Abstract
Red blood cells (RBCs) from patients with sickle cell disease (SCD) lyse in deoxygenated isosmotic non-electrolyte solutions. Haemolysis has features which suggest that it is linked to activation of the pathway termed Psickle. This pathway is usually described as a non-specific cationic conductance activated by deoxygenation, HbS polymerisation and RBC sickling. The current work addresses the hypothesis that this haemolysis will provide a novel diagnostic and prognostic test for SCD, dependent on the altered properties of the RBC membrane resulting from HbS polymerisation. A simple test represented by this haemolysis assay would be useful especially in less affluent deprived areas of the world where SCD is most prevalent. RBCs from HbSS and most HbSC individuals showed progressive lysis in deoxygenated isosmotic sucrose solution at pH 7.4 to a level greater than that observed with RBCs from HbAS or HbAA individuals. Cytochalasin B prevented haemolysis. Haemolysis was temperature- and pH-dependent. It required near physiological temperatures to occur in deoxygenated sucrose solutions at pH 7.4. At pH 6, haemolysis occurred even in oxygenated samples. Haemolysis was reduced in patients on long-term (>5 months) hydroxyurea treatment. Several manoeuvres which stabilise soluble HbS (aromatic aldehydes o-vanillin or 5-hydroxymethyl, and urea) reduced haemolysis, an effect not due to increased oxygen affinity. Conditions designed to elicit HbS polymerisation in cells from sickle trait patients (deoxygenated hyperosmotic sucrose solutions at pH 6) supported their haemolysis. These findings are consistent with haemolysis requiring HbS polymerisation and support the hypothesis that this may be used as a test for SCD.
Key points
Red blood cells (RBCs) from the two main genotypes of sickle cell disease (SCD) patients showed haemolysis in deoxygenated isosmotic sucrose solution, to a greater extent than those from sickle trait individuals (HbAS). RBCs from normal individuals (HbAA) did not lyse.
Several treatments reduced haemolysis in RBCs from HbSS patients – cytochalasin B, aromatic aldehydes (o-vanillin and 5-hydroxymethylfurfural), urea, low temperature and high pH. These effects were not due to increased oxygen affinity of haemoglobin. Long-term treatment of patients with hydroxyurea was also associated with reduced levels of haemolysis.
Treatment with aromatic aldehydes and urea reduced sickling of HbSS RBCs in deoxygenated saline solutions. Further, RBCs from sickle trait individuals (HbAS) could be induced to sickle under acidic (pH 6), hypertonic (400 mosmol kg−1) conditions. When these conditions were reproduced in sucrose solutions, HbAS RBCs also showed considerable haemolysis.
Results suggest that haemolysis of HbS-containing RBCs is associated with HbS polymerisation, perhaps damaging the RBC membrane. These findings suggest that haemolysis may be used as a simple, novel technique to diagnose SCD, using the altered membrane permeability of RBCs rather than the presence of HbS per se. As extent of haemolysis varies between individuals and is reduced following hydroxyurea treatment, the method may also be useful prognostically.
Introduction
Sickle cell disease (SCD) is one of the commonest severe inherited disorders (Steinberg et al. 2001; Rees et al. 2010). It results from the presence in patients’ red blood cells (RBCs) of the mutated haemoglobin (Hb) HbS, rather than the normal adult HbA. HbS results from a point mutation in codon 6 of the β chain of Hb, such that the glutamic acid residue at this position is replaced by valine. The majority (about two-thirds) of SCD patients are homozygous for HbS (HbSS) whilst the second main group (about one-third) are represented by individuals heterozygous for HbS and another mutated Hb HbC, in which the same glutamic acid residue is replaced instead by lysine. There are, in addition, a number of less common SCD genotypes such as HbS-β thalassaemia.
Unlike HbA, HbS may polymerise on deoxygenation, forming long rods which affect rheology and distort the RBC shape into sickles and a variety of other peculiar shapes (Eaton & Hofrichter 1987). The polymerisation event leads to multiple clinical complications which are features of the disease. These complications fall into two main types: a chronic anaemia and acute ischaemic disorders (such as stroke, osteonecrosis and acute chest syndrome) (Steinberg et al. 2001). The clinical condition is noticeably heterogeneous, however, so that some patients have few complications whilst others are severely affected (Platt et al. 1991, 1994; Ohene-Frempong et al. 1998; Vichinsky et al. 2000). The ability to recognise which individuals are more at risk of extensive pathology would enable resources to be targeted at these more needy individuals. This strategy would be particularly valuable in more economically deprived areas of the world (such as Western Africa) where the disease is most prevalent.
HbS-containing RBCs are also characterised by abnormal membrane permeability, and, in particular, increased cation ‘leaks’ (Joiner 1993; Gibson & Ellory 2002; Lew & Bookchin 2005). This feature is important because it contributes to increased solute loss (mainly K+ and Cl−) and RBC shrinkage (as water follows osmotically). Consequently, HbS concentration ([HbS]) becomes elevated. As the lag time to polymerisation following deoxygenation is inversely proportional to a high power of [HbS] (Eaton & Hofrichter 1987), HbS polymerisation is markedly encouraged even in modestly shrunken RBCs. Shrunken RBCs are therefore much more likely to undergo polymerisation in the hypoxic microcirculation with the increased potential that they become lodged, leading to microvascular occlusions. As a consequence, the high cation permeability has been much studied, with considerable efforts targeted at reducing solute loss and maintaining RBC hydration, thereby protecting cells from HbS polymerisation and ameliorating clinical signs (Rosa et al. 1980; Clark et al. 1982; Brugnara et al. 1995; Stocker et al. 2003; Stuart & Nagel 2004). To date, however, these attempts have proved unsuccessful. For example, the clotrimazole analogue senicapoc (ICA-17043) was found to improve RBC hydration in SCD patients but did not reduce pain crisis frequency (Ataga et al. 2011). One of the main pathways involved in RBC dehydration has been characterised as a deoxygenation-induced cation conductance (Joiner et al. 1988; Joiner 1993), activated by HbS polymerisation and RBC shape change (Mohandas et al. 1986), but the molecular identity of which remains unknown. This pathway, sometimes called Psickle (Lew & Bookchin 2005), is especially significant because it mediates Ca2+ entry with subsequent activation of Ca2+-activated K+ channels (or Gardos channels) (Gardos 1958), which bring about rapid solute loss.
