Abstract
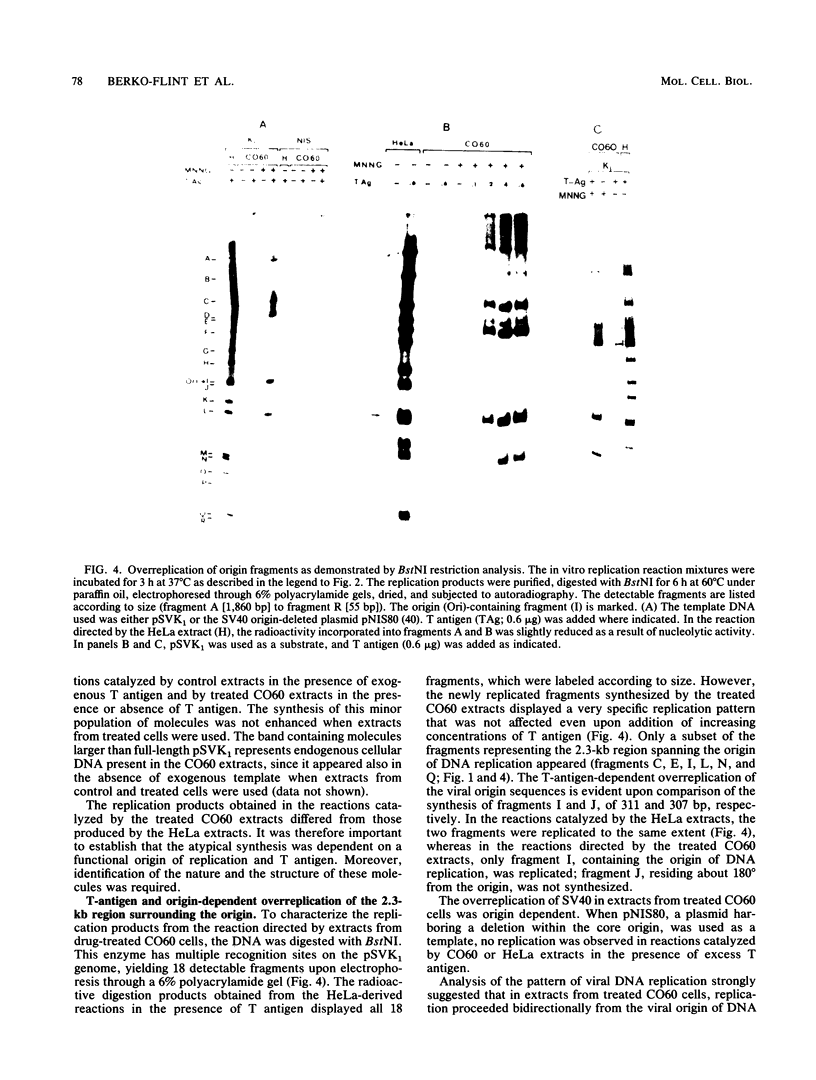
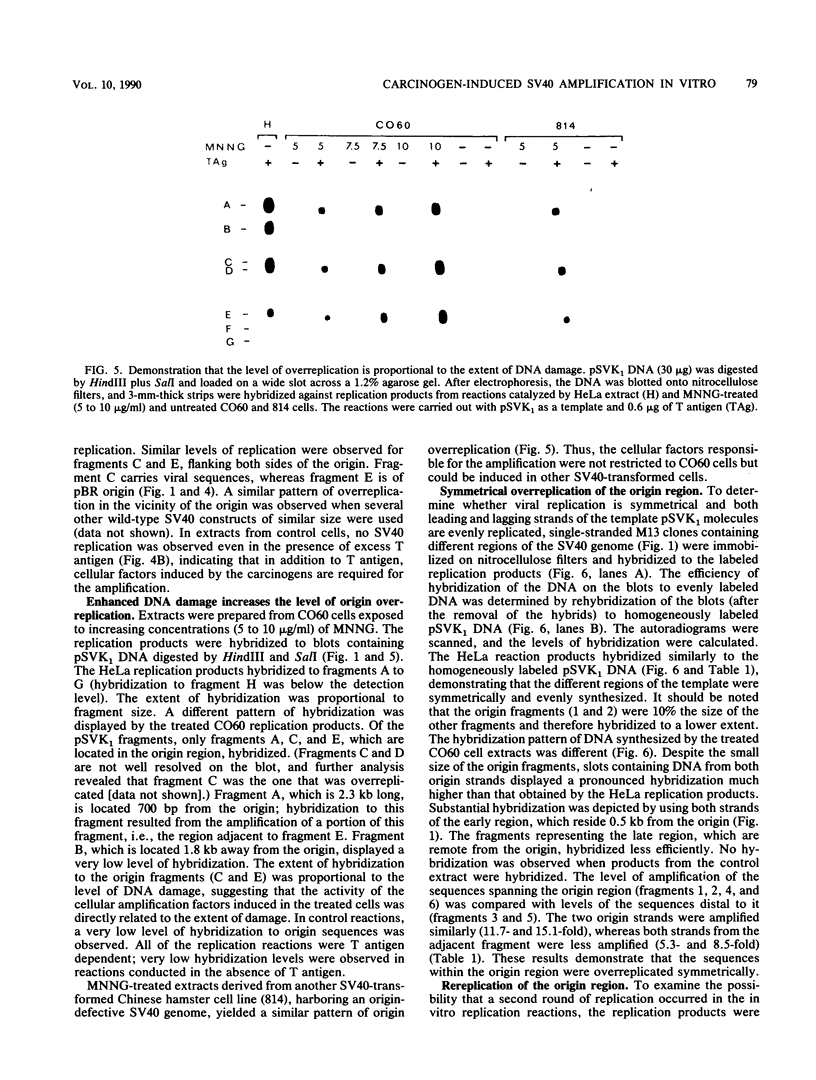
An in vitro system to study carcinogen-induced amplification in simian virus 40 (SV40)-transformed Chinese hamster (CO60) cells is described. SV40 amplification in this system resembled in many aspects the viral overreplication observed in drug-treated CO60 cells. Cytosolic extracts from N-methyl-N'-nitro-N-nitrosoguanidine-treated cells supported de novo DNA synthesis in the presence of excess exogenous T antigen and the SV40-containing plasmid pSVK1. The pattern of viral replication in these extracts was unique, since only the 2.4-kilobase-pair region spanning the origin was overreplicated, whereas distal sequences were not replicated significantly. Extracts from control cells supported only marginal levels of replication. In HeLa extracts, complete SV40 DNA molecules were replicated efficiently. The overreplication of the origin region in CO60 cell extracts was bidirectional and symmetrical. A fraction of the newly synthesized DNA molecules underwent a second round of replication, yielding MboI-sensitive fragments representing the 2.4-kilobase-pair region around the origin. The mechanisms controlling the amplification of the viral origin region, the nature of the cellular factors induced in the carcinogen-treated cells, and their putative association with general drug-induced SOS-like responses are discussed.
Full text
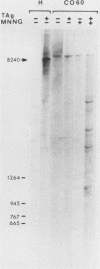
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C. Transient gene expression control: effects of transfected DNA stability and trans-activation by viral early proteins. Mol Cell Biol. 1985 May;5(5):1034–1042. doi: 10.1128/mcb.5.5.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. Oncogenes and human cancer: cause or consequence? Carcinogenesis. 1986 Jul;7(7):1037–1042. doi: 10.1093/carcin/7.7.1037. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Braithwaite A. W., Sturzbecher H. W., Addison C., Palmer C., Rudge K., Jenkins J. R. Mouse p53 inhibits SV40 origin-dependent DNA replication. Nature. 1987 Oct 1;329(6138):458–460. doi: 10.1038/329458a0. [DOI] [PubMed] [Google Scholar]
- Classon M., Henriksson M., Sümegi J., Klein G., Hammarskjöld M. L., Hammaskjöld M. L. Elevated c-myc expression facilitates the replication of SV40 DNA in human lymphoma cells. Nature. 1987 Nov 19;330(6145):272–274. doi: 10.1038/330272a0. [DOI] [PubMed] [Google Scholar]
