Abstract
We propose that canine visceral leishmaniasis (CVL) is a systemic fibrotic disease, as evidenced by the wide distribution of fibrosis that we have found in the dogs suffering from chronic condition. The inflammatory cells apparently direct fibrosis formation. Twenty-four cases (symptomatic dogs) were identified from a total of one hundred and five cases that had been naturally infected with Leishmania chagasi and had been documented during an epidemiological survey of CVL carried out by the metropolitan area of the municipality of Belo Horizonte, MG, Brazil. The histological criterion was intralobular liver fibrosis, as has been described previously in dogs with visceral leishmaniasis. In addition to the findings in the liver, here we describe and quantify conspicuous and systemic deposition of collagen in other organs, including spleen, cervical lymph nodes, lung and kidney of all the infected symptomatic dogs. Thus we report that there is a systematic fibrotic picture in these animals, where inflammatory cells appear to direct fibrosis in all organs that have been studied. Therefore we propose that CVL is a systemic fibrotic disease.
Keywords: canine visceral leishmaniasis, fibrosis, histopathology
Fibrosis is defined as the excessive deposition of extracellular matrix (ECM) in an organ or organs, and can occur throughout the body. ECM contributes a significant proportion of the volume in any tissue. Three groups of macromolecules are physically associated to form ECM: (i) fibrous structural proteins, such as collagens and elastins; (ii) a diverse group of adhesion glycoproteins, including fibronectin and laminin; and (iii) a gel of proteoglycans and hyaluronan. Interstitial matrix and basal membrane are the two basic general organizations where the former is present in spaces between epithelial / endothelial and smooth muscle cells and in connective tissue. It consists of fibrillar (types I, II and II) and non-fibrillar collagen (IV, V and VI), elastin, fibronectin, proteoglycans, hyaluronate and other components (Wynn 2007)
Canine visceral leishmaniasis (CVL) is caused by Leishmania infantum (syn. L. chagasi in America) and is transmitted by phlebotomine sand fly bites (Lutzomyia longipalpis species in America (Deane & Deane 1964; Mauricio et al. 2000). Protozoa of the genus Leishmania are dimorphic obligate intracellular parasites that reside within mononuclear phagocytes in the mammalian host. Classic histopathological lesions have been described mainly in organs rich in cells of the mononuclear phagocytic system, such as liver, spleen, lymph nodes, bone marrow, gastrointestinal tract and skin. In general, an intense chronic inflammatory reaction consisting of infiltration by mononuclear cells (macrophages, plasma cells and lymphocytes) occurs in the liver and spleen (Tryphonas et al. 1977; Anosa & Idowu 1983; Keenan et al. 1984; Tafuri et al. 1996)
A chronic inflammatory reaction characterized by intralobular granuloma formations in the liver has been described previously in experimentally infected dogs (Gonzalez et al. 1988; Oliveira et al. 1993) and has also been seen in dogs naturally with L. chagasi in Brazil (Tafuri et al. 1996) and Colombia (Sanchez et al. 2004). Moreover, authors such as Lima et al. 2007 and Melo et al. 2008 found that >92% of naturally infected dogs showed intralobular hepatic granuloma formation comprising of plasma cells, macrophages (epithelioid cells) and lymphocytes. Other organs also frequently involved in this systemic disease include skin (Deane & Deane 1955; Tafuri et al. 2001; Solano-Gallego et al. 2004; Figueiredo et al. 2010), bone marrow (Solano-Gallego et al. 2004), lymph nodes (Martinez-Moreno et al. 1993; Lima et al. 2004) gastrointestinal tract (Lennox et al. 1972; Tryphonas et al. 1977; Anderson et al. 1980; Slappendel 1988; Ferrer et al. 1991; Adamama-Moraitou et al. 2007; Pinto et al. 2011) and kidneys (Tafuri et al. 1989; Nieto et al. 1992). Thus, CVL is a chronic disease characterized by a systemic inflammatory reaction where the cellular exudate is mainly composed of mononuclear cells.
Fibrosis is an important health problem, but the pathogenic principles which underlie it remain largely unknown. It can develop either spontaneously or more frequently, as a consequence of underlying diseases. Irrespective of the primary cause, fibrotic tissue is always infiltrated by mononuclear immune cells (Wynn 2007; Wick et al. 2010). We have attempted to characterize whether dogs with hepatic intralobular fibrosis (Melo et al. 2008) also have systemic fibrosis when they suffer from a chronic inflammatory disease, such as visceral leishmaniasis.
Materials and methods
Dogs
Twenty-four mongrel dogs with Leishmania (Leishmania) chagasi identified previously by Melo et al. (2008) were used for this work. These dogs were identified during an epidemiological survey of CVL carried out by the metropolitan area of the municipality of Belo Horizonte, MG (southern Brazil). Enzyme-linked immunosorbent assays (ELISA; optical density >100 >1:400 dilutions) were positive for all infected animals. Serum samples were also analysed using a commercial kit containing an immunochromatographic strip with recombinant leishmanial antigen k39 and a dominant amastigote antigen of L. chagasi (rK39), highly sensitive and specific for Leishmania donovani complex infection (Burns et al. 1993; Houghton et al. 1998; Kumar et al. 2002). Sera from all infected dogs were positive for this test. Another six dogs with serological and parasitological examinations negative for Leishmania were obtained as control animals.
Groups and clinical aspects of all dogs
Infected dogs were clinically classified and divided into two groups: Group 1, symptomatic dogs – 24 animals that exhibited at least one classical sign of the disease, including lymphadenopathy, cutaneous alterations (alopecia, dry exfoliative dermatitis or ulcers), onychogryphosis, keratoconjunctivitis, weight loss or cachexia, and anaemia (Alvar et al. 2004); Group 2, control dogs – 6 uninfected animals that were serologically and parasitologically negative for Leishmania.
Ethics and control animals
In Brazil, animals with CVL are usually left untreated. The treatment continues to be prohibited by the Brazilian Health Ministry (Portaria Interministerial 1.426 de 11 de Julho de 2008). According to the Ministry of Health Policy (recommended by World Health Organization), seropositive dogs (ELISA testing with Biomanguinhos Test-FIOCRUZ-RJ) must be euthanatized. Otherwise, dogs are not euthanized without the Ethics Committee approval, particularly control (uninfected) dogs. The study was approved by the CETEA/UFMG (Comite de Etica em Experimentação Animal/Universidade Federal de Minas Gerais), protocol 190/06 (valid until 12 March 2013). All procedures involving animals were conducted according to the guidelines of the Colégio Brasileiro de Experimentação Animal (COBEA).
Parasitological diagnosis of Leishmania infection, necropsy and histopathology
The dogs were euthanized with 2.5% (1.0 ml/kg) intravenous thiopental and T61™ (0.3 ml/kg). Touch aspirates of bone marrow were obtained for parasitological diagnosis of infected and control animals. The smears were air-dried and stained with 10% Giemsa. Leishmania amastigotes were detected in all infected animals by light microscopy using oil immersion (×1000 final magnification). Control animals were parasitological negative.
Fragments of organs (livers, spleens, cervical lymph nodes, lungs and kidneys) were removed for histopathology. They were fixed in 10% neutral-buffered formalin, dehydrated, cleared, embedded in paraffin, cut into 3 μm sections and stained with haematoxylin and eosin (HE).