Based on the novel observation that an unusual permeability is induced in normal RBCs from HbAA individuals when suspended at low ionic strength (LIS) (Bernhardt et al. 1991, 2001), we hypothesised that a similar effect may also occur in HbS-containing RBCs but that its permeability may be exacerbated upon HbS polymerisation. In fact we found that HbS-containing RBCs undergo haemolysis in deoxygenated isosmotic solutions of certain non-electrolytes (Browning et al. 2007). Although the pathway is reminiscent of the LIS-induced permeability, isosmotic non-electrolyte solutions do not induce haemolysis in normal RBCs (Browning et al. 2007). Haemolysis is not observed in HbS-containing RBCs when they are oxygenated, under conditions in which HbS is soluble. It is accompanied by entry of non-electrolytes, and probably results from swelling and osmotic lysis. Haemolysis also shares many of the properties with the cation-permeable Psickle, such as partial inhibition by 4,4′-diisothiocyano-2,2′-stilbenedisulphonate (DIDS), similar oxygen dependence and ‘stochastic’ activation (Browning et al. 2007; Ellory et al. 2008; Dalibalta et al. 2010). As haemolysis appears to be dependent on the presence and polymerisation of HbS, we further speculated that it may be possible to use this property for diagnosis of SCD. As the extent of the lytic phenomenon appears to be linked to Psickle, one of the main abnormal cation permeabilities implicated in SCD pathogenesis, in addition it may also be valuable prognostically to identify patients most likely to suffer severe clinical complications.
In this paper, we further characterise haemolysis in non-electrolyte solutions, with the objective of developing a robust and simple assay for SCD diagnosis and prognosis, amenable to use in under-developed areas of the world.
Methods
Solutions and chemicals
All solutions had an osmolality of 290 ± 5 mosmol kg−1 and pH 7.4 at 37 °C unless otherwise stated. The basic saline (MBS) used to wash blood samples comprised (in mm): NaCl 145, Mops 10, glucose 5, with pH adjusted with Tris base. Mops-buffered nitrate saline (NMBS), used to deplete red cells of intracellular chloride, contained NaNO3 instead of NaCl. Haemolysis solutions were also Mops-buffered and usually isosmotic but with salts replaced with non-electrolyte, usually sucrose. Sucrose was chosen over other non-electrolytes as preliminary experiments indicated that it produced more reproducible results than other sugars such as lactose. In some experiments, pH was altered between 6 and 8, using Tris base or Tris-HCl. Experiments with RBCs from sickle trait individuals (HbAS) were also performed in solutions made hyperosmotic (400 mosmol kg−1) through addition of extra NaCl or sucrose and at lower pH (pH 6). Haemolysis solutions were usually supplemented with ouabain (0.1 mm), bumetanide (0.01 mm) and clotrimazole (CLT, 0.01 mm), or EGTA (2 mm to chelate contaminant Ca2+) in place of CLT. An ice-cold stop solution containing the same non-electrolyte used during lysis was used to collect aliquots to determine the extent of haemolysis. Where indicated, haemolysis solutions were supplemented with up to 5 mm o-vanillin or 5-hydroxymethylfurfural (5HMF), urea (at 200 mm) or cytochalasin B (10 μm). For experiments involving the aromatic aldehydes and cytochalasin B, RBCs were usually pretreated with the reagents for 20 min prior to deoxygenation. All chemicals used were obtained from Sigma-Aldrich (Poole, UK).
Blood
Discarded blood samples were provided from King's College Hospital with ethical permission (REC reference number: 11/LO/0065). Blood was collected in EDTA-containing syringes and stored at 4°C until required (normally within 72 h). Prior to use, blood samples were washed three times by centrifugation (600 g, 3 min) in MBS to remove platelets and plasma. RBCs were then washed an additional four times in NMBS to achieve Cl− substitution. A final wash was made to transfer RBCs to the requisite non-electrolyte haemolysis solution. After the final wash RBC samples were left on ice as a packed preparation (approximately 90% haematocrit, Hct). Unless stated otherwise, all experiments were performed on RBCs from homozygous HbSS patients. Potassium loss from RBCs during washing was measured by lysing packed cells in 0.005% Triton X-100 in water, following which [K+] was measured using a K+ electrode (VetLyte Na+K+Cl− Analyzer; IDCXX Laboratories, Westbrook, ME, USA) and [Hb] measured by optical density (540 nm in Drabkins solution). Comparing K+ content of RBCs per g Hb showed that K+ loss on washing was 4.2 ± 2.5% (n= 3) of the total initial value.
