Abstract
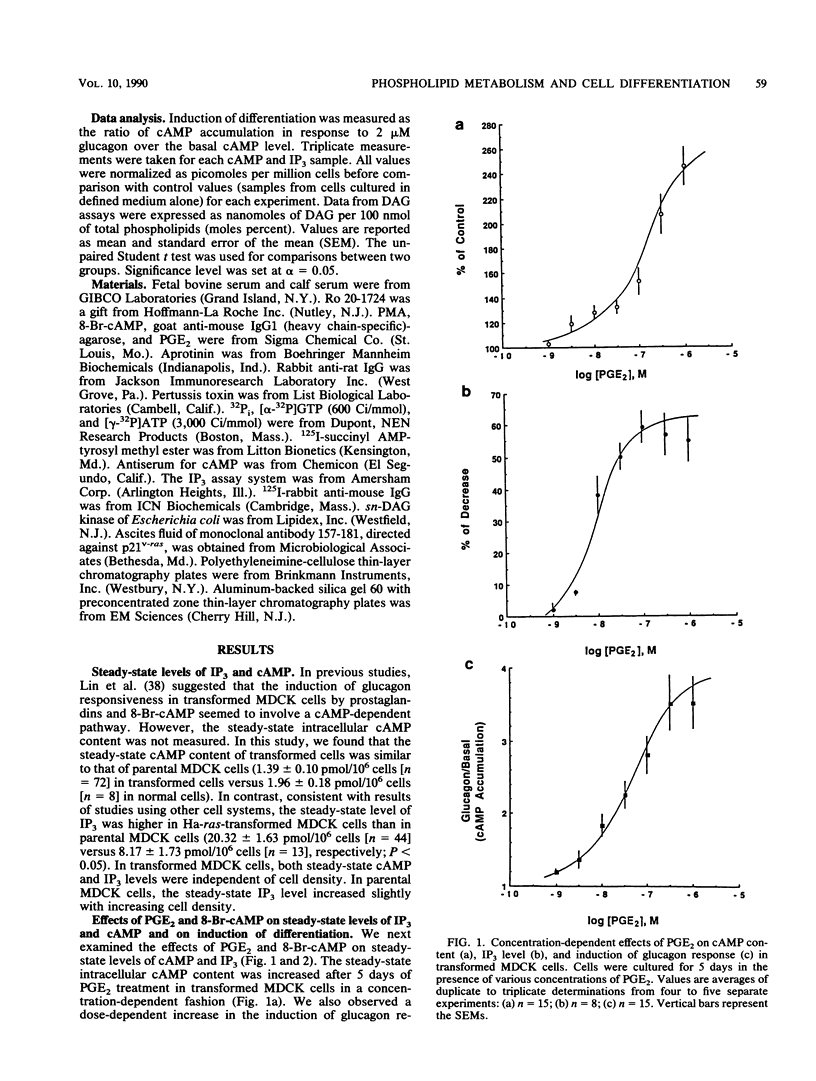
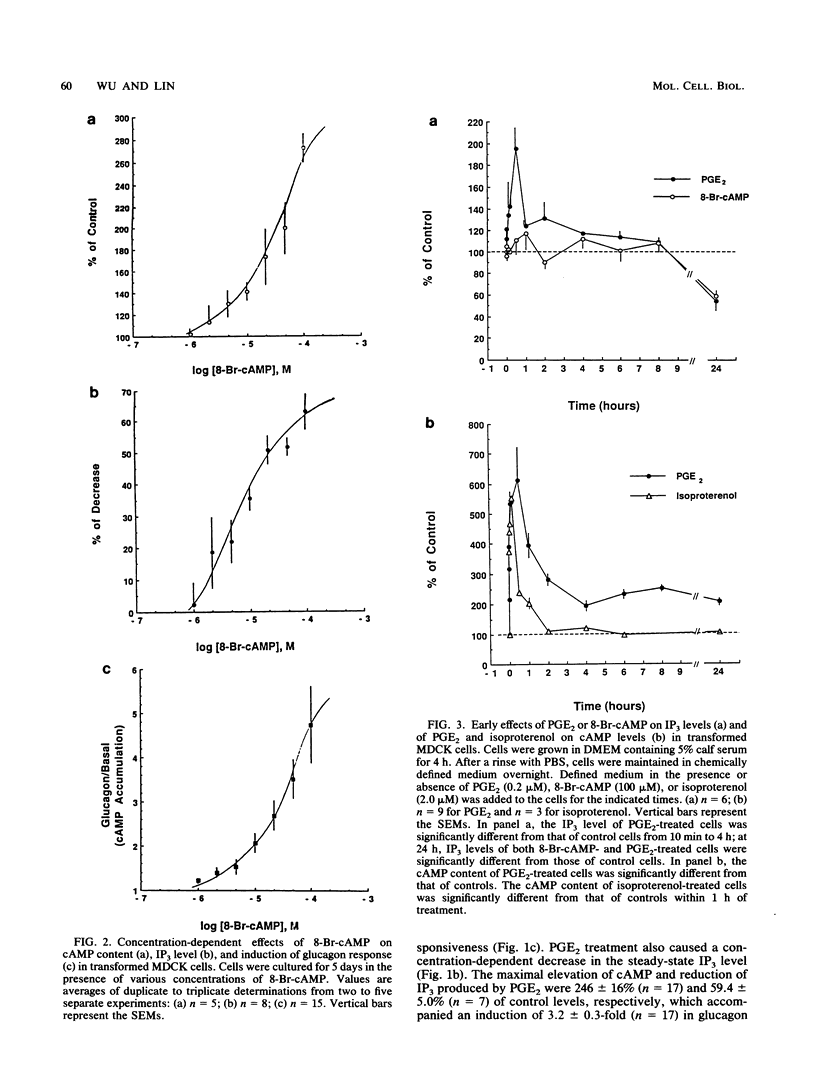
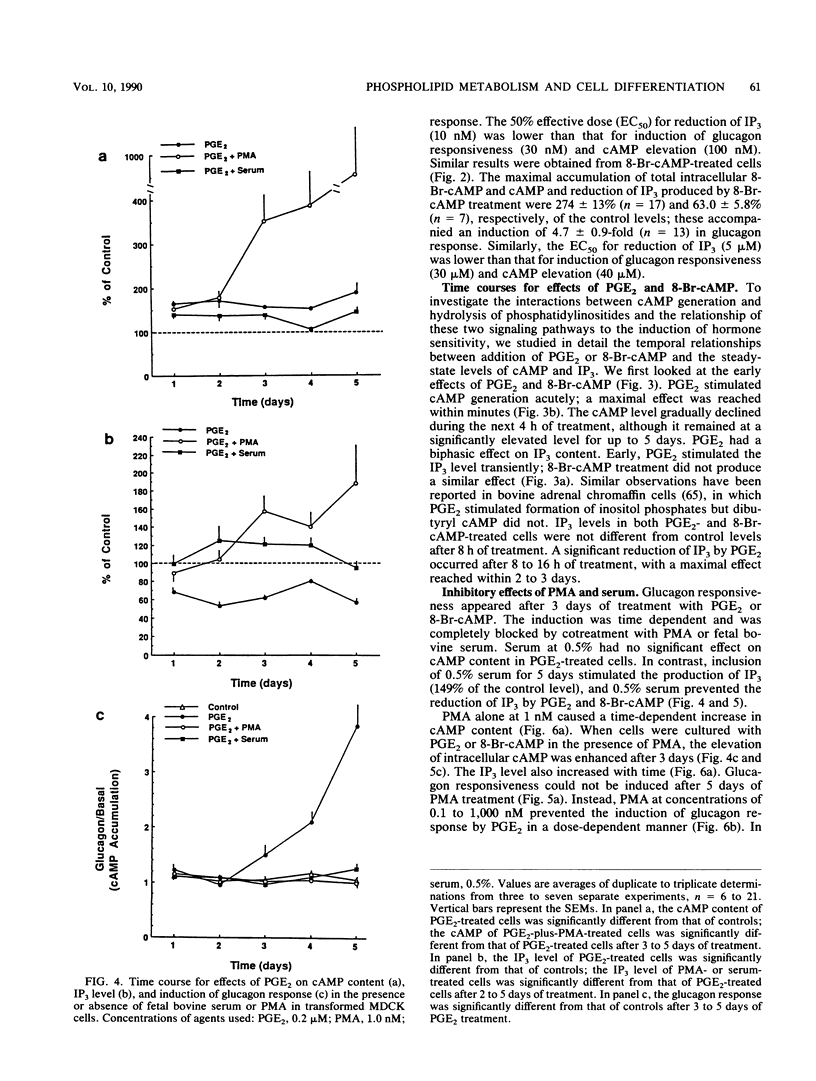
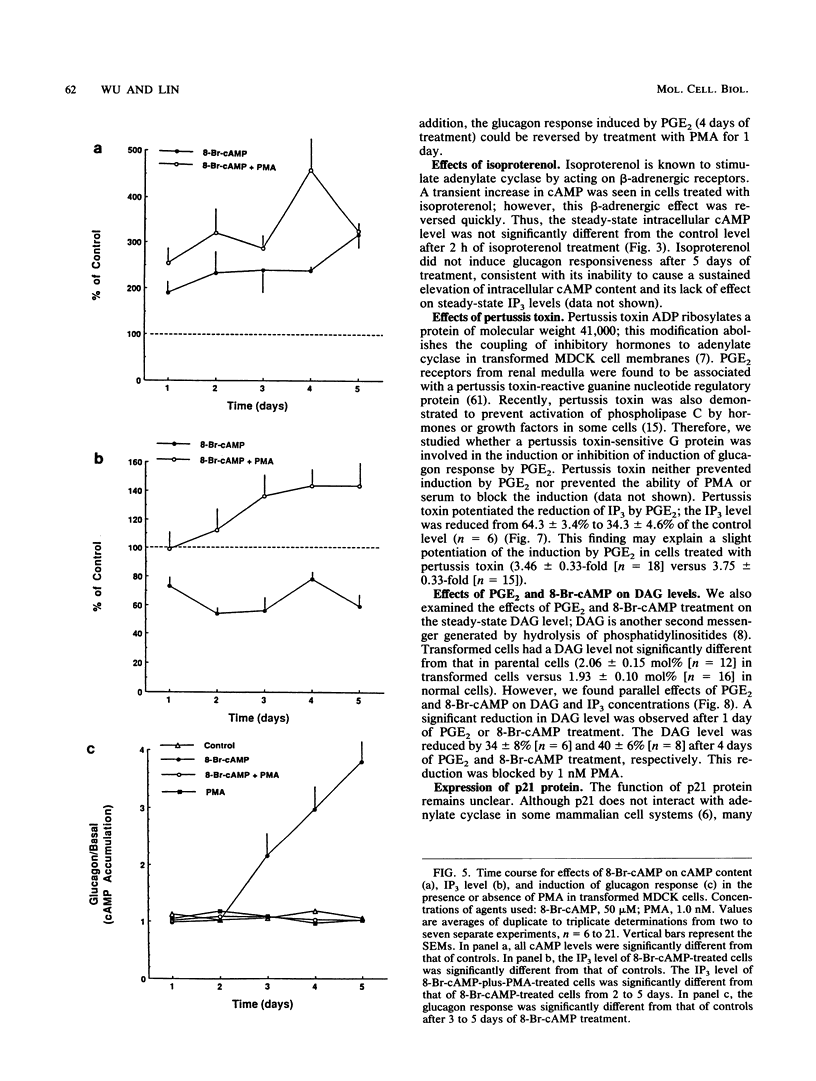
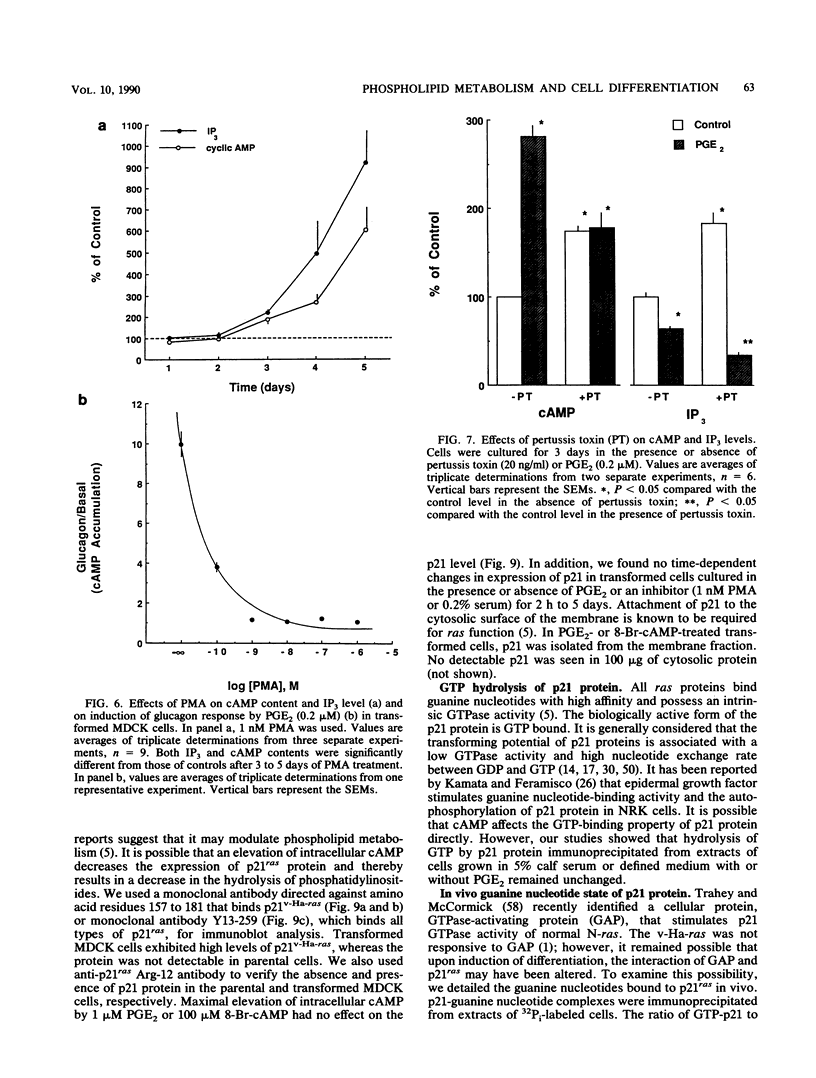
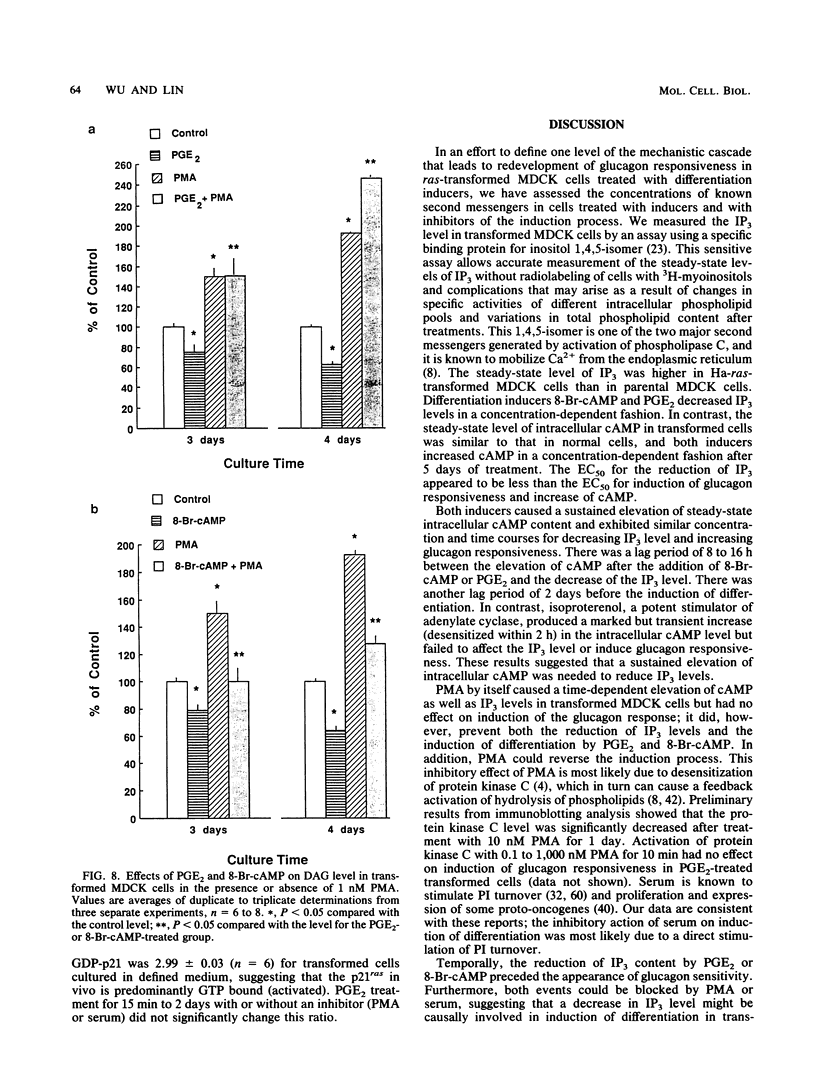
We used Ha-ras-transformed Madin-Darby canine kidney (MDCK) cells as a model to study possible signal transduction mechanisms underlying the induction of glucagon responsiveness by the differentiation inducers prostaglandin E2 (PGE2) and 8-bromo-cyclic (8-Br-cAMP) AMP and the inhibition of induction by phorbol ester or a serum factor. The steady-state level of inositol 1,4,5-trisphosphate (IP3) was higher in Ha-ras-transformed MDCK cells than in parental MDCK cells. In contrast, the steady-state level of intracellular cAMP of transformed cells was similar to that of normal cells. PGE2 and 8-Br-cAMP increased cAMP content but decreased IP3 levels in a concentration-dependent fashion after 5 days of treatment. We examined the time course for effects of PGE2 and 8-Br-cAMP and found that there was a lag period of 8 to 16 h between elevation of cAMP after the addition of 8-Br-cAMP or PGE2 and the decrease of IP3 levels. Another lag period of 2 days existed before the induction of differentiation. Both the reduction of IP3 levels and the induction of glucagon responsiveness were blocked by phorbol-12-myristate-13-acetate or serum, suggesting that a decrease in the IP3 level might be causally involved in induction of differentiation in transformed MDCK cells. However, induction of differentiation was not due to changes in the expression or guanine nucleotide-binding properties of p21 protein. It is likely that cAMP has a direct regulatory effect on the phospholipid signaling pathway. We conclude that perturbation of the inositol phosphate signaling pathway may be responsible for the induction of differentiation by PGE2 and 8-Br-cAMP in transformed MDCK cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Adari H., Lowy D. R., Willumsen B. M., Der C. J., McCormick F. Guanosine triphosphatase activating protein (GAP) interacts with the p21 ras effector binding domain. Science. 