- DiMaio D., Nathans D. Regulatory mutants of simian virus 40. Effect of mutations at a T antigen binding site on DNA replication and expression of viral genes. J Mol Biol. 1982 Apr 15;156(3):531–548. doi: 10.1016/0022-2836(82)90265-0. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Nathans D. Purification of simian virus 40 large T antigen by immunoaffinity chromatography. J Virol. 1985 Mar;53(3):1001–1004. doi: 10.1128/jvi.53.3.1001-1004.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsett D., Deichaite I., Winocour E. Circular and linear simian virus 40 DNAs differ in recombination. Mol Cell Biol. 1985 Apr;5(4):869–880. doi: 10.1128/mcb.5.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel M., Sachs L. Induction of virus synthesis in polyoma transformed cells by ultraviolet light and mitomycin C. Virology. 1970 Jan;40(1):174–177. doi: 10.1016/0042-6822(70)90391-0. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn R., Schlehofer J. R., Yalkinoglu A. O., Zur Hausen H. Selective DNA-amplification induced by carcinogens (initiators): evidence for a role of proteases and DNA polymerase alpha. Int J Cancer. 1985 Jul 15;36(1):85–91. doi: 10.1002/ijc.2910360114. [DOI] [PubMed] [Google Scholar]
- Herrlich P., Angel P., Rahmsdorf H. J., Mallick U., Pöting A., Hieber L., Lücke-Huhle C., Schorpp M. The mammalian genetic stress response. Adv Enzyme Regul. 1986;25:485–504. doi: 10.1016/0065-2571(86)90030-0. [DOI] [PubMed] [Google Scholar]
- Ishimi Y., Claude A., Bullock P., Hurwitz J. Complete enzymatic synthesis of DNA containing the SV40 origin of replication. J Biol Chem. 1988 Dec 25;263(36):19723–19733. [PubMed] [Google Scholar]
- Johnston R. N., Feder J., Hill A. B., Sherwood S. W., Schimke R. T. Transient inhibition of DNA synthesis results in increased dihydrofolate reductase synthesis and subsequent increased DNA content per cell. Mol Cell Biol. 1986 Oct;6(10):3373–3381. doi: 10.1128/mcb.6.10.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J. SV40 DNA replication. J Biol Chem. 1988 Dec 5;263(34):17889–17892. [PubMed] [Google Scholar]
- Klein G., Klein E. Conditioned tumorigenicity of activated oncogenes. Cancer Res. 1986 Jul;46(7):3211–3224. [PubMed] [Google Scholar]
- Kleinberger T., Etkin S., Lavi S. Carcinogen-mediated methotrexate resistance and dihydrofolate reductase amplification in Chinese hamster cells. Mol Cell Biol. 1986 Jun;6(6):1958–1964. doi: 10.1128/mcb.6.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberger T., Flint Y. B., Blank M., Etkin S., Lavi S. Carcinogen-induced trans activation of gene expression. Mol Cell Biol. 1988 Mar;8(3):1366–1370. doi: 10.1128/mcb.8.3.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberger T., Sahar E., Lavi S. Carcinogen-mediated co-activation of two independent genes in Chinese hamster cells. Carcinogenesis. 1988 Jun;9(6):979–985. doi: 10.1093/carcin/9.6.979. [DOI] [PubMed] [Google Scholar]
