalizing on the fact that this mutation creates a polymorphism for the restriction endonuclease AvaII, family analysis demonstrated that the Mmineral springs alpha 1AT allele was transmitted in an autosomal-codominant fashion. Evaluation of genomic DNA showed that the index case was homozygous for the alpha 1AT Mmineral springs allele. Cytoplasmic blot analysis of blood monocytes of the Mmineral springs homozygote demonstrated levels of alpha 1AT mRNA transcripts comparable to those in cells of a normal M1 (Val213) homozygote control. Evaluation of in vitro translation of Mmineral springs alpha 1AT mRNA transcripts demonstrated a normal capacity to direct the translation of alpha 1AT. Evaluation of secretion of alpha 1AT by the blood monocytes by pulse-chase labeling with [35S]methionine, however, demonstrated less secretion by the Mmineral springs cells than normal cells. To characterize the posttranslational events causing the alpha 1AT-secretory defect associated with the alpha 1AT Mmineral springs gene, retroviral gene transfer was used to establish polyclonal populations of murine fibroblasts containing either a normal human M1 alpha 1AT cDNA or an Mmineral springs alpha 1AT cDNA and expressing comparable levels of human alpha 1AT mRNA transcripts. Pulse-chase labeling of these cells with [35S]methionine demonstrated less secretion of human alpha 1AT from the Mmineral springs cells than from the M1 cells, and evaluation of cell lysates also demonstrated lower amounts of intracellular human alpha 1AT in the Mmineral springs cells than in the normal M1 control cells. Thus, the Gly-67 --> Glu mutation that characterizes Mmineral springs causes reduced alpha 1AT secretion on the basis of aberrant posttranslational alpha 1AT biosynthesis by a mechanism distinct from that associated with the alpha 1AT Z allele, whereby intracellular aggregation of the mutant protein is etiologic of the alpha 1AT-secretory defect. Furthermore, for the alpha 1AT protein that does reach the circulation, this mutation markedly affects the ability of the molecule to inhibit neutrophil elastase; i.e., the alpha 1AT Mmineral springs allele predisposes to emphysema on the basis of serum apha 1AT deficiency coupled with alpha AT dysfunction.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates P. F., Swift R. A. Double cos site vectors: simplified cosmid cloning. Gene. 1983 Dec;26(2-3):137–146. doi: 10.1016/0378-1119(83)90183-x. [DOI] [PubMed] [Google Scholar]
- Bathurst I. C., Stenflo J., Errington D. M., Carrell R. W. Translation and processing of normal (PiMM) and abnormal (PiZZ) human alpha 1-antitrypsin. FEBS Lett. 1983 Mar 21;153(2):270–274. doi: 10.1016/0014-5793(83)80622-x. [DOI] [PubMed] [Google Scholar]
- Beatty K., Bieth J., Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem. 1980 May 10;255(9):3931–3934. [PubMed] [Google Scholar]
- Brantly M., Courtney M., Crystal R. G. Repair of the secretion defect in the Z form of alpha 1-antitrypsin by addition of a second mutation. Science. 1988 Dec 23;242(4886):1700–1702. doi: 10.1126/science.2904702. [DOI] [PubMed] [Google Scholar]