Collagen detection and criteria for selective samples inclusion
For collagen studies, fragments of all organs (livers, spleens, cervical lymph nodes, lungs and kidneys) were stained with Gomori's ammoniacal silver and Picrosirius red. In silver staining, the fibrillar collagen becomes black, and in Picrosirius, some fibres (probably type I) stain red or yellow, whereas reticular collagen (probably type III) appears green under polarized light. For Picrosirius staining, fibrillar collagen was estimated at ×110 resolution using polarized light. (De Paola 1956; Junqueira et al. 1979)
The histological criterion for the diagnosis was intralobular liver fibrosis as has been previously described in dogs with visceral leishmaniasis. Based on an earlier histological study by Melo et al. (2008) of fragments of livers from one hundred and five mongrel dogs naturally infected with L. chagasi in Brazil, the collagen deposition, despite being identified by HE, Gomori trichrome and Heidenhain blue staining, was most evident with Gomori's ammoniacal silver staining. Thus, we selected 24 cases with an intense and diffuse hepatic intralobular fibrosis after using this staining method to confirm the presence of easily detectable hepatic fibrosis. Then, we used the same histochemical method to study the fibropoesis (collagen deposition) for tissue sections of spleens, cervical lymph nodes, lungs and kidneys of the same 24 dogs. The aim was to determine whether this fibrosis process could be found systemically, that is, in the other organs.
Histomorphometric analysis
Stained sections were analysed morphometrically to characterize the intralobular collagen deposition, excluding perivascular collagen, using an Axiolab light microscope (Carl Zeiss, Oberkochen, Germany) with ×440 resolution. The images were transferred to a computer video screen and relayed to an image analysis system (Kontron Elektronic/Carl Zeiss). The total area occupied by stained collagen fibres was measured using a digital pad from real images and segmented to generate binary images. The results were expressed in square micrometres (Caliari 1997; Goncalves et al. 2003; Melo et al. 2008)
Immunohistochemical method for labelling amastigotes of Leishmania and morphometric analysis
Deparaffined slides were hydrated and incubated with 4% hydrogen peroxide (30vv) in 0.01 M phosphate-buffered saline (PBS; pH 7.2) to block endogenous peroxidase activity, followed by incubation with normal goat serum (1:100 dilution) to block non-specific immunoglobulin absorption. Heterologous hyperimmune serum from dogs naturally infected with L. chagasi (IFAT titre >1:40) was diluted 1:100 with 0.01 M PBS and employed as the primary antibody. Slides were incubated in a humid chamber at 4 °C for 18–22 h, washed with PBS, incubated with biotinylated goat anti-mouse and anti-rabbit immunoglobulin (LSAB2 kit; Dako, Carpinteria, CA, USA), washed in PBS and incubated with streptavidin–peroxidase complex (LSAB2 kit; Dako) for 20 min at room temperature. Slides were treated with 0.024% diaminobenzidine (Sigma Saint Louis, MO, USA) and 0.16% hydrogen peroxidase (30 vv), dehydrated, cleared, counterstained with Harris's haematoxylin and mounted with cover slips. This immunohistochemistry method was carried out using a secondary antibody not specific to canine immunoglobulin. This characterised a cross-immune reaction and provided an alternative method for detecting Leishmania amastigotes in paraffin-embedded canine tissues as previously described by Tafuri et al. (2004). Leishmania amastigotes were readily observed within macrophages in all tissue fragments of all the selected organs of naturally infected dogs.
For the histomorphometric study, forty randomly chosen images (horizontal and vertical movements were selected using the microscope stage – XY translation to avoid overlapping fields) from histological slides of liver tissue fragments. These allowed for assessment of the number of immunolabelled amastigotes. Kontron Elektronick/Carl Zeiss image analyzer (KS300 software) as described by Caliari (1997) and Melo et al. (2008) was utilized, as was an Axiolab light microscope (Zeiss) at a resolution of ×440 (parasites were represented in μm2).
Statistical analysis
The Mann–Whitney U-test was used to compare segments between the two groups. For correlation analysis of fibrosis and parasite load, Pearson parametric analysis was used. The graphpad instat 3.0 and prism 4.0 software programs (San Diego, CA, USA) were used in these comparisons. Statistical significance was set at P < 0.05.
Results
The most frequent clinical signs were lymphadenopathy (81%), weight loss (35%), skin alterations such as onychogryphosis (35%), ulcers (30%), chronic dry desquamation (30%), alopecia (22%) mainly restricted to the ears and limbs, followed by clinical anaemia (15%) and loneness (10%).
Microscopically, histological examination after HE staining showed a general chronic inflammatory reaction involving the entire architecture of the organs as discussed below.
Liver
The histological analysis included the capsule, portal tracts and central veins or perisinusoidal spaces. The portal space showed no fibrosis, but there was mild infiltration of lymphocytes, plasma cells and macrophages with or without amastigotes. Kupffer cells showed hyperplasia and hypertrophy. Intralobular granulomas comprised of plasma cells, epithelioid cells and lymphocytes were found in almost all cases (n = 21; 87%). The search for Leishmania parasites by HE, and more specifically by immunohistochemistry, located amastigotes in all liver samples, mainly in Kupffer cells, but also in macrophage granulomas (Table 1).
Table 1.
Frequency of hepatic lesions of dogs naturally infected with Leishmania (L.) chagasi from Belo Horizonte, Minas Gerais (MG) state, Brazil
| Histological alterations | n = 24 | Percentage |
|---|---|---|
| Degenerative lesions (hidropic and/or esteatosis) | 24 | 100 |
| Chronic portal inflammatory reaction | 24 | 100 |
| Glisson capsule with a chronic inflammatory reaction | 13 | 54.1 |
| Hypertrophy and hyperplasia of kupffer cells | 23 | 95.8 |
| Hepatic intralobular granulomas | 21 | 87.5 |
| Haemosiderosis | 8 | 33.3 |
| Sinusoidal congestion | 21 | 87.5 |
| Parasitism | 21 | 87.5 |
Spleen
A diffuse chronic inflammatory reaction was observed in the capsule, subcapsular region, trabecular system and red pulp. The capsule and the trabecular system were thickened, and the trabecular vessels were dilated and congested. The red pulp showed profound modifications due to the marginal macrophage proliferation, and a granulomatous inflammatory reaction was seen in some cases. Macrophages located in granulomas were loaded with parasites with vacuolated cytoplasm. Brown crystals (hemosiderin pigments) were frequently observed in red pulp inside macrophages. The white pulp showed variable alterations. We observed cases (n = 17; 77.2%) with follicular hyperplasia, where germinal centres became contiguous in the outer cortex and moved irregularly into the red pulp. Cells with large nuclei, branched chromatin and prominent nucleoli (clear zone) delimited by medium-sized lymphocytes (dark zone) characterized the germinal centres. However, in other cases (n = 8; 33.3%), the white pulp was formed of only few lymphocytes around the central arteriole. Parasitized macrophages replaced the lymphocytes normally seen around the central arteriole thus emphasising the relative depletion of T-dependent areas of the white pulp. In all cases (n = 24; 100%), intense parasitism was found within the thickness of the capsule, subcapsular and perifollicular regions (marginal zone; Table 2).
Table 2.
Frequency of spleen lesions of dogs naturally infected with Leishmania (L.) chagasi from Belo Horizonte, Minas Gerais (MG) state, Brazil
| Histological alterations | n = 24 | Percentage |
|---|---|---|
| Inflammation of the capsule | 18 | 75 |
| Thickening of the capsule | 21 | 87.5 |
| Hypertrophy and hyperplasia of white pulp | 17 | 70.8 |
| Hypertrophy and hyperplasia of red pulp | 22 | 91.6 |
| Depletion of T-dependent areas | 8 | 33.3 |
| Granulomas | 3 | 12.5 |
| Congestion | 17 | 70.8 |
| Haemosiderosis | 16 | 66.6 |
| Parasitism | 24 | 100 |
Cervical lymph node
The main lesion observed was hypertrophy of the cortical and medullary regions. The latter were closely packed with differentiated plasma cells, medium-sized and large lymphocytes (lymphoblasts), and macrophages. However, the hypertrophy and hyperplasia of macrophages (most ‘epithelioid cells’ on account of their large vesicular nuclei, branched chromatin and abundant cytoplasm) were the main node histological alterations observed (n = 22; 91.6%; Table 3).
Table 3.