- Lambert M. E., Pellegrini S., Gattoni-Celli S., Weinstein I. B. Carcinogen induced asynchronous replication of polyoma DNA is mediated by a trans-acting factor. Carcinogenesis. 1986 Jun;7(6):1011–1017. doi: 10.1093/carcin/7.6.1011. [DOI] [PubMed] [Google Scholar]
- Lavi S., Etkin S. Carcinogen-mediated induction of SV40 DNA synthesis in SV40 transformed Chinese hamster embryo cells. Carcinogenesis. 1981;2(5):417–423. doi: 10.1093/carcin/2.5.417. [DOI] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro: specificity of initiation and evidence for bidirectional replication. Mol Cell Biol. 1985 Jun;5(6):1238–1246. doi: 10.1128/mcb.5.6.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor H., Neer A. Effects of cycloheximide on virus RNA replication in an inducible line of polyoma-transformed rat cells. Cell. 1975 Jul;5(3):311–318. doi: 10.1016/0092-8674(75)90106-3. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Wobbe C. R., Weissbach L., Dean F. B., Hurwitz J. Role of DNA polymerase alpha and DNA primase in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1986 May;83(9):2869–2873. doi: 10.1073/pnas.83.9.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S., Oishi M. UV Irradiation induces an activity which stimulates Simian virus 40 rescue upon cell fusion. Mol Cell Biol. 1984 Jun;4(6):1159–1162. doi: 10.1128/mcb.4.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelich G., Stillman B. Coordinated leading and lagging strand synthesis during SV40 DNA replication in vitro requires PCNA. Cell. 1988 Apr 8;53(1):117–126. doi: 10.1016/0092-8674(88)90493-x. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., D'Urso G. An origin unwinding activity regulates initiation of DNA replication during mammalian cell cycle. Science. 1988 Sep 16;241(4872):1486–1489. doi: 10.1126/science.2843984. [DOI] [PubMed] [Google Scholar]
- Ronai Z. A., Lambert M. E., Johnson M. D., Okin E., Weinstein I. B. Induction of asynchronous replication of polyoma DNA in rat cells by ultraviolet irradiation and the effects of various inhibitors. Cancer Res. 1987 Sep 1;47(17):4565–4570. [PubMed] [Google Scholar]
- Ronai Z. A., Weinstein I. B. Identification of a UV-induced trans-acting protein that stimulates polyomavirus DNA replication. J Virol. 1988 Mar;62(3):1057–1060. doi: 10.1128/jvi.62.3.1057-1060.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured cells. J Biol Chem. 1988 May 5;263(13):5989–5992. [PubMed] [Google Scholar]
- Schlehofer J. R., Ehrbar M., zur Hausen H. Vaccinia virus, herpes simplex virus, and carcinogens induce DNA amplification in a human cell line and support replication of a helpervirus dependent parvovirus. Virology. 1986 Jul 15;152(1):110–117. doi: 10.1016/0042-6822(86)90376-4. [DOI] [PubMed] [Google Scholar]