1988 Apr 22;240(4851):518–521. doi: 10.1126/science.2833817. [DOI] [PubMed] [Google Scholar]
- Alonso T., Morgan R. O., Marvizon J. C., Zarbl H., Santos E. Malignant transformation by ras and other oncogenes produces common alterations in inositol phospholipid signaling pathways. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4271–4275. doi: 10.1073/pnas.85.12.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Ballester R., Rosen O. M. Fate of immunoprecipitable protein kinase C in GH3 cells treated with phorbol 12-myristate 13-acetate. J Biol Chem. 1985 Dec 5;260(28):15194–15199. [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Beckner S. K., Hattori S., Shih T. Y. The ras oncogene product p21 is not a regulatory component of adenylate cyclase. Nature. 1985 Sep 5;317(6032):71–72. doi: 10.1038/317071a0. [DOI] [PubMed] [Google Scholar]
- Beckner S. K., Wright D. E., Lin M. C. Decreased potency of glucagon on transformed-induced MDCK cells does not reflect an alteration of adenylate cyclase components. Endocrinology. 1987 Oct;121(4):1438–1446. doi: 10.1210/endo-121-4-1438. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Birrer M. J., Segal S., DeGreve J. S., Kaye F., Sausville E. A., Minna J. D. L-myc cooperates with ras to transform primary rat embryo fibroblasts. Mol Cell Biol. 1988 Jun;8(6):2668–2673. doi: 10.1128/mcb.8.6.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop W. R., Bell R. M. Functions of diacylglycerol in glycerolipid metabolism, signal transduction and cellular transformation. Oncogene Res. 1988 Feb;2(3):205–218. [PubMed] [Google Scholar]
- Della Bianca V., De Togni P., Grzeskowiak M., Vicentini L. M., Di Virgilio F. Cyclic AMP inhibition of phosphoinositide turnover in human neutrophils. Biochim Biophys Acta. 1986 May 29;886(3):441–447. doi: 10.1016/0167-4889(86)90180-1. [DOI] [PubMed] [Google Scholar]