Frequency of cervical lymph node lesions of dogs naturally infected with Leishmania (L.) chagasi from Belo Horizonte, Minas Gerais (MG) state, Brazil
| Histological alterations | n = 24 | Percentage |
|---|---|---|
| Lymphadenitis capsular | 23 | 95.8 |
| Subcapsular sinus inflammation | 20 | 83.3 |
| Hypertrophy and hyperplasia of lymphoid nodules | 22 | 91.6 |
| Hypertrophy and hyperplasia of macrophages | 24 | 100 |
| Congestion | 20 | 83.3 |
| Haemosiderosis | 14 | 58.3 |
| Parasitism | 19 | 79.1 |
Lungs
The central feature observed was chronic and diffuse interstitial pneumonia (n = 23, 95.8%). The lesions had an intra-alveolar accumulation of various macrophages and interstitial thickening due to accumulations of mononuclear cells and fibrous tissue. In general, the cellular exudate was characterized predominantly by plasma cells, macrophages and lymphocytes with rare eosinophils and neutrophils. Macrophages were very numerous in septa, rare in alveolar spaces and had large shapeless nuclei with irregular borders. However, Leishmania amastigotes were seen in these cells in five cases. Hyperplasia of smooth muscle was observed in all intralobular septal walls (Table 4).
Table 4.
Frequency of lung lesions of dogs naturally infected with Leishmania (L.) chagasi from Belo Horizonte, Minas Gerais (MG) state, Brazil
| Histological alterations | n = 24 | Percentage |
|---|---|---|
| Pneumonitis in systematized foci | 2 | 8.33 |
| Intense chronic diffuse pneumonitis | 23 | 95.8 |
| Thickening of alveolar septa | 21 | 87.5 |
| Parasitism | 5 | 20.8 |
Kidney
In general, the glomeruli showed a diffuse membranoproliferative glomerulonephritis (MPGN) in the majority of the cases (n = 18; 75%). Cellularity of the glomerular tufts was increased due to proliferation of endothelial, epithelial or mesangial cells. The glomerular capillary walls had thickened basement membranes, as also the parietal and visceral endothelial and epithelial swelling. In most of the cases, thickening of Bowman's capsule occurred due to hyperplasia of parietal epithelial cells, thickening of the basement membrane and periglomerular fibrosis. Some glomeruli with tuft atrophy due to scarring were found. Non-glomerular alterations included tubular proteinuria and intense and diffuse chronic interstitial inflammation (plasma cells, macrophages and lymphocytes; Table 5).
Table 5.
Frequency of kidney lesions of dogs naturally infected with Leishmania (L.) chagasi from Belo Horizonte, Minas Gerais (MG) state, Brazil
| Histological alterations | n = 24 | Percentage |
|---|---|---|
| Degenerative diseases | 5 | 20.8 |
| Membranoproliferative glomerulonephritis (MPGN) | 18 | 75 |
| Interstitial nephritis | 12 | 50 |
| Glomerular atrophy/fibrosis | 10 | 41.6 |
| Glomerular fibrosis (scarring) | 4 | 16.6 |
| Haemosiderosis | 1 | 4.1 |
| Parasitism | 11 | 45.8 |
Fibrosis pattern
Gomori's ammoniac silver stained numerous reticular fibers black (argyrophilic fibers) in all organs described above. In livers it was mostly found in the portal space region and sinusoids in the hepatic lobe walls. Fibres were diffusely spread in several directions to form a compact network; some fibres encircled groups of hepatocytes or even single cells. Hepatic reticular fibres in all infected dogs were thicker than those in the control group (Figure 1a–f). Quantification showed significantly increased deposition of hepatic collagen in infected dogs' samples (symptomatic) compared with control dogs (control dogs' collagen deposition average/μm2 = 787,115; infected dogs' average 2750,083; P < 0.0001). There was a positive correlation between hepatic collagen deposition and parasite load in livers (r2 = 0.20).
Figure 1.
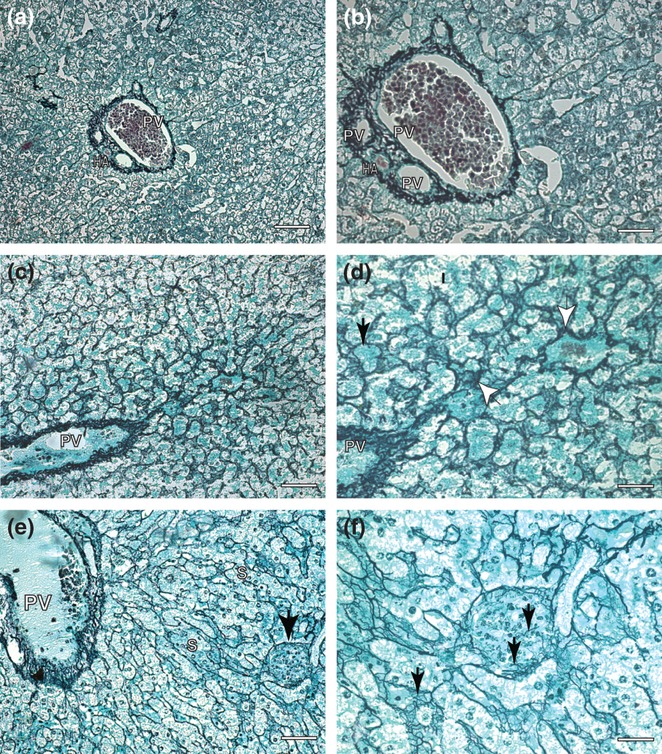
Liver sections of control and symptomatic dog naturally infected with Leishmania (L.) chagasi: (a,b). Control dog: (a) Lower magnification showing portal tract and collagen fibers in black color. Ammoniacal silver-staining (Bars = 62 μm); (b) higher magnification showing a delicate reticulin fibers detected by ammoniacal silver-staining. Ammoniacal silver-staining (Bars = 16 μm). Infected dog. “Rogers′ Cirrhosis (c–f). (c) Lower magnification showing portal tract in the left and collagen fibers in black color in the hepatic lobule. Ammoniacal silver-staining (Bars = 62 μm); (d) higher magnifications showing a conspicuous collagen thickening in the space of Disse and hepatic cells had become isolated from the sinusoidal blood by the fibropoiesis (black arrows). Observe dense and coiled fibers extend through sinusoids (white arrows). Ammoniacal silver-staining (Bars = 16 μm). (e) Beyond an intense fibrilopoesis, note a presence of one intralobular granuloma at the right corner of the picture. Ammoniacal silver-staining (Bars = 32 μm). (f) Detail of figure (e) showing granuloma with rare reticulin fibers inside. Ammoniacal silver-staining (Bars = 16 μm). PV, portal vein; S, sinusoids vessels; HA, hepatic artery.
In the spleens of all infected dogs, fibrilopoesis occurred mainly in the capsule and parenchyma. In fact, argyrophilic fibres were observed in the capsule and trabeculi, with the inside of the red pulp maintaining its tissue architecture. Besides being of higher intensity, the fibres were thicker, more dense and coiled than controls, forming a conspicuous network (Figure 2a–e). Quantification showed a significantly increased deposition of spleen collagen in infected dogs' samples (symptomatic) compared with control dogs (control dogs' collagen deposition average/μm2 = 556,405; infected dogs' average 2165,575; P < 0.0001). There was a positive correlation between splenic collagen deposition and the parasite load in spleens (r2 = 0.113)
Figure 2.