- Brantly M., Nukiwa T., Crystal R. G. Molecular basis of alpha-1-antitrypsin deficiency. Am J Med. 1988 Jun 24;84(6A):13–31. doi: 10.1016/0002-9343(88)90154-4. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Laurell C. B., Brennan S. O., Owen M. C., Vaughan L., Boswell D. R. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Carrell R. W. alpha 1-Antitrypsin: molecular pathology, leukocytes, and tissue damage. J Clin Invest. 1986 Dec;78(6):1427–1431. doi: 10.1172/JCI112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constans J., Viau M., Gouaillard C. Pi M4: an additional Pi M subtype. Hum Genet. 1980;55(1):119–121. doi: 10.1007/BF00329137. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Johnson A. M., Fagerhol M. K. Report of Nomenclature Meeting for alpha 1-antitrypsin, INSERM, Rouen/Bois-Guillaume-1978. Hum Genet. 1980;53(3):429–433. doi: 10.1007/BF00287070. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Woo S. L., Mansfield T. DNA restriction fragments associated with alpha 1-antitrypsin indicate a single origin for deficiency allele PI Z. Nature. 1985 Jul 4;316(6023):79–81. doi: 10.1038/316079a0. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Brantly M. L., Hubbard R. C., Curiel D. T., States D. J., Holmes M. D. The alpha 1-antitrypsin gene and its mutations. Clinical consequences and strategies for therapy. Chest. 1989 Jan;95(1):196–208. doi: 10.1378/chest.95.1.196. [DOI] [PubMed] [Google Scholar]
- Curiel D. T., Chytil A., Courtney M., Crystal R. G. Serum alpha 1-antitrypsin deficiency associated with the common S-type (Glu264----Val) mutation results from intracellular degradation of alpha 1-antitrypsin prior to secretion. J Biol Chem. 1989 Jun 25;264(18):10477–10486. [PubMed] [Google Scholar]
- Curiel D. T., Holmes M. D., Okayama H., Brantly M. L., Vogelmeier C., Travis W. D., Stier L. E., Perks W. H., Crystal R. G. Molecular basis of the liver and lung disease associated with the alpha 1-antitrypsin deficiency allele Mmalton. J Biol Chem. 1989 Aug 15;264(23):13938–13945. [PubMed] [Google Scholar]
- Curiel D., Brantly M., Curiel E., Stier L., Crystal R. G. Alpha 1-antitrypsin deficiency caused by the alpha 1-antitrypsin Nullmattawa gene. An insertion mutation rendering the alpha 1-antitrypsin gene incapable of producing alpha 1-antitrypsin. J Clin Invest. 1989 Apr;83(4):1144–1152. doi: 10.1172/JCI113994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish R., Trapnell B. C., Crystal R. G., Tolstoshev P. Copy number of a human type I alpha 2 collagen gene. J Biol Chem. 1982 Nov 25;257(22):13816–13822. [PubMed] [Google Scholar]
- Dykes D. D., Miller S. A., Polesky H. F. Distribution of alpha 1-antitrypsin variants in a US white population. Hum Hered. 1984;34(5):308–310. doi: 10.1159/000153485. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Errington D. M., Bathurst I. C., Janus E. D., Carrell R. W. In vitro synthesis of M and Z forms of human alpha 1-antitrypsin. FEBS Lett. 1982 Nov 1;148(1):83–86. doi: 10.1016/0014-5793(82)81247-7. [DOI] [PubMed] [Google Scholar]
- Esser V., Russell D. W. Transport-deficient mutations in the low density lipoprotein receptor. Alterations in the cysteine-rich and cysteine-poor regions of the protein block intracellular transport. J Biol Chem. 1988 Sep 15;263(26):13276–13281. [PubMed] [Google Scholar]
- Fagerhol M. K., Cox D. W. The Pi polymorphism: genetic, biochemical, and clinical aspects of human alpha 1-antitrypsin. Adv Hum Genet. 1981;11:1-62, 371-2. [PubMed] [Google Scholar]
- Foreman R. C. Disruption of the Lys-290--Glu-342 salt bridge in human alpha 1-antitrypsin does not prevent its synthesis and secretion. FEBS Lett. 1987 May 25;216(1):79–82. doi: 10.1016/0014-5793(87)80760-3. [DOI] [PubMed] [Google Scholar]
- Foreman R. C., Judah J. D., Colman A. Xenopus oocytes can synthesise but do not secrete the Z variant of human alpha 1-antitrypsin. FEBS Lett. 1984 Mar 12;168(1):84–88. doi: 10.1016/0014-5793(84)80211-2. [DOI] [PubMed] [Google Scholar]