Measurements of RBC haemolysis
Generally 2 ml of isosmotic haemolysis solution was transferred to an Eschweiler tonometer suspended in a thermally regulated water bath (37°C, unless otherwise stated) and supplemented with ouabain, bumetanide and clotrimazole (as above). The tonometer was flushed with humidified gas, N2 or air, and allowed to equilibrate for 10 min. To start the haemolysis assay, 60 μl of the washed packed cells (final Hct about 2%) was transferred to the tonometer. The contents of the tonometer were gently mixed (speed of rotation usually 60 min−1) and continuously flushed with the appropriate gas (unless stated otherwise). At pre-determined intervals, serial aliquots (0.2 ml) of the cell suspension were removed and added to 0.8 ml of ice-cold stop solution in an Eppendorf tube and immediately centrifuged (12,000 g, 10 s). The supernatant was transferred to a cuvette and the extent of haemolysis was determined by measuring the optical density at 540 nm. Maximal (100%) lysis was measured by transferring 0.2 ml of RBC suspension to a 0.8 ml solution containing 0.1% Triton X-100, followed by centrifugation and determination of the optical density (540 nm) of the supernatant.
Measurements of K+ permeability
Potassium permeability was measured using 86Rb+ as a tracer for K+ (Dunham & Ellory 1981; Speake et al. 1997). In these experiments, RBCs were incubated in deoxygenated isosmotic sucrose solution, pH 7.4, in the presence of bumetanide, ouabain and clotrimazole (as above) at an Hct of about 1%. Rubidium (86Rb+) was added in 15 mm KCl solution to give a final [K+] of 0.75 mm. Influx was performed over 10 min after which RBCs were washed four times in ice-cold isotonic MgCl2 stop solution.
Measurements of oxygen saturation
Aliquots of RBC suspensions were removed from tonometers and O2 saturation was measured using a Clark-type electrode coupled to an oxygen meter (CB1-D3; Hansatech, King's Lynn, UK). The measuring chamber contained saponin (3%, w/v) to lyse RBC samples and potassium ferricyanide (6%, w/v) to oxidise haem Fe2+ to Fe3+ and so displace Hb-bound O2. O2 content of fully oxygenated blood was measured in cell suspensions equilibrated with air.
Measurements of RBC sickling
These were performed in MBS because non-electrolyte solutions cause RBC crenation so that shape change due to HbS polymerisation is impossible to ascertain. RBCs were incubated in tonometers at 2% Hct for up to 60 min after which samples were fixed in the same solution as that used during incubation but with the addition of 0.3% glutaraldehyde. Sickling was assessed by light microscopy within 10 min of fixing. Several hundred RBCs (typically 300–400) were counted using an Improved Neubauer haemocytometer (in five 1 mm × 1 mm squares, the central one and the four corners). Control experiments showed that this protocol was sufficient to maintain RBC shape for several days. Although counting was not blinded, three different individuals found similar percentages of sickling.
Statistics
Unless otherwise stated data are presented as mean ± SEM from n separate experiments. Statistical differences were determined using Student's unpaired t test with P < 0.05 considered significant.
Results
Haemolysis in isosmotic sucrose solutions
In the first series of experiments, the influence of genotype on haemolysis in deoxygenated isosmotic sucrose solutions (pH 7.4) was investigated (Fig. 1A). To date, RBC samples from >100 SCD patients have been tested and all showed significant haemolysis. RBCs in samples from homozygous HbSS patients lysed to the greatest extent. Haemolysis was progressive over about 40 min and then usually slowed to a plateau. This increase in haemolysis was absent when HbSS samples were incubated at pH 7.4 in oxygenated isosmotic sucrose solutions (Fig. 1B). RBCs from the second most common SCD genotype, heterozygotes for HbS and HbC (HbSC), also showed progressive haemolysis but to a lesser extent (Fig. 1A). For HbSS patients, mean haemolysis over 60 min was 17.2 ± 0.9% (n= 34) with a range of 8.8–30.4%, and about half this in samples from HbSC individuals with a mean 9.0 ± 0.5% (n= 16) and a range of 6.2–14.1% (Fig. 1C). The increase in haemolysis became significant by 30 min for both genotypes (P < 0.01), perhaps reflecting the time taken to deoxygenate RBCs fully (cf. Fig. 7) using normal nitrogen flows and tonometer speeds.
Figure 1. Haemolysis of red blood cells from individuals of different genotypes.
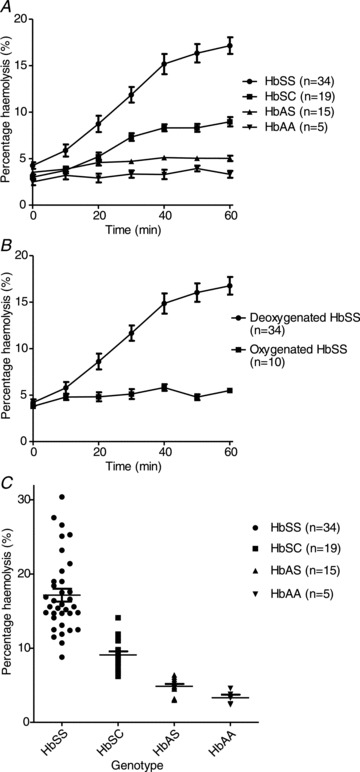
RBCs were incubated in isosmotic sucrose solution (pH 7.4, 37°C). Serial aliquots were removed as indicated and percentage haemolysis was determined by optical density. A, effect of genotype, all under deoxygenated conditions. B, effect of oxygen tension on haemolysis of RBCs from homozygous HbSS patients. C, scatter diagram showing haemolysis after 60 min for the four main genotypes (HbSS, HbSC, HbAS and HbAA). Data represent means ± SEM for 5–34 individuals, as indicated on the panels.