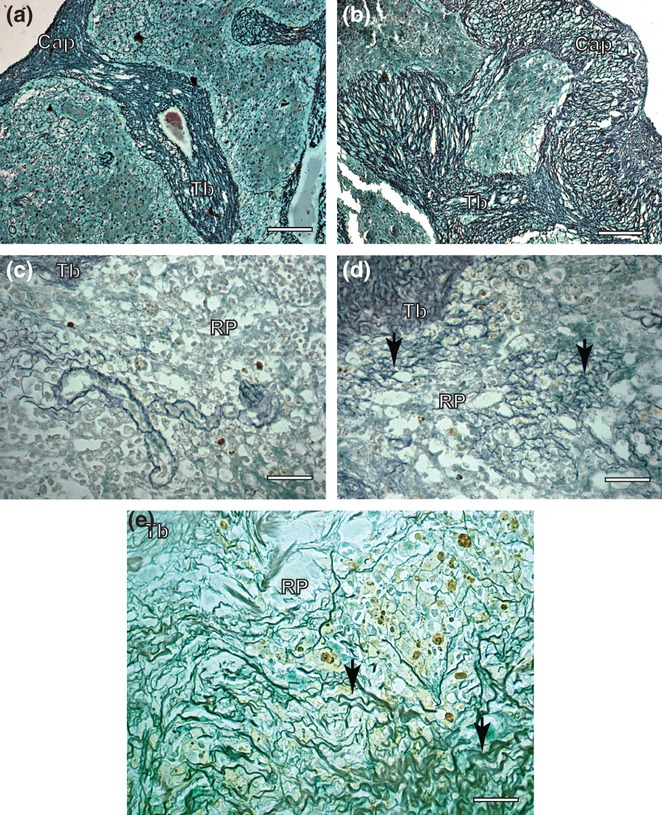
Spleen sections of control and symptomatic dog naturally infected with Leishmania (L.) chagasi. (a) Control dog: observe capsule and trabecules system composed by reticular fibers Ammoniacal silverstaining (Bars = 32 μm); (b) Infected dog: note intense argyrophilic fibers deposition in the capsule and trabecules. Ammoniacal silver-staining (Bars = 32 μm); (c) Control dog: observe inside red pulp reticulin fibers as a delicate network. Ammoniacal silver-staining (Bars = 16 μm); (d,e) Infected dog: inside of red pulp besides in higher intensity, the fibers were thicker, dense and coiled than controls, forming a conspicuous network (black arrows). Ammoniacal silver-staining (Bars = 16 μm); Cap, capsule; Tb, trabecule; RP, red pulp.
In cervical lymph nodes fibrilopoesis was observed mainly in the medullary layer characterized by thicker collagen argyrophilic fibre deposition, but the architecture of the lymph nodes was essentially preserved (Figure 3a,e). There was increased deposition of lymph node collagen in infected dogs' samples (symptomatic) compared with control dogs (control dogs' collagen deposition average/μm2 = 787,115; infected dogs' average 2008,242; P < 0.0001). There was a positive correlation between lymph node collagen deposition and the parasite load in the nodes (r2 = 0.016).
Figure 3.
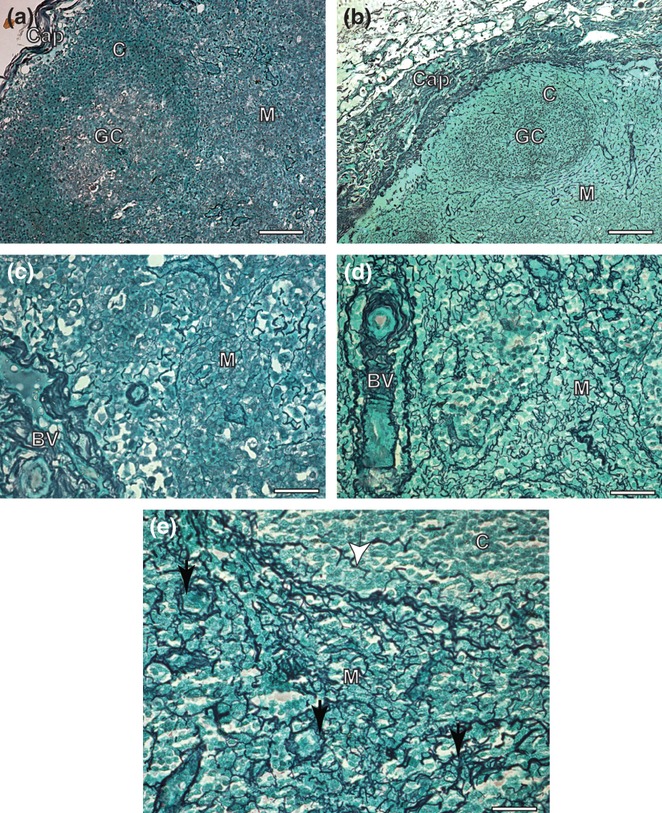
Cervical lymph node sections of control and symptomatic dog naturally infected with Leishmania (L.) chagasi. (a) Control dog: observe capsule composed by a delicate reticular fibers Ammoniacal silverstaining (Bars = 32 μm); (b) Infected dog: note intense argyrophilic fibers deposition in the capsule associated to an intense chronic inflammatory response. Ammoniacal silver-staining (Bars = 32 μm); (C) Control dog: observe inside medular layer reticulin fibers as a delicate network. Ammoniacal silver-staining (Bars = 16 μm); (d,e) Infected dog: inside of medular layer besides in higher intensity, the fibers were thicker, dense and coiled than controls, forming a conspicuous network (black arrows). Note few reticulin fibers deposition inside cortical zone. Ammoniacal silver-staining (Bars = 16 μm); Cap, capsule; C, cortical; GC, Germinative Center; M, medular; BV, blood vessels.
Gomori's ammoniacal silver staining revealed numerous reticular fibres within the alveolar septa. The reticular fibres were deeply stained and formed very dense tangled structures. More pronounced deposition of reticular fibres was present where inflammatory foci were more intense and thickening was more accentuated when compared with the lung tissue of control animals (Figure 4a–e). There was increased deposition of lung collagen in infected dogs' samples (symptomatic) compared with control dogs (control dogs' collagen deposition average/μm2 = 446,298 infected dogs' average 3775,503; P < 0.0001). There was a negative correlation between hepatic collagen deposition and the parasite load in lungs (r2 = 0.006).
Figure 4.
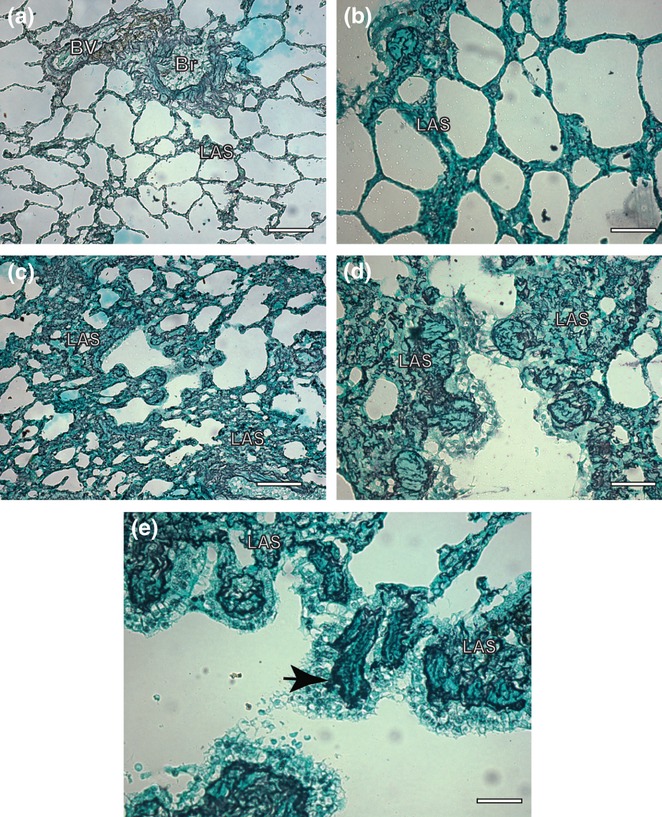
Lung sections of control and symptomatic dog naturally infected with Leishmania (L.) chagasi. (a,b). Control dog: observe a delicate reticular fibers network within the alveolar septa Ammoniacal silverstaining (Bars = 32 μm and Bars = 16 μm, respectively); (c,d) Infected dog: note intense argyrophilic fibers deposition in the within the alveolar septa associated to an intense chronic inflammatory response. Ammoniacal silver-staining (Bars = 32 μm and Bars = 16 μm, respectively); (e) Infected dog: besides in higher intensity, the fibers were thicker, dense and coiled than controls, forming a conspicuous network (black arrow). Ammoniacal silver-staining (Bars = 16 μm); LAS, lung alveolar septa; Br, bronchioles; BV, blood vessels.