- Der C. J., Finkel T., Cooper G. M. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986 Jan 17;44(1):167–176. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Wallace M. A., Wojcikiewicz R. J. Evidence for involvement of guanine nucleotide-binding regulatory proteins in the activation of phospholipases by hormones. FASEB J. 1988 Jul;2(10):2569–2574. doi: 10.1096/fasebj.2.10.2838362. [DOI] [PubMed] [Google Scholar]
- Faletto D. L., Arrow A. S., Macara I. G. An early decrease in phosphatidylinositol turnover occurs on induction of Friend cell differentiation and precedes the decrease in c-myc expression. Cell. 1985 Nov;43(1):315–325. doi: 10.1016/0092-8674(85)90037-6. [DOI] [PubMed] [Google Scholar]
- Feig L. A., Cooper G. M. Relationship among guanine nucleotide exchange, GTP hydrolysis, and transforming potential of mutated ras proteins. Mol Cell Biol. 1988 Jun;8(6):2472–2478. doi: 10.1128/mcb.8.6.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmus J., Buick R. N. Relationship of c-myc expression to differentiation and proliferation of HL-60 cells. Cancer Res. 1985 Feb;45(2):822–825. [PubMed] [Google Scholar]
- Fleischman L. F., Chahwala S. B., Cantley L. ras-transformed cells: altered levels of phosphatidylinositol-4,5-bisphosphate and catabolites. Science. 1986 Jan 24;231(4736):407–410. doi: 10.1126/science.3001936. [DOI] [PubMed] [Google Scholar]
- Fukami K., Matsuoka K., Nakanishi O., Yamakawa A., Kawai S., Takenawa T. Antibody to phosphatidylinositol 4,5-bisphosphate inhibits oncogene-induced mitogenesis. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9057–9061. doi: 10.1073/pnas.85.23.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geny B., LePeuch C., Cost H., Basset M., Cockcroft S. Phorbol esters inhibit inositol phosphate and diacylglycerol formation in proliferating HL60 cells. Relationship to differentiation. FEBS Lett. 1988 Jun 20;233(2):239–243. doi: 10.1016/0014-5793(88)80434-4. [DOI] [PubMed] [Google Scholar]