Figure 7. Effect of aromatic aldehydes, urea and temperature on oxygen saturation of red blood cells from homozygous HbSS patients.
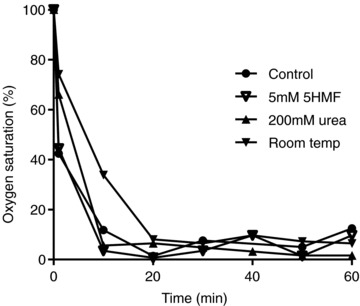
RBCs, initially oxygenated, were placed in isosmotic sucrose solutions (all pH 7.4) in gently rotating tonometers and flushed with humidified nitrogen. Aliquots of RBC suspensions were removed serially and for determination of oxygen saturation. Four conditions were tested. Three were measured at 37°C: control samples, and with the addition of 5HMF (5 mm) or urea (200 mm). In the fourth, the effect of incubation at 25°C (room temperature) was determined. The data are from a single experiment representative of three others.
It was noticeable, however, that there was variation in both the rate and the extent between samples. For HbSS samples the level of haemolysis varied about 3-fold. In these, rates of haemolysis were usually highest over 10–40 min, and over this time period they varied from about 7% per hour to 50% per hour with a mean of 19 ± 2% per hour (n= 34). A lesser number of the rarer SCD genotypes were also studied. Haemolysis of RBCs from HbS-β thalassaemia and HbS-α thalassaemia patients lay within the range observed for HbSS homozygotes (n= 3).
Haemolysis was much reduced in RBCs from normal individuals (HbAAs, n= 5) and those with sickle trait (HbAS, n= 15). In these cases, the rise in haemolysis over 60 min was 1.1 ± 0.3% (n= 17) and this increase was not significant (Fig. 1A). Although RBCs from different individuals showed variation in haemolysis, the increase in haemolysis over 60 min was never less than 7% in RBCs from HbSS individuals whilst the change in haemolysis of RBCs from HbAA and HbAS individuals was always <2.5% (Fig. 1C) – differences which were distinguishable by eye. In the case of RBCs from HbSC individuals, 12 samples showed an increase in haemolysis of >5%. The haemolysis test was therefore able to distinguish readily between homozygous (HbSS) SCD patients and non-SCD individuals (normal HbAA and sickle trait HbAS). There was some overlap in haemolysis, however, between heterozygous HbSC patients and sickle trait (Fig. 1C).
Effect of transport inhibitors and osmolality on haemolysis
As haemolysis is accompanied by entry of non-electrolyte into the RBCs, it is likely that it is precipitated by osmotic swelling. The Na+/K+ pump, Na+–K+–2Cl− cotransporter, K+–Cl− cotransporter and the Gardos channel represent the four main cation transport systems in the RBC membrane. In RBCs suspended in non-electrolyte media, all four would mediate solute loss and would potentially reduce haemolysis. In most experiments, therefore, ouabain (0.1 mm), bumetanide (0.01 mm) and clotrimazole (0.01 mm, or 2 mm EGTA) were routinely included, and Cl− was substituted with NO3−, to inhibit efflux of intracellular ions via these pathways. To determine whether these inhibitors were a prerequisite for haemolysis, experiments were carried out in their absence. In haemolysis assays including all three inhibitors, lysis of RBCs from HbSS patients was 24.2 ± 1.0% (all n= 3), with values of 24.2 ± 2.0, 22.4 ± 4.6 and 21.7 ± 6.8% in the absence of ouabain, bumetanide or clotrimazole, respectively (all non-significant compared with control with inhibitors). Thus, ouabain had no effect on rate of haemolysis whilst inhibition of Na+–K+–2Cl− cotransporter or the Gardos channel resulted in only a marginal, insignificant increase in haemolysis.
It was also possible that swelling the cells through incubation in hypotonic non-electrolyte solution to increase the osmotic driving force for water entry, or conversely using hyperosmotic solutions to increase the driving force for non-electrolyte entry, may increase haemolysis. This was tested by using sucrose solutions diluted with water (up to 20% hypotonic) or with higher sucrose concentrations (up to 20% hypertonic). In these experiments, haemolysis in isosmotic sucrose solution was 22.9 ± 0.9%, increasing to 25.2 ± 3.6% in 20% hypotonic sucrose and to 22.4 ± 1.2% (all n= 3) in 20% hypertonic sucrose. Again these changes were insignificant.
Effect of cytochalasin B on haemolysis
Previously it has been reported that deoxygenated RBCs from SCD patients are impermeable to the non-electrolytes arabinose, erythritol and mannitol (Clark & Rossi 1990). These compounds are all smaller than sucrose, having about half the molecular weight, so their entry through a pathway permeable to sucrose would be anticipated. In this previous report, however, experiments were carried out in cytochalasin B-treated RBCs presumably to prevent entry of these particular non-electrolytes through the RBC glucose transporter. Without pretreatment with cytochalasin B, haemolysis occurred in deoxygenated isosmotic arabinose, erythritol and mannitol solutions and at similar rates to those in deoxygenated sucrose solutions (Fig. 2A). When RBCs were pretreated with cytochalasin B (10 μm) haemolysis was much reduced, shown here for deoxygenated isosmotic sucrose solutions (Fig. 2B). Pretreatment of RBCs with cytochalasin B (10 μm) also greatly reduced haemolysis in deoxygenated arabinose, erythritol and mannitol solutions, by a mean of 86 ± 7% (n= 6).