Under HE analysis of all kidney fragments of all infected dogs, we found a MPGN constituted by mesangial cell proliferation, basement membrane thickening, leucocyte infiltration and accentuation of lobular architecture. We also frequently observed the presence of many glomeruli showing advanced scarring, sometimes to the point of complete sclerosis. Thus, fibrilopoesis was found mainly in glomeruli (cortex area) and small proportions in tubules of cortex and medullary layer (Figure 5a–g). There was increased deposition of collagen in infected dogs' samples (symptomatic) compared with control dogs (control dogs' collagen deposition average/μm2 = 928,789; infected dogs' average 3079,159; P < 0.0001). There was a positive correlation between hepatic collagen deposition and the parasite load in kidneys (r2 = 0.339). A resume of all data on the measurements of each dog is given in Table 6.
Figure 5.
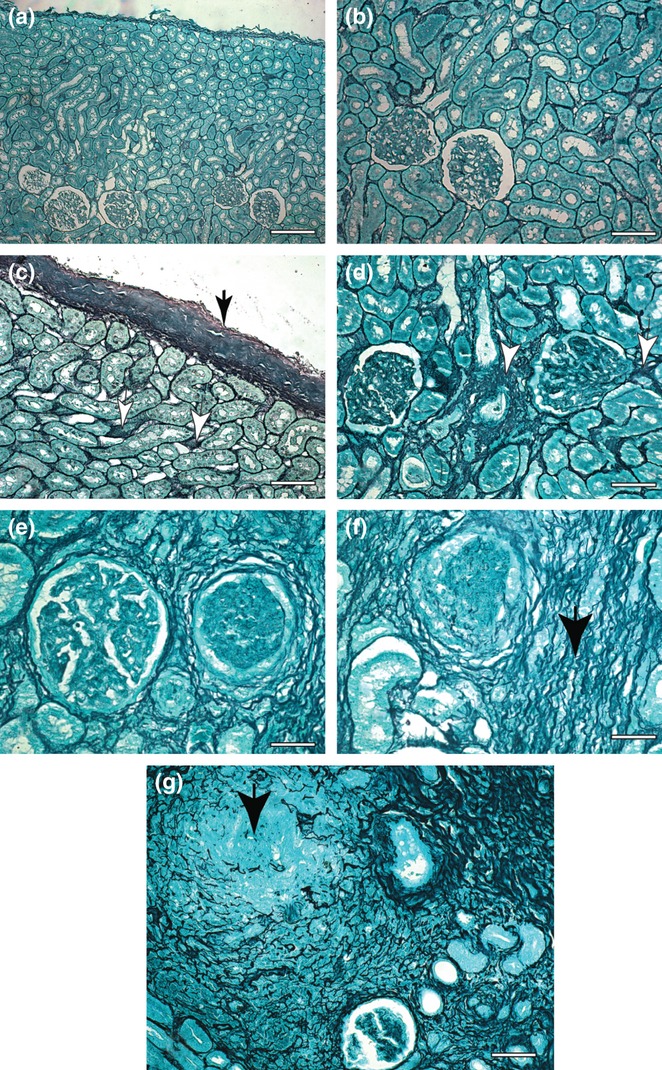
Kidney sections of control and symptomatic dog naturally infected with Leishmania (L.) chagasi. (a,b) Control dog: observe a delicate reticular fibers network in the capsule and cortex area. Ammoniacal silverstaining (Bars = 62 μm and Bars = 32 μm, respectively); (c,d) Infected dog: note intense argyrophilic fibers deposition in the capsule (black arrow) and cortex area (white arrow). Ammoniacal silver-staining (Bars = 32 μm and Bars = 16 μm, respectively); (e,f) Infected dog: (e) note two glomeruli, at right it appears with accentuation of lobular architecture and at left it appears with advanced scarring. Ammoniacal silverstaining (Bars = 16 μm). (f) Besides in higher intensity, the fibers were thicker, dense and coiled than controls, forming a conspicuous network (black arrow) into the cortex area. Ammoniacal silver-staining (Bars = 16 μm); (g) Infected dog: a case where we found glomeruli showing advanced scarring, sometimes to in the point of complete sclerosis (black arrow). Note an intense fibrilopoesis around the tubules of cortex area at the upper right side of the picture. Ammoniacal silver-staining (Bars = 16 μm).
Table 6.
Morphometrical analysis (average/μm2) of collagen deposition (Gomori ammoniacal silver staining) in liver, kidney, cervical lymph node, spleen and lung of naturally infected dogs with Leishmania (L.) chagasi from Belo Horizonte, Minas Gerais (MG) state, Brazil
| Dogs | Liver | Spleen | Cervical lymph node | Lung | Kidney |
|---|---|---|---|---|---|
| Control | 787,115 | 556,405 | 963,188 | 446,298 | 928,789 |
| Infected | 2750,083* | 2165,575* | 2008,242* | 3775,503* | 3079,159* |
P < 0,001
Discussion
Canine visceral leishmaniasis is a chronic disease defined as an immune response that persists for several months, in which inflammation, tissue remodelling and repair processes occur simultaneously. The distribution of the parasite is extensive throughout the body as it is found in liver, spleen, lymph nodes, bone marrow, gut, kidney, skin and other tissues. The presence of parasites and the immune response of the vertebrate host seen in many different tissues and organs provokes a general chronic inflammatory reaction characterized by a mononuclear cell infiltration consisting mainly of plasma cells (plasmocytosis), macrophages (with epithelioid and giant cell formations macrophage derived cells) and lymphocytes (lymphoblasts). In contrast to acute inflammatory reactions characterized by rapidly resolving vascular changes, oedema and neutrophilic inflammation, fibrosis typically occurs with chronic inflammation. Fibrosis is defined by overgrowth, hardening and/or scarring of various tissues, being attributed to excess deposition of ECM components, including collagen (Wynn 2007; Melo et al. 2008). In addition to the liver, we have described and quantified collagen deposition in other organs, such as spleens, cervical lymph nodes, lungs and kidneys of L. chagasi-infected dogs (symptomatic dogs).
Previous studies on one hundred and five adult mongrel dogs naturally infected with L. chagasi found intralobular fibrosis in all of them, with more collagen deposition being confirmed in the infected animals than in the controls by histomorphometric analysis (Melo et al. 2008, 2009). Moreover, their study of symptomatic dogs (n = 69) showed significantly increased hepatic fibrilopoesis (intralobular fibrosis) compared with asymptomatic dogs (n = 36). They also found a highly significant positive correlation between hepatic collagen deposition and parasite load. Thus, based on previously published results, our group decided to continue these studies on 24 cases of dogs with an intense intralobular fibrosis, to determine whether symptomatic dogs naturally infected with L. chagasi with this intense hepatic fibrilopoesis show collagen deposition (fibrosis) systemically in other organs. We have confirmed using the same methodology described by Melo et al. (2008) with Gomori's ammoniacal silver staining method that all infected dogs have marked fibrilopoesis compared with uninfected dogs (controls) in spleens, cervical lymph nodes, lungs and kidneys.
Hepatic intralobular fibrosis in CVL has been described previously in the literature (Tafuri et al. 1996; Melo et al. 2008, 2009). Reticular collagen fibres in dogs with visceral leishmaniasis were thicker than in uninfected dogs. Their fibrilopoesis was found mostly in sinusoids and in the hepatic lobe walls. Reticular fibres were spread diffusely in several directions forming a compact network; some fibres encircled groups of hepatocytes or even a single cell, producing the aspect of cirrhosis called ‘cirrhosis dites monocelular’ (Nattan-Larrier 1918), and some were diffuse, mostly in areas, such as those previously described in hepatic cirrhosis (Rogers 1908) (Figure 1c,d).