- Guillemette G., Balla T., Baukal A. J., Spät A., Catt K. J. Intracellular receptors for inositol 1,4,5-trisphosphate in angiotensin II target tissues. J Biol Chem. 1987 Jan 25;262(3):1010–1015. [PubMed] [Google Scholar]
- Huang M., Chida K., Kamata N., Nose K., Kato M., Homma Y., Takenawa T., Kuroki T. Enhancement of inositol phospholipid metabolism and activation of protein kinase C in ras-transformed rat fibroblasts. J Biol Chem. 1988 Dec 5;263(34):17975–17980. [PubMed] [Google Scholar]
- Imai A., Hattori H., Takahashi M., Nozawa Y. Evidence that cyclic AMP may regulate Ca2+-mobilization and phospholipases in thrombin-stimulated human platelets. Biochem Biophys Res Commun. 1983 Apr 29;112(2):693–700. doi: 10.1016/0006-291x(83)91518-8. [DOI] [PubMed] [Google Scholar]
- Kamata T., Feramisco J. R. Epidermal growth factor stimulates guanine nucleotide binding activity and phosphorylation of ras oncogene proteins. Nature. 1984 Jul 12;310(5973):147–150. doi: 10.1038/310147a0. [DOI] [PubMed] [Google Scholar]
- Kato H., Kawai S., Takenawa T. Disappearance of diacylglycerol kinase translocation in ras-transformed cells. Biochem Biophys Res Commun. 1988 Aug 15;154(3):959–966. doi: 10.1016/0006-291x(88)90233-1. [DOI] [PubMed] [Google Scholar]
- Kelvin D. J., Simard G., Sue-A-Quan A., Connolly J. A. Growth factors, signaling pathways, and the regulation of proliferation and differentiation in BC3H1 muscle cells. II. Two signaling pathways distinguished by pertussis toxin and a potential role for the ras oncogene. J Cell Biol. 1989 Jan;108(1):169–176. doi: 10.1083/jcb.108.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelvin D. J., Simard G., Tai H. H., Yamaguchi T. P., Connolly J. A. Growth factors, signaling pathways, and the regulation of proliferation and differentiation in BC3H1 muscle cells. I. A pertussis toxin-sensitive pathway is involved. J Cell Biol. 1989 Jan;108(1):159–167. doi: 10.1083/jcb.108.