- Frants R. R., Eriksson A. W. alpha1-antitrypsin: common subtypes of Pi M. Hum Hered. 1976;26(6):435–440. doi: 10.1159/000152838. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Zimmerman R. L., Rennard S. I., Crystal R. G. Antielastases of the human alveolar structures. Implications for the protease-antiprotease theory of emphysema. J Clin Invest. 1981 Oct;68(4):889–898. doi: 10.1172/JCI110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver R. I., Jr, Chytil A., Karlsson S., Fells G. A., Brantly M. L., Courtney M., Kantoff P. W., Nienhuis A. W., Anderson W. F., Crystal R. G. Production of glycosylated physiologically "normal" human alpha 1-antitrypsin by mouse fibroblasts modified by insertion of a human alpha 1-antitrypsin cDNA using a retroviral vector. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1050–1054. doi: 10.1073/pnas.84.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver R. I., Jr, Mornex J. F., Nukiwa T., Brantly M., Courtney M., LeCocq J. P., Crystal R. G. Alpha 1-antitrypsin deficiency and emphysema caused by homozygous inheritance of non-expressing alpha 1-antitrypsin genes. N Engl J Med. 1986 Mar 20;314(12):762–766. doi: 10.1056/NEJM198603203141207. [DOI] [PubMed] [Google Scholar]
- Hirschberg C. B., Snider M. D. Topography of glycosylation in the rough endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1987;56:63–87. doi: 10.1146/annurev.bi.56.070187.000431. [DOI] [PubMed] [Google Scholar]
- Janoff A. Elastases and emphysema. Current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis. 1985 Aug;132(2):417–433. doi: 10.1164/arrd.1985.132.2.417. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueppers F., Christopherson M. J. Alpha1-antitrypsin: further genetic heterogeneity revealed by isoelectric focusing. Am J Hum Genet. 1978 Jul;30(4):359–365. [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Kong N. Glucose removal from N-linked oligosaccharides is required for efficient maturation of certain secretory glycoproteins from the rough endoplasmic reticulum to the Golgi complex. J Cell Biol. 1984 May;98(5):1720–1729. doi: 10.1083/jcb.98.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebermann H., Tokuoka R., Deisenhofer J., Huber R. Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984 Aug 15;177(3):531–557. [PubMed] [Google Scholar]
- Long G. L., Chandra T., Woo S. L., Davie E. W., Kurachi K. Complete sequence of the cDNA for human alpha 1-antitrypsin and the gene for the S variant. Biochemistry. 1984 Oct 9;23(21):4828–4837. doi: 10.1021/bi00316a003. [DOI] [PubMed] [Google Scholar]
- McCracken A. A., Kruse K. B., Brown J. L. Molecular basis for defective secretion of the Z variant of human alpha-1-proteinase inhibitor: secretion of variants having altered potential for salt bridge formation between amino acids 290 and 342. Mol Cell Biol. 1989 Apr;9(4):1406–1414. doi: 10.1128/mcb.9.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornex J. F., Chytil-Weir A., Martinet Y., Courtney M., LeCocq J. P., Crystal R. G. Expression of the alpha-1-antitrypsin gene in mononuclear phagocytes of normal and alpha-1-antitrypsin-deficient individuals. J Clin Invest. 1986 Jun;77(6):1952–1961. doi: 10.1172/JCI112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukiwa T., Brantly M. L., Ogushi F., Fells G. A., Crystal R. G. Characterization of the gene and protein of the common alpha 1-antitrypsin normal M2 allele. Am J Hum Genet. 1988 Sep;43(3):322–330. [PMC free article] [PubMed] [Google Scholar]