Splenic fibrilopoesis occurred mainly in the capsule also in the parenchyma. Besides their higher intensity, the fibres in all infected dogs were thicker than controls, forming a conspicuous network (Figure 2b,c). As previously described in livers (Melo et al. 2008, 2009), there was a positive correlation between this fibrilopoesis and parasite load in the spleen. Another feature was the splenic congestion found in 71% of the animals. Alexandre-Pires et al. 2006 noted that the spleen reticular meshwork constitutes a fine-meshed filtration bed built up of dendrite rich fibroblastic reticular cells associated with argyrophilic extracellular reticular fibres. Weiss et al. 1986 also described striking activation of reticular cells in relation to the spleen during murine malaria infection (the stromal cells that form the splenic filtration bed) in the form of intense protein secretion, branching and mitosis. They postulated that these reticular cells could be responsible for synthesizing collagen III (reticular fibres), besides controlling the circulation of the spleen. Additionally, Alexandre-Pires et al. 2006 showed that with leishmaniasis infection, a new reticular web condition is most probably involved in impeding and slowing the passage of blood and blood elements through the filtration beds and, consequently, the transit of parasites through the spleen. The collapse of the venous sinus architecture might be a response to the initial congested and massively enlarged spleen typical of initial visceral leishmanioses.
In CVL, lymphadenopathy is also a common clinical sign of the disease. Lymphadenopathy is usually defined as an increase in lymph node size, and it could appear as a regional or generalized alteration (Rogers et al. 1993). In agreement with others (Ciaramella et al. 1997; Lima et al. 2004; Costa et al. 2008), a generalized enlargement of lymph nodes, especially the cervical nodes, has been found in higher indices in dogs with visceral leishmaniasis. These papers indicated that cervical nodes are more reactive because of their anatomical position. Thus, we studied cervical nodes of dogs with fibrosis and found in every case an intense fibrilopoesis associated with chronic lymphadenitis. Moreover, despite the presence of diffuse chronic inflammation involving the capsule throughout the medullary zones, hypertrophy and hyperplasia of medullary cords and sinus cells occurred all 24 cases (100%), In addition, intense fibrilopoesis was greater than in uninfected dogs. Fibrosis was mainly observed in the medullary layer, characterized by thicker collagen argyrophilic fibre deposition, but the essential architecture was preserved. It is possible that the higher fibrilopoesis in this tissue is more evident because of the histological architecture of the lymph node. In contrast in the, cortical tissue formed of dense lymphoid tissue cells (nodules and follicles with germinal centres) there were no signs of collagen deposition as seen in the medullary layer.
Interstitial pneumonitis in visceral leishmaniasis was initially described in humans by Andrade (1959) and later confirmed by Raso & Siqueira (1964) and Duarte et al. (1989). In dogs, pulmonary lesions are very similar to those observed in humans and were described previously by some authors as an interstitial pneumonitis characterized mainly by thickening of alveolar walls associated with the presence of a chronic cellular exudate largely consisting of macrophages (Tryphonas et al. 1977; Anderson et al. 1980; Goncalves et al. 2003). Later, similar lesions were described in hamsters (Duarte & Corbett 1984) and dogs naturally infected with Leishmania chagasi (Duarte et al. 1986; Goncalves et al. 2003). In agreement with Goncalves et al. (2003), we found in all cases a chronic and diffuse interstitial pneumonitis. The thickened interalveolar septa were replaced by the cellular exudate (mostly macrophages, lymphocytes and plasma cells) associated with collagen deposition. Indeed, morphometrical analysis of the silver-stained reticular fibres in the alveolar septa confirmed the early qualitative microscopic evaluation, revealing more numerous reticular fibres in the infected animals than the control group. The parasitological study using immunohistochemistry gave five positive cases (20.8%), which is in accord with other studies that describe low pulmonary parasitism in the lungs of human beings (Duarte et al. 1989) and dogs (Duarte et al. 1986). However, other methodologies used in this study might indicate that this view needs to be modified. In our laboratory (data not shown), we have analysed DNA extracted from paraffin-embedded lung tissue by PCR, which could amplify the expected leishmania products of 120 bp in 50% of the naturally infected dogs examined, despite the absence of evidence of parasites in the same fragments of lung tissue analysed by histopathology and immunocytochemistry techniques. This means that there were amastigote forms or DNA of Leishmania in the lungs that were not visualized by optical microscopy.
Membranoproliferative glomerulonephritis is manifested histologically by alterations in the glomerular basement membrane and mesangium and by proliferation of glomerular cells. In agreement with Tafuri et al. (1989, 2001) and Costa et al. (2003), we found a MPGN mesangial cell proliferation, basement membrane thickening, leucocyte infiltration and accentuation of lobular architecture in all animals. As CVL is a chronic disease that is not rare, some glomeruli showed advanced scarring, even to the point of complete sclerosis. We also observed foci of marked interstitial fibrosis associated with atrophy and dropout of many of the tubules in the cortex, and diminution and loss of portions of the peritubular capillary network. Thus, fibrilopoesis was found in the glomeruli (cortex area) and tubules of cortex and medullar layers. The small- and medium-sized arteries are frequently thick walled, with narrowed lumina, probably secondary to glomerular hypertension. Both glomerular and interstitial fibrilopoesis were positively correlated with parasite load.
Fibrosis is defined as overgrowth, hardening and/or scarring of various tissues, which is attributed to excess deposition of ECM components, including collagen (Wynn 2008). We have described a significant systematical collagen deposition in organs of dogs naturally infected with L. chagasi. Resident fibroblasts or ‘activated fibroblasts’, well known as myofibroblasts (α-smooth muscle actin and collagen αI positive, secrete collagen type I and III), are the primary source of collagen-producing cells in kidneys, lungs and livers. In fibrotic kidneys, activation of cortical fibroblasts (the main area affected in our cases) into myofibroblasts correlates with development of tubulointerstitial fibrosis. Normally, cortical fibroblasts are a population of quiescent cells with a low turnover rate, but they proliferate, produce excess matrix proteins and become principal mediators of the fibrotic response to injury. In fibrotic lungs, the majority of myofibroblasts arise from pre-existing fibroblasts, such as peribronchiolar and perivascular adventitial fibroblasts. In fibrotic livers, different from other organs, the majority of fibroblasts arise not from fibroblasts but from hepatic stellate cells. Thus, besides a variable source of fibrogenic cells in different organs, excess of type I and III collagen deposition by fibroblasts/myofibroblasts could be considered the end result of chronic inflammatory reactions resulting in fibrosis, as observed in CVL.
The recruitment of inflammatory cells to the site of the injury is one of the mechanisms of the fibrogenesis, where fibroblasts are activated. Moreover, this has been strictly related to CD4+ Th2 cytokines such as IL-4, IL-5, IL-13 and IL-21 and TGFβ1 (Song et al. 2000; Wynn 2004; Wick et al. 2010). Another source of fibrogenic cells are activated macrophages, which regulate fibrogenesis by providing cytokines/chemokines and growth factors that modulate proliferation and collagen synthesis. However, activated macrophages display distinct biological features. They can be activated in a classical pathway induced by LPS or IFN-γ, being effector cells against intracellular parasites (increasing NO levels), or they can be activated in an alternative pathway induced by IL-4 or glucocorticoid. Being fibrotic cells, they can enhance fibrogenesis (Song et al. 2000; Strauss-Ayali et al. 2007; Marra et al. 2009). Activated by IL-4, alternative macrophages are also associated with the expression of matrix proteins, such as fibronectin, protein beta IGH3 (βIGH3) and increased expression of arginase 1 (Arg-1; Gratchev et al. 2001; Varin & Gordon 2009). Melo et al. (2009) showed that adhesive fibronectin deposition in CVL was significantly more highly expressed in the livers of naturally infected dogs with L. chagasi. It could indicate that this EMC modification can be incriminated in activating macrophages in the alternative pathway contributing to the fibrilopoesis, in all the organs of infected dogs studied in this work.