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal J. C., Aaronson S. A. Activation of ras p21 transforming properties associated with an increase in the release rate of bound guanine nucleotide. Mol Cell Biol. 1986 Dec;6(12):4214–4220. doi: 10.1128/mcb.6.12.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal J. C., Moscat J., Aaronson S. A. Novel source of 1,2-diacylglycerol elevated in cells transformed by Ha-ras oncogene. Nature. 1987 Nov 19;330(6145):269–272. doi: 10.1038/330269a0. [DOI] [PubMed] [Google Scholar]
- Lacal J. C., de la Peña P., Moscat J., Garcia-Barreno P., Anderson P. S., Aaronson S. A. Rapid stimulation of diacylglycerol production in Xenopus oocytes by microinjection of H-ras p21. Science. 1987 Oct 23;238(4826):533–536. doi: 10.1126/science.2821623. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lee W. M., Schwab M., Westaway D., Varmus H. E. Augmented expression of normal c-myc is sufficient for cotransformation of rat embryo cells with a mutant ras gene. Mol Cell Biol. 1985 Dec;5(12):3345–3356. doi: 10.1128/mcb.5.12.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. C., Beckner S. K., Darfler F. J. Characterization of hormone-sensitive Madin-Darby canine kidney cells. Methods Enzymol. 1985;109:360–365. doi: 10.1016/0076-6879(85)09101-7. [DOI] [PubMed] [Google Scholar]
- Lin M. C., Darfler F. J., Beckner S. K. Induction of glucagon sensitivity in a transformed kidney cell line by prostaglandin E2 and its inhibition by epidermal growth factor. Mol Cell Biol. 1987 Dec;7(12):4324–4328. doi: 10.1128/mcb.7.12.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara I. G. Elevated phosphocholine concentration in ras-transformed NIH 3T3 cells arises from increased choline kinase activity, not from phosphatidylcholine breakdown. Mol Cell Biol. 1989 Jan;9(1):325–328. doi: 10.1128/mcb.9.