Figure 2. Effect of arabinose, erythritol, mannitol and cytochalasin B on haemolysis of red blood cells from homozygous HbSS patients.
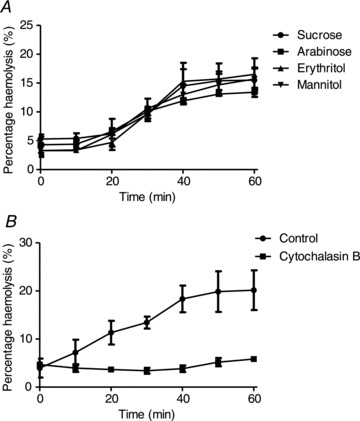
Haemolysis was determined in deoxygenated, isosmotic non-electrolyte solutions (pH 7.4, 37°C), as described in the legend to Fig. 1. A, untreated RBCs incubated either in sucrose or in isosmotic solutions of arabinose, erythritol or mannitol. B, effect of cytochalasin B on haemolysis in deoxygenated isosmotic sucrose solution. RBCs were usually first pretreated with cytochalasin B (10 μm for 20 min) prior to deoxygenation and the reagent was also present throughout. Data represent means ± SEM for three individuals.
Effect of temperature on haemolysis
The preceding experiments were all carried out at 37°C. Temperature is expected to alter the permeability of RBCs from SCD patients through effects either on HbS polymerisation or on permeation through potential transport pathways (such as Psickle) once they have been activated (Galkin et al. 2007). It also affects RBC permeability in LIS media (Blackstock & Stewart 1986; Bernhardt et al. 1991). Change in temperature may therefore affect haemolysis and was investigated further (Fig. 3). Haemolysis was minimal at 25°C and significantly reduced at 32°C compared to 37°C, whilst at higher temperature (45°C), haemolysis was increased. By contrast to effects in RBCs from SCD patients, those from normal individuals (HbAA) showed almost no haemolysis at 45°C, with lysis in this case increasing from 1 to 1.6% over 60 min.
Figure 3. Effect of temperature on haemolysis of red blood cells from homozygous HbSS patients.
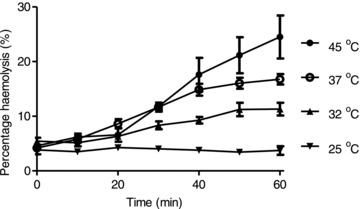
Haemolysis was determined in deoxygenated, isosmotic sucrose solutions, pH 7.4, as described in the legend to Fig. 1, at 45, 37, 32 and 25°C. Data represent means ± SEM for three individuals.
In normal RBCs incubated in LIS solutions, K+ permeability paradoxically rises at lower temperatures, although this effect is reported to require rather lower temperatures (<10°C; Blackstock & Stewart 1986; Bernhardt et al. 1991) than used here. Nevertheless, it was possible that inhibition of lysis of RBCs from SCD patients in sucrose solutions at lower temperatures may have been caused by increased K+ efflux preventing osmotic lysis. In deoxygenated sucrose solution at pH 7.4, however, unidirectional K+ influx fell from 6.63 ± 0.65 mmol (l cells h)−1 at 37°C to 3.91 ± 0.66 mmol (l cells h)−1 (means ± SEM, n= 4) at room temperature, an inhibition of 41 ± 7%, indicating that inhibition of haemolysis was not due to increased K+ permeability at this lower temperature.
Effect of HbS stabilisation on haemolysis
Haemolysis was tested on RBC samples from HbSS patients receiving hydroxyurea therapy (typically 500 mg daily for >5 months). In this case, haemolysis was reduced by about 50% after 60 min compared to individuals not receiving this drug (Fig. 4), an effect which did not correlate with a rise in HbF levels. A rapid effect of hydroxyurea within 2 weeks prior to induction of HbF has also been described (Bridges et al. 1996). A direct action of hydroxyurea on RBC function was therefore investigated on RBCs from untreated patients to which hydroxyurea was added only during the haemolysis experiments. A concentration of hydroxyurea of 2 mm was chosen as peak plasma levels during therapy do not exceed this value even during intravenous administration (Rodriguez et al. 1998). After 60 min, haemolysis was 15.2 ± 0.4 and 15.2 ± 0.3% (n= 3; n.s.) in the absence and presence of hydroxyurea, respectively, thus discounting a direct action on the RBC function, at least as regards this assay.
Figure 4. Effect of treatment with hydroxyurea on haemolysis of red blood cells from homozygous HbSS patients.
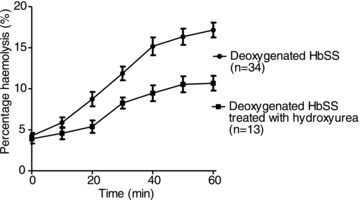
Haemolysis was determined in deoxygenated, isosmotic sucrose solutions, pH 7.4, as described in the legend to Fig. 1, in RBCs from untreated patients and those receiving hydroxyurea therapy (typically 500 mg daily). Data represent means ± SEM for 13 individuals treated with hydroxyurea.