In the literature, some of the studies which discuss the link between inflammation and fibrosis are not as straightforward (Iredale 2007; Stramer et al. 2007). However, in both our previous and present work, we found a systematic fibrotic picture in a chronic CVL where inflammatory cells appear to direct fibrosis.
Acknowledgments
This work was financed by the Conselho Nacional de Desenvolvimento da Pesquisa Tecnológica e Científica (CNPq/473601/2009-5), Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG/CBB 00219/09-80 and APQ-01355-09) and Pro-reitoria de Pesquisa (PRPq) da Universidade Federal de Minas Gerais (UFMG), Brazil.
Conflict of Interests
The authors have declared that no competing interests exist.
References
- Adamama-Moraitou KK, Rallis TS, Koytinas AF, Tontis D, Plevraki K, Kritsepi M. Asymptomatic colitis in naturally infected dogs with Leishmania infantum: a prospective study. Am. J. Trop. Med. Hyg. 2007;76:53–57. [PubMed] [Google Scholar]
- Alexandre-Pires G, Pais D, Correia M, Pina JA. Leishmaniosis–a report about the microvascular and cellular architecture of the infected spleen in Canis familiaris. Microsc. Res. Tech. 2006;69:227–235. doi: 10.1002/jemt.20267. [DOI] [PubMed] [Google Scholar]
- Alvar J, Canavate C, Molina R, Moreno J, Nieto J. Canine leishmaniasis. Adv. Parasitol. 2004;57:1–88. doi: 10.1016/S0065-308X(04)57001-X. [DOI] [PubMed] [Google Scholar]
- Anderson DC, Buckner RG, Glenn BL, MacVean DW. Endemic canine leishmaniasis. Vet. Pathol. 1980;17:94–96. doi: 10.1177/030098588001700110. [DOI] [PubMed] [Google Scholar]
- Andrade ZA. [Interstitial pneumonitis in kala-azar] Hospital. 1959;55:371–381. [PubMed] [Google Scholar]
- Anosa VO, Idowu AL. The clinico-haematological features and pathology of leishmaniasis in a dog in Nigeria. Zbl. Vet. Med. B. 1983;30:600–608. doi: 10.1111/j.1439-0450.1983.tb01886.x. [DOI] [PubMed] [Google Scholar]
- Burns JM, Jr, Shreffler WG, Benson DR, Ghalib HW, Badaro R, Reed SG. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc. Natl Acad. Sci. USA. 1993;90:775–779. doi: 10.1073/pnas.90.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliari MV. Princípios de Morfometria Digital: KS300 Para Iniciantes, Vol. 1. Belo Horizonte: UFMG; 1997. [Google Scholar]
- Ciaramella P, Oliva G, Luna RD, et al. A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum. Vet. Rec. 1997;141:539–543. doi: 10.1136/vr.141.21.539. [DOI] [PubMed] [Google Scholar]
- Costa FA, Goto H, Saldanha LC, et al. Histopathologic patterns of nephropathy in naturally acquired canine visceral leishmaniasis. Vet. Pathol. 2003;40:677–684. doi: 10.1354/vp.40-6-677. [DOI] [PubMed] [Google Scholar]
- Costa MM, Lima WG, Figueiredo MM, Michalick MS, Tafuri WL. Cervical, mandibular, and parotid lymph nodes of dogs naturally infected with Leishmania infantum: a histopathologic and immunohistochemistry study and its correlation with facial skin lesions. Vet. Pathol. 2008;45:613–616. doi: 10.1354/vp.45-5-613. [DOI] [PubMed] [Google Scholar]
- De Paola DA. 1956. A Punção-Biópsia do Fígado. Valor da Pesquisa Histoquímica; pp. 8–13. HSE. [Google Scholar]
- Deane LM, Deane MP. Leishmaniose visceral urbana (no cão e no homem) em Sobral, Ceará. O Hosp. 1955;47:75–87. [Google Scholar]
- Deane LM, Deane MP. [Visceral leishmaniasis in Central and South America] Arq. Hig. Saude Publica. 1964;29:89–94. [PubMed] [Google Scholar]
- Duarte MI, Corbett CE. Histopathological and ultrastructural aspects of interstitial pneumonitis of experimental visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 1984;78:683–688. doi: 10.1016/0035-9203(84)90242-6. [DOI] [PubMed] [Google Scholar]
- Duarte MI, Laurenti MD, Brandao Nunes VL, Rego Junior AF, Oshiro ET, Corbett CE. Interstitial pneumonitis in canine visceral leishmaniasis. Rev. Inst. Med. Trop. Sao Paulo. 1986;28:431–436. doi: 10.1590/s0036-46651986000600009. [DOI] [PubMed] [Google Scholar]
- Duarte MI, da Matta VL, Corbett CE, Laurenti MD, Chebabo R, Goto H. Interstitial pneumonitis in human visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 1989;83:73–76. doi: 10.1016/0035-9203(89)90712-8. [DOI] [PubMed] [Google Scholar]
- Ferrer L, Juanola B, Ramos JA, Ramis A. Chronic colitis due to Leishmania infection in two dogs. Vet. Pathol. 1991;28:342–343. doi: 10.1177/030098589102800414. [DOI] [PubMed] [Google Scholar]
- Figueiredo MM, Moura EP, Costa MM, Ribeiro VM, Michalick MS, Tafuri WL. Histopathological and parasitological investigations of ear healthy skin of dogs naturally and experimentally infected with LeishmaniaLeishmaniachagasi. Histol. Histopathol. 2010;25:877–887. doi: 10.14670/HH-25.877. [DOI] [PubMed] [Google Scholar]
- Goncalves R, Tafuri WL, Melo MN, Raso P. Chronic interstitial pneumonitis in dogs naturally infected with LeishmaniaLeishmaniachagasi: a histopathological and morphometric study. Rev. Inst. Med. Trop. Sao Paulo. 2003;45:153–158. doi: 10.1590/s0036-46652003000300007. [DOI] [PubMed] [Google Scholar]
- Gonzalez JL, Rollan E, Novoa C, Castano M. Structural and ultrastructural hepatic changes in experimental canine leishmaniasis. Histol. Histopathol. 1988;3:323–329. [PubMed] [Google Scholar]
- Gratchev A, Guillot P, Hakiy N, et al. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein betaIG-H3. Scand. J. Immunol. 2001;53:386–392. doi: 10.1046/j.1365-3083.2001.00885.x. [DOI] [PubMed] [Google Scholar]
- Houghton RL, Petrescu M, Benson DR, et al. A cloned antigen (recombinant K39) of Leishmania chagasi diagnostic for visceral leishmaniasis in human immunodeficiency virus type 1 patients and a prognostic indicator for monitoring patients undergoing drug therapy. J. Infect. Dis. 1998;177:1339–1344. doi: 10.1086/515289. [DOI] [PubMed] [Google Scholar]
- Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J. Clin. Invest. 2007;117:539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histoch. J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Keenan CM, Hendricks LD, Lightner L, Webster HK, Johnson AJ. Visceral leishmaniasis in the German shepherd dog. I. Infection, clinical disease, and clinical pathology. Vet. Pathol. 1984;21:74–79. doi: 10.1177/030098588402100113. [DOI] [PubMed] [Google Scholar]
- Kumar P, Pai K, Tripathi K, Pandey HP, Sundar S. Immunoblot analysis of the humoral immune response to Leishmania donovani polypeptides in cases of human visceral leishmaniasis: its usefulness in prognosis. Clin. Diagn. Lab. Immunol. 2002;9:1119–1123. doi: 10.1128/CDLI.9.5.1119-1123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox WJ, Smart ME, Little PB. Canine leishmaniasis in Canada. Can. Vet. J. 1972;13:188–190. [PMC free article] [PubMed] [Google Scholar]