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najam N., Clair T., Bassin R. H., Cho-Chung Y. S. Cyclic AMP suppresses expression of v-rasH oncogene linked to the mouse mammary tumor virus promoter. Biochem Biophys Res Commun. 1986 Jan 14;134(1):436–442. doi: 10.1016/0006-291x(86)90582-6. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Johnson G. S., Anderson W. B. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- Porfiri E., Hoffbrand A. V., Wickremasinghe R. G. Granulocytic differentiation of HL60 human promyelocytic leukemia cells is preceded by a reduction in levels of inositol lipid-derived second messengers. Exp Hematol. 1988 Aug;16(7):641–646. [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Ridgway A. A., De Vouge M. W., Mukherjee B. B. Dibutyryl cAMP inhibits expression of transformation-related properties in Kirsten murine sarcoma virus transformed Balb/c-3T3 cells despite continued presence of p21v-Ki-ras. Biochem Cell Biol. 1988 Jan;66(1):54–65. doi: 10.1139/o88-007. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Williams D., Maryak J., Vass W., Goldberg R. J., Parks W. P. Type C particle-positive and type C particle-negative rat cell lines: characterization of the coding capacity of endogenous sarcoma virus-specific RNA. J Virol. 1976 Dec;20(3):570–582. doi: 10.1128/jvi.20.3.570-582.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seuwen K., Lagarde A., Pouysségur J. Deregulation of hamster fibroblast proliferation by mutated ras oncogenes is not mediated by constitutive activation of phosphoinositide-specific phospholipase C. EMBO J. 1988 Jan;7(1):161–168. doi: 10.1002/j.1460-2075.1988.tb02796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal I. S., Gibbs J. B., D'Alonzo J. S., Temeles G. L., Wolanski B. S., Socher S. H., Scolnick E. M. Mutant ras-encoded proteins with altered nucleotide binding exert dominant biological effects. Proc Natl Acad Sci U S A. 1986 Feb;83(4):952–956. doi: 10.1073/pnas.83.