- Nukiwa T., Brantly M., Ogushi F., Fells G., Satoh K., Stier L., Courtney M., Crystal R. G. Characterization of the M1(Ala213) type of alpha 1-antitrypsin, a newly recognized, common "normal" alpha 1-antitrypsin haplotype. Biochemistry. 1987 Aug 25;26(17):5259–5267. doi: 10.1021/bi00391a008. [DOI] [PubMed] [Google Scholar]
- Nukiwa T., Satoh K., Brantly M. L., Ogushi F., Fells G. A., Courtney M., Crystal R. G. Identification of a second mutation in the protein-coding sequence of the Z type alpha 1-antitrypsin gene. J Biol Chem. 1986 Dec 5;261(34):15989–15994. [PubMed] [Google Scholar]
- Nukiwa T., Takahashi H., Brantly M., Courtney M., Crystal R. G. alpha 1-Antitrypsin nullGranite Falls, a nonexpressing alpha 1-antitrypsin gene associated with a frameshift to stop mutation in a coding exon. J Biol Chem. 1987 Sep 5;262(25):11999–12004. [PubMed] [Google Scholar]
- Ogushi F., Fells G. A., Hubbard R. C., Straus S. D., Crystal R. G. Z-type alpha 1-antitrypsin is less competent than M1-type alpha 1-antitrypsin as an inhibitor of neutrophil elastase. J Clin Invest. 1987 Nov;80(5):1366–1374. doi: 10.1172/JCI113214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlino E., Cortese R., Ciliberto G. The human alpha 1-antitrypsin gene is transcribed from two different promoters in macrophages and hepatocytes. EMBO J. 1987 Sep;6(9):2767–2771. doi: 10.1002/j.1460-2075.1987.tb02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Cole F. S., Kilbridge P., Rossing T. H., Colten H. R. Expression of the alpha 1-proteinase inhibitor gene in human monocytes and macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(3):795–799. doi: 10.1073/pnas.82.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Kay R. M., Cole F. S., Rossing T. H., Van Thiel D., Colten H. R. The cellular defect in alpha 1-proteinase inhibitor (alpha 1-PI) deficiency is expressed in human monocytes and in Xenopus oocytes injected with human liver mRNA. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6918–6921. doi: 10.1073/pnas.82.20.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Pierce J. A., Eradio B., Dew T. A. Antitrypsin phenotypes in St. Louis. JAMA. 1975 Feb 10;231(6):609–612. [PubMed] [Google Scholar]
- Rabin M., Watson M., Kidd V., Woo S. L., Breg W. R., Ruddle F. H. Regional location of alpha 1-antichymotrypsin and alpha 1-antitrypsin genes on human chromosome 14. Somat Cell Mol Genet. 1986 Mar;12(2):209–214. doi: 10.1007/BF01560668. [DOI] [PubMed] [Google Scholar]
- Sifers R. N., Brashears-Macatee S., Kidd V. J., Muensch H., Woo S. L. A frameshift mutation results in a truncated alpha 1-antitrypsin that is retained within the rough endoplasmic reticulum. J Biol Chem. 1988 May 25;263(15):7330–7335. [PubMed] [Google Scholar]
- Sifers R. N., Hardick C. P., Woo S. L. Disruption of the 290-342 salt bridge is not responsible for the secretory defect of the PiZ alpha 1-antitrypsin variant. J Biol Chem. 1989 Feb 15;264(5):2997–3001. [PubMed] [Google Scholar]
- Verbanac K. M., Heath E. C. Biosynthesis, processing, and secretion of M and Z variant human alpha 1-antitrypsin. J Biol Chem. 1986 Jul 25;261(21):9979–9989. [PubMed] [Google Scholar]
- Vogel B. E., Minor R. R., Freund M., Prockop D. J. A point mutation in a type I procollagen gene converts glycine 748 of the alpha 1 chain to cysteine and destabilizes the triple helix in a lethal variant of osteogenesis imperfecta. J Biol Chem. 1987 Oct 25;262(30):14737–14744. [PubMed] [Google Scholar]
- Wewers M. D., Casolaro M. A., Sellers S. E., Swayze S. C., McPhaul K. M., Wittes J. T., Crystal R. G. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987 Apr 23;316(17):1055–1062. doi: 10.1056/NEJM198704233161704. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]