A number of other manoeuvres were tested which reduce the tendency of HbS to polymerise. The aromatic aldehydes o-vanillin and 5HMF have been shown to interact with HbS, reducing polymerisation (Zaugg et al. 1977; Abraham et al. 1991). The effect of these reagents on haemolysis in deoxygenated isosmotic sucrose solutions (pH 7.4) is shown in Fig. 5A and B. At 5 mm, both reagents completely prevented any increase in haemolysis (Fig. 5A). Both also showed dose-dependent effects. For 5HMF, 1 mm reduced haemolysis by about 50% whilst both 3 and 5 mm reduced haemolysis to low levels (Fig. 5B). For both aromatic aldehydes, it was not necessary to pretreat RBCs prior to deoxygenation to observe a reduction in haemolysis (data not shown). As well as aromatic aldehydes, high concentrations of urea (200 mm) were also used to stabilise unpolymerised HbS. Again, haemolysis was largely prevented (Fig. 6).
Figure 5. Effect of aromatic aldehydes on haemolysis and oxygen saturation of red blood cells from homozygous HbSS patients.
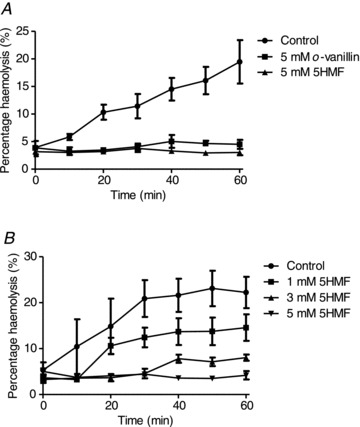
Haemolysis was determined in deoxygenated, isosmotic sucrose solutions (pH 7.4, 37°C), as described in the legend to Fig. 1. A, effect of 5 mm o-vanillin or 5-hydroxymethylfurfural (5HMF). B, effect of different concentrations of 5HMF: 1, 3 and 5 mm. RBCs were usually first pretreated with these aromatic aldehydes for 20 min prior to deoxygenation and the reagents was also present throughout. Plots represent means ± SEM for three individuals.
Figure 6. Effect of urea on haemolysis of red blood cells from homozygous HbSS patients.
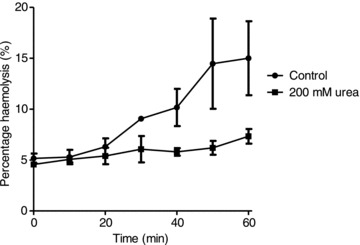
Haemolysis was determined in deoxygenated, isosmotic sucrose solutions (pH 7.4, 37°C), as described in the legend to Fig. 1, in the absence or presence of urea (200 mm). Data represent means ± SEM (n= 4).
Effect of inhibitors of haemolysis on oxygen saturation
Aromatic aldehydes, urea and lower temperatures will all increase the O2 affinity of Hb. It was therefore important to ascertain that inhibition of haemolysis by these procedures did not act through stabilising oxyHbS, thus preventing its polymerisation. This was tested by measuring O2 saturation (Fig. 7). Deoxygenation occurred rapidly and at similar rates in the absence or presence of o-vanillin, 5HMF or urea, or at room temperature, compared to 37°C.
Effect of pH on haemolysis
Psickle activation and permeability is also pH dependent (Joiner et al. 1993). We therefore investigated haemolysis over a range of pH values. Under deoxygenated conditions, reducing pH from 7.4 to 6 had a small effect on the extent and rate of lysis of RBCs from HbSS patients. For example, in a single experiment representative of three others, the rate of haemolysis over 30 min under deoxygenated conditions was 1.1% min−1 at pH 7.4 and 1.4% min−1 at pH 6, with total lysis being 32.3 and 39.9%, respectively. Under oxygenated conditions, however, larger differences were apparent (Fig. 8A). Again, at pH 7.4, oxygenated RBCs showed little haemolysis compared to pH 7.4 deoxygenated RBCs (Fig. 8A). When oxygenated, at pH 6.5, haemolysis was modest, whilst at pH 6 RBCs underwent similar rates of haemolysis to that observed for RBCs when deoxygenated at pH 7.4 (Fig. 8A). Both o-vanillin and 5HMF (5 mm) also prevented haemolysis in oxygenated sucrose solutions at pH 6 (Fig. 8B). Both these aromatic aldehydes therefore inhibited haemolysis in oxygenated sucrose solutions of pH 6 as they did at pH 7.4 in deoxygenated samples (cf. Fig. 5). Haemolysis of RBCs from HbSS patients incubated in oxygenated MBS (i.e. containing predominantly NaCl rather than sucrose) at pH 6 rose by only 2% over 60 min, showing that membrane instability due to the low pH was not responsible for the effect. Conversely, RBCs from normal HbAA individuals were unstable at pH 6 when deoxygenated, with haemolysis rising to 13.0 ± 1.3% (n= 4) after 60 min.
Figure 8. Effect of pH on haemolysis of red blood cells from homozygous HbSS patients.

Haemolysis was determined in isosmotic sucrose solutions, all at 37°C but at various pH and oxygen states, as described in the legend to Fig. 1. A, comparison of haemolysis under deoxygenated conditions pH 7.4 with that in oxygenated conditions at pH 7.4, 6.5 and 6.0. B, effect of 5 mm hydroxymethylfurfural (5HMF) or o-vanillin on haemolysis of oxygenated RBCs at pH 6. Data represent means ± SEM for three individuals.