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., DeGudicibus S. R., Smith M. R. Cellular ras activity and tumor cell proliferation. Exp Cell Res. 1987 Jul;171(1):232–242. doi: 10.1016/0014-4827(87)90266-7. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y., Noda M., Kitayama H., Ikawa Y. Possible involvement of two signaling pathways in induction of neuron-associated properties by v-Ha-ras gene in PC12 cells. J Biol Chem. 1988 Aug 25;263(24):12102–12108. [PubMed] [Google Scholar]
- Tagliaferri P., Clair T., DeBortoli M. E., Cho-Chung Y. S. Two classes of cAMP analogs synergistically inhibit p21 ras protein synthesis and phenotypic transformation of NIH/3T3 cells transfected with Ha-MuSV DNA. Biochem Biophys Res Commun. 1985 Aug 15;130(3):1193–1200. doi: 10.1016/0006-291x(85)91741-3. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Ishitoya J., Nagai Y. Inhibitory effect of prostaglandin E2, forskolin, and dibutyryl cAMP on arachidonic acid release and inositol phospholipid metabolism in guinea pig neutrophils. J Biol Chem. 1986 Jan 25;261(3):1092–1098. [PubMed] [Google Scholar]
- Taub M., Chuman L., Saier M. H., Jr, Sato G. Growth of Madin-Darby canine kidney epithelial cell (MDCK) line in hormone-supplemented, serum-free medium. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3338–3342. doi: 10.1073/pnas.76.7.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele C. J., Reynolds C. P., Israel M. A. Decreased expression of N-myc precedes retinoic acid-induced morphological differentiation of human neuroblastoma. 1985 Jan 31-Feb 6Nature. 313(6001):404–406. doi: 10.1038/313404a0. [DOI] [PubMed] [Google Scholar]