- Schutzbank T., Robinson R., Oren M., Levine A. J. SV40 large tumor antigen can regulate some cellular transcripts in a positive fashion. Cell. 1982 Sep;30(2):481–490. doi: 10.1016/0092-8674(82)90245-8. [DOI] [PubMed] [Google Scholar]
- Segawa K., Yamaguchi N. Induction of c-Ha-ras transcription in rat cells by simian virus 40 large T antigen. Mol Cell Biol. 1987 Jan;7(1):556–559. doi: 10.1128/mcb.7.1.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. E., Bohmann D., Sergeant A. The SV40 enhancer influences viral late transcription in vitro and in vivo but not on replicating templates. EMBO J. 1985 Dec 1;4(12):3247–3252. doi: 10.1002/j.1460-2075.1985.tb04073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale S. T., Tjian R. T-antigen-DNA polymerase alpha complex implicated in simian virus 40 DNA replication. Mol Cell Biol. 1986 Nov;6(11):4077–4087. doi: 10.1128/mcb.6.11.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R., Debatisse M., Giulotto E., Wahl G. M. Recent progress in understanding mechanisms of mammalian DNA amplification. Cell. 1989 Jun 16;57(6):901–908. doi: 10.1016/0092-8674(89)90328-0. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Gluzman Y. Replication and supercoiling of simian virus 40 DNA in cell extracts from human cells. Mol Cell Biol. 1985 Aug;5(8):2051–2060. doi: 10.1128/mcb.5.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlsty T. D., Brown P. C., Schimke R. T. UV radiation facilitates methotrexate resistance and amplification of the dihydrofolate reductase gene in cultured 3T6 mouse cells. Mol Cell Biol. 1984 Jun;4(6):1050–1056. doi: 10.1128/mcb.4.6.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerie K., Delers A., Bruck C., Thiriart C., Rosenberg H., Debouck C., Rosenberg M. Activation of human immunodeficiency virus type 1 by DNA damage in human cells. Nature. 1988 May 5;333(6168):78–81. doi: 10.1038/333078a0. [DOI] [PubMed] [Google Scholar]
- Wang E. H., Friedman P. N., Prives C. The murine p53 protein blocks replication of SV40 DNA in vitro by inhibiting the initiation functions of SV40 large T antigen. Cell. 1989 May 5;57(3):379–392. doi: 10.1016/0092-8674(89)90913-6. [DOI] [PubMed] [Google Scholar]
- Wobbe C. R., Dean F., Weissbach L., Hurwitz J. In vitro replication of duplex circular DNA containing the simian virus 40 DNA origin site. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5710–5714. doi: 10.1073/pnas.82.17.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Li J. J., Kelly T. J. Initiation of simian virus 40 DNA replication in vitro: large-tumor-antigen- and origin-dependent unwinding of the template. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3643–3647. doi: 10.1073/pnas.84.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]