Effect of HbS stabilisation on HbS polymerisation and sickling
We then investigated the effect of these inhibitors of haemolysis on HbS polymerisation and RBC sickling. This was performed in MBS rather than sucrose solutions because RBC shape perturbation in the latter makes it impossible to assess the extent of sickling. Results are shown in Fig. 9. As expected, in RBCs from HbSS patients, there were few sickling cells under oxygenated conditions at pH 7.4. Deoxygenation elicited marked sickling, which was significantly inhibited by o-vanillin, 5HMF and urea, but not by cytochalasin B.
Figure 9. Sickling shape change in red blood cells from homozygous HbSS SCD patients and individuals with sickle trait (HbAS).
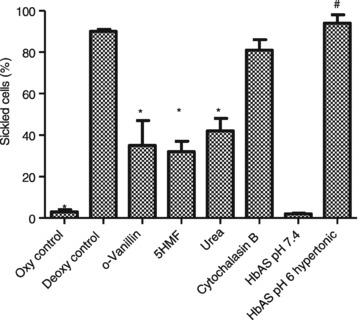
The percentage of RBCs undergoing sickling after 60 min was assessed by light microscopy in RBC aliquots fixed under the same conditions as incubation using 0.3% glutaraldehyde. Aside from one experiment with sickle trait RBCs, salines were isosmotic, pH 7.4. RBCs from HbSS individuals were held under six different conditions: (i) oxygenated controls; (ii) deoxygenated controls; deoxygenated in the presence of (iii) o-vanillin (5 mm), (iv) 5-hydroxymethylfurfural (5HMF, 5 mm), (v) urea (200 mm) and (vi) cytochalasin B (10 μm). RBCs from sickle trait individuals (HbAS) were also deoxygenated (vii) in isosmotic saline, pH 7.4, and (viii) in hypertonic acidic saline (400 mosmol kg−1, pH 6). Bars represent means ± SEM of 3–5 separate experiments on RBCs from different individuals. *Significant difference (P < 0.01) with oxygenated HbSS controls; # with HbAS under isosmotic conditions, pH 7.4.
In addition, RBCs from sickle trait individuals (HbAS) were also tested. When deoxygenated in isosmotic saline, pH 7.4, there was little sickling. RBCs can be induced to sickle by means of a ‘sickling pulse’ (Bookchin & Lew 1981). Using hypertonic saline (400 mosmol kg−1) at low pH (pH 6) it was possible to induce sickling in sickle trait RBCs to a similar extent to that observed in RBCs from HbSS individuals (Fig. 9). Under similar hypertonic acid conditions but in deoxygenated sucrose solutions (hyperosmotic, 400 mosmol kg−1, pH 6), haemolysis was also induced, reaching 19.7 ± 5.6% (mean ± SEM, n= 8) after 60 min.
Discussion
The present findings are consistent with the hypothesis that haemolysis in deoxygenated isosmotic non-electrolyte solutions is a property restricted to RBCs containing sufficient [HbS] to undergo polymerisation and distort the cell membrane. Haemolysis is stimulated by factors which promote HbS polymerisation (deoxygenation, HbSS, HbSC, low pH, high temperature) and reduced by those which stabilise soluble Hb (oxygenation, HbAS, HbAA, low temperature, hydroxyurea, aromatic aldehydes, urea). In parallel experiments performed in saline, RBC sickling, indicative of HbS polymerisation, was observed under conditions which supported haemolysis. Haemolysis thus appears to require the presence of reasonably high concentrations of polymerised HbS. That haemolysis appears to depend on the altered membrane permeability of HbS-containing RBCs following Hb polymerisation, rather than the presence of HbS per se, constitutes a unique feature. As such, this simple assay may have valuable diagnostic applications. As polymerised HbS and altered RBC permeability are also implicated in the pathogenesis of SCD, it may also have prognostic implications.
Haemolysis was observed in all HbSS samples (Browning et al. 2007; Ellory et al. 2008; Dalibalta et al. 2010). It was also significant and readily detectable in RBCs from the second most common genotype of SCD, HbSC, and in some of the rarer genotypes of SCD. The extent and rate of haemolysis, however, varied between samples, notably being reduced in RBCs from SCD patients treated with hydroxyurea. We are currently investigating to determine whether there is any correlation with severity of clinical complications.
Reduction of haemolysis in RBCs from hydroxyurea-treated patients raises the question of the mechanism by which this reagent has its effect. The increased expression of HbF is well known (Platt et al. 1994) and would reduce the tendency of HbS to polymerise. This may be the mechanism by which it reduces haemolysis in sucrose solutions (Fig. 4). On the other hand, rapid effects of hydroxyurea occurring within 2 weeks of commencing treatment have been reported for some RBC parameters (e.g. adhesion and K+ content) (Bridges et al 1996). Induction of sizeable HbF would usually take longer (Steinberg et al. 1997) – although there are reports of elevation of HbF over this short timescale (Charache et al. 1987; Rodgers et al. 1990). Alternative actions of hydroxyurea may be postulated such as reduction in RBC damage during their circulation through rapid down-regulation of endothelial adhesion molecules. A direct effect of hydroxyurea on RBCs over the 60 min time course of the haemolysis assay was not observed, however.