- Trahey M., McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987 Oct 23;238(4826):542–545. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Hamamori Y., Yamashita T., Fukumoto Y., Takai Y. Involvement of three intracellular messenger systems, protein kinase C, calcium ion and cyclic AMP, in the regulation of c-fos gene expression in Swiss 3T3 cells. FEBS Lett. 1986 Nov 10;208(1):39–42. doi: 10.1016/0014-5793(86)81527-7. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Umegaki K., Smith W. L. Association of a solubilized prostaglandin E2 receptor from renal medulla with a pertussis toxin-reactive guanine nucleotide regulatory protein. J Biol Chem. 1986 Oct 15;261(29):13430–13437. [PubMed] [Google Scholar]
- Watson S. P., McConnell R. T., Lapetina E. G. The rapid formation of inositol phosphates in human platelets by thrombin is inhibited by prostacyclin. J Biol Chem. 1984 Nov 10;259(21):13199–13203. [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. Elevated levels of diacylglycerol and decreased phorbol ester sensitivity in ras-transformed fibroblasts. Nature. 1987 Jan 22;325(6102):359–361. doi: 10.1038/325359a0. [DOI] [PubMed] [Google Scholar]
- Wright T. M., Rangan L. A., Shin H. S., Raben D. M. Kinetic analysis of 1,2-diacylglycerol mass levels in cultured fibroblasts. Comparison of stimulation by alpha-thrombin and epidermal growth factor. J Biol Chem. 1988 Jul 5;263(19):9374–9380. [PubMed] [Google Scholar]
- Yokohama H., Tanaka T., Ito S., Negishi M., Hayashi H., Hayaishi O. Prostaglandin E receptor enhancement of catecholamine release may be mediated by phosphoinositide metabolism in bovine adrenal chromaffin cells. J Biol Chem. 1988 Jan 25;263(3):1119–1122. [PubMed] [Google Scholar]
- Yu C. L., Tsai M. H., Stacey D. W. Cellular ras activity and phospholipid metabolism. Cell. 1988 Jan 15;52(1):63–71. doi: 10.1016/0092-8674(88)90531-4. [DOI] [PubMed] [Google Scholar]
- de Chaffoy de Courcelles D., Roevens P., van Belle H. Agents that elevate platelet cAMP stimulate the formation of phosphatidylinositol 4-phosphate in intact human platelets. FEBS Lett. 1986 Jan 20;195(1-2):115–118. doi: 10.1016/0014-5793(86)80142-9. [DOI] [PubMed] [Google Scholar]