- Lima WG, Michalick MS, de Melo MN, Luiz Tafuri W. Canine visceral leishmaniasis: a histopathological study of lymph nodes. Acta Trop. 2004;92:43–53. doi: 10.1016/j.actatropica.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Lima WG, Oliveira PS, Caliari MV, et al. Histopathological and immunohistochemical study of type 3 complement receptors (CD11b/CD18) in livers and spleens of asymptomatic and symptomatic dogs naturally infected with LeishmaniaLeishmaniachagasi. Vet. Immunol. Immunopathol. 2007;117:129–136. doi: 10.1016/j.vetimm.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Marra F, Aleffi S, Galastri S, Provenzano A. Mononuclear cells in liver fibrosis. Semin. Immunopathol. 2009;31:345–358. doi: 10.1007/s00281-009-0169-0. [DOI] [PubMed] [Google Scholar]
- Martinez-Moreno A, Martinez-Cruz MS, Blanco A, Hernandez-Rodriguez S. Immunological and histological study of T- and B-lymphocyte activity in canine visceral leishmaniosis. Vet. Parasitol. 1993;51:49–59. doi: 10.1016/0304-4017(93)90195-s. [DOI] [PubMed] [Google Scholar]
- Mauricio IL, Stothard JR, Miles MA. The strange case of Leishmania chagasi. Parasitol. Today. 2000;16:188–189. doi: 10.1016/s0169-4758(00)01637-9. [DOI] [PubMed] [Google Scholar]
- Melo F, Amaral M, Oliveira P, et al. Diffuse intralobular liver fibrosis in dogs naturally infected with LeishmaniaLeishmaniachagasi. Am. J. Trop. Med. Hyg. 2008;79:198–204. [PubMed] [Google Scholar]
- Melo FA, Moura EP, Ribeiro RR, et al. Hepatic extracellular matrix alterations in dogs naturally infected with LeishmaniaLeishmaniachagasi. Int. J. Exp. Pathol. 2009;90:538–548. doi: 10.1111/j.1365-2613.2009.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattan-Larrier L. Les cirrhoses hepatiques due au Kalazar. Bull. l'Acad Med. 1918;89:402–408. [Google Scholar]
- Nieto CG, Navarrete I, Habela MA, Serrano F, Redondo E. Pathological changes in kidneys of dogs with natural Leishmania infection. Vet. Parasitol. 1992;45:33–47. doi: 10.1016/0304-4017(92)90025-5. [DOI] [PubMed] [Google Scholar]
- Oliveira GG, Santoro F, Sadigursky M. The subclinical form of experimental visceral leishmaniasis in dogs. Mem. Inst. Oswaldo Cruz. 1993;88:243–248. doi: 10.1590/s0074-02761993000200011. [DOI] [PubMed] [Google Scholar]
- Pinto AJ, Figueiredo MM, Silva FL, Martins T, Michalick MS, Tafuri WL. Histopathological and parasitological study of the gastrointestinal tract of dogs naturally infected with Leishmania infantum. Acta Vet. Scand. 2011;53:67. doi: 10.1186/1751-0147-53-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raso P, Siqueira JTDe. [Contribution to the Knowledge of the Pathological Anatomy of Visceral Leishmaniasis, with Special Reference to the Pulmonary and Cardiac Lesions] O Hosp. 1964;65:1291–1309. [PubMed] [Google Scholar]
- Rogers L. A peculiar intralobular cirrhosisof the liver pro- duced by the protozoal parasite of Kala-Azar. Ann. Trop. Med. Parasitol. 1908;2:147–152. [Google Scholar]
- Rogers KS, Barton CL, Landis M. Canine and feline lymph nodes. Part II. Diagnosis, evaluation and lymphadenopathy. Compendium. 1993;15:1493–1501. [Google Scholar]
- Sanchez MA, Diaz NL, Zerpa O, Negron E, Convit J, Tapia FJ. Organ-specific immunity in canine visceral leishmaniasis: analysis of symptomatic and asymptomatic dogs naturally infected with Leishmania chagasi. Am. J. Trop. Med. Hyg. 2004;70:618–624. [PubMed] [Google Scholar]
- Slappendel RJ. Canine leishmaniasis. A review based on 95 cases in The Netherlands. Vet. Q. 1988;10:1–16. doi: 10.1080/01652176.1988.9694140. [DOI] [PubMed] [Google Scholar]
- Solano-Gallego L, Fernandez-Bellon H, Morell P, et al. Histological and immunohistochemical study of clinically normal skin of Leishmania infantum-infected dogs. J. Comp. Pathol. 2004;130:7–12. doi: 10.1016/s0021-9975(03)00063-x. [DOI] [PubMed] [Google Scholar]
- Song W, Jackson K, McGuire PG. Degradation of type IV collagen by matrix metalloproteinases is an important step in the epithelial-mesenchymal transformation of the endocardial cushions. Dev. Biol. 2000;227:606–617. doi: 10.1006/dbio.2000.9919. [DOI] [PubMed] [Google Scholar]
- Stramer BM, Mori R, Martin P. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J. Invest. Dermatol. 2007;127:1009–1017. doi: 10.1038/sj.jid.5700811. [DOI] [PubMed] [Google Scholar]
- Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J. Leukoc. Biol. 2007;82:244–252. doi: 10.1189/jlb.0307191. [DOI] [PubMed] [Google Scholar]
- Tafuri WL, Michalick MS, Dias M, et al. [Optical and electron microscopic study of the kidney of dogs naturally and experimentally infected with LeishmaniaLeishmaniachagasi] Rev. Inst. Med. Trop. Sao Paulo. 1989;31:139–145. doi: 10.1590/s0036-46651989000300002. [DOI] [PubMed] [Google Scholar]
- Tafuri WL, Barbosa AJ, Michalick MS, et al. Histopathology and immunocytochemical study of type 3 and type 4 complement receptors in the liver and spleen of dogs naturally and experimentally infected with LeishmaniaLeishmaniachagasi. Rev. Inst. Med. Trop. Sao Paulo. 1996;38:81–89. doi: 10.1590/s0036-46651996000200001. [DOI] [PubMed] [Google Scholar]
- Tafuri WL, de Oliveira MR, Melo MN. Canine visceral leishmaniosis: a remarkable histopathological picture of one case reported from Brazil. Vet. Parasitol. 2001;96:203–212. doi: 10.1016/s0304-4017(00)00436-2. [DOI] [PubMed] [Google Scholar]
- Tafuri WL, Santos RL, Arantes RM, Goncalves R, de Melo MN, Michalick MS. An alternative immunohistochemical method for detecting Leishmania amastigotes in paraffin-embedded canine tissues. J. Immunol. Methods. 2004;292:17–23. doi: 10.1016/j.jim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Tryphonas L, Zawidzka Z, Bernard MA, Janzen EA. Visceral leishmaniasis in a dog: clinical, hematological and pathological observations. Can. J. Comp. Med. 1977;41:1–12. [PMC free article] [PubMed] [Google Scholar]
- Varin A, Gordon S. Alternative activation of macrophages: immune function and cellular biology. Immunobiology. 2009;214:630–641. doi: 10.1016/j.imbio.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Weiss L, Geduldig U, Weidanz W. Mechanisms of splenic control of murine malaria: reticular cell activation and the development of a blood–spleen barrier. Am. J. Anat. 1986;176:251–285. doi: 10.1002/aja.1001760303. [DOI] [PubMed] [Google Scholar]
- Wick G, Backovic A, Rabensteiner E, Plank N, Schwentner C, Sgonc R. The immunology of fibrosis: innate and adaptive responses. Trends Immunol. 2010;31:110–119. doi: 10.1016/j.it.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]