RBCs from HbAA individuals were protected and RBCs from sickle trait individuals (HbAS) also showed less haemolysis. In this respect, the present haemolysis assay has advantages for diagnosing SCD patients over the widely used sickledex test (Canning & Huntsman 1970), which is routinely used to confirm the presence of HbS suggested by its mobility in HPLC assays and which does not distinguish RBCs from HbAS and HbSS individuals. There was overlap in haemolysis between samples from HbSC and HbAS individuals, which may limit the usefulness of the haemolysis assay in distinguishing between these two genotypes. It may be possible to alter incubation conditions, however – for example, by including aromatic aldehydes or cytochalasin B, or altering pH – to increase the difference between these genotypes. We are currently investigating these possibilities. In this context, it may also be relevant to note that RBCs from HbSC individuals have a higher proportion of HbS, containing 50% HbS and 50% HbC, whilst those from HbAS RBCs usually have about 40% HbS and 60% HbA (Bunn et al. 1982). The combined total intracellular Hb concentration is also higher in HbSC RBCs (Bunn et al. 1982). These two features account for the mild, often subclinical signs experienced by individuals with sickle trait, as simple deoxygenation is usually insufficient to elicit HbS polymerisation and sickling. They may underlie differences observed in the extent of haemolysis.
Only about one-fifth of RBCs undergo haemolysis during each episode of deoxygenation (Browning et al. 2007). This aspect is similar to the ‘stochastic’ nature of Psickle activation in shrinkage assays to examine Gardos channel activation following Ca2+ entry in response to deoxygenation (Lew et al. 1997). It has been hypothesised that, on their formation, HbS polymers randomly make contact with key regulatory sites at the RBC membrane controlling ion permeability. The same appears to apply to non-electrolyte-induced haemolysis. If this is the case, the haemolysis assay may well provide a simple measure of Psickle activity.
Non-electrolyte permeability via the Psickle pathway was previously discounted, at least for arabinose, erythritol and mannitol (Clark & Rossi 1990). In these previous experiments, however, RBCs were pretreated with 10 μm cytochalasin B. We show that this treatment also abolished haemolysis. When untreated, RBCs from SCD patients did in fact lyse in isosmotic solutions of these non-electrolytes. Cytochalasin B is well known to bind to actin filaments (Theodoropoulos et al. 1994) and also acts as an inhibitor of hexose transport (Estensen & Plageman 1972). It inhibits the binding of G-actin to the cytoplasmic face of RBC membranes (Cohen et al. 1978). Other actions relevant to RBC permeability, however, include effects on a number of other signal transduction pathways, including alterations in protein kinase activity (Pedersen et al. 2001). Although its site of action to prevent haemolysis is not clear, it did not prevent sickling (Fig. 9).
In most experiments, haemolysis was investigated using deoxygenation at pH 7.4 and 37°C. The extent of haemolysis could be modulated by a number of manoeuvres, however. Low pH (pH 6) promotes HbS polymerisation, and induced haemolysis in isosmotic sucrose solutions even in oxygenated samples. Psickle activity is elevated at lower pH (Joiner et al. 1993) and low pH also activates a conductance in RBCs from HbSS individuals similar to that induced by deoxygenation (Ma et al. 2012). For RBCs from HbAS individuals, we also found that encouraging HbS polymerisation and shape change using hypertonic salines and reduced pH (Bookchin & Lew 1981) also supported haemolysis in sucrose solutions. Conversely, the aromatic aldehydes o-vanillin and 5HMF reduce HbS polymerisation by forming Schiff bases with the terminal –NH2 groups of the β chains (Zaugg et al. 1977; Abraham et al. 1991). In this way, they also act to increase the oxygen affinity of Hb and thereby stabilise soluble HbS. They were thus originally proposed as potential therapeutic reagents for SCD patients but are probably insufficiently specific. They, too, were effective in the haemolysis assays, inhibiting lysis both in deoxygenated cells at pH 7.4 and in oxygenated samples at pH 6. A third reagent, urea, which interacts with protein by a different mechanism (Makhatadze & Privalov 1992) but which also stabilises soluble HbS, also prevented haemolysis. A trivial explanation for the inhibitory effect of these compounds on haemolysis via preventing deoxygenation of HbS was excluded by measuring oxygen saturation.
It is not possible to assess sickling by ordinary microscopy in sucrose solutions as the cells become crenated and classical sickle shapes are not observed. In parallel experiments, however, we looked at sickling in saline. In most cases, haemolysis was precipitated by conditions which stimulated HbS polymerisation, whilst being inhibited by manoeuvres which reduced sickling. The main exception to this is represented by cytochalasin B, with which obvious sickling still occurred on deoxygenation. In this case, we speculate that interference with actin polymerisation (or possibly some other action of cytochalasin B) interferes with activation of the Psickle-like pathway, thereby inhibiting haemolysis downstream of the HbS polymerisation event.
In conclusion, the present results indicate that progressive, substantial haemolysis in isosmotic non-electrolyte solutions is unique to RBCs containing sufficient HbS to undergo polymerisation and shape change. The current findings support the hypothesis that the non-electrolyte haemolysis assay constitutes a novel test for SCD, dependent on the altered properties of the RBC membrane induced by HbS polymerisation, rather than the simple presence of HbS per se.
Acknowledgments
This work was supported by Action Medical Research and the Medical Research Council. There are no conflicts of interest, financial or otherwise, for any of the authors.
Glossary
- CLT
clotrimazole
- Hb
haemoglobin
- Hct
haematocrit
- 5HMF
5-hydroxymethylfurfural
- LIS
low ionic strength
- RBCs
red blood cells
- SCD
sickle cell disease
Author contributions
All experiments were carried out in J.S.G.'s laboratory. J.S.G., D.C.R., J.C.E., J.A.B. and C.M. were responsible for design and conception of the experiments. C.M., A.H. and A.O. performed the experiments. C.M., D.C.R., J.C.E. and J.S.G. analysed and interpreted the data. J.S.G., D.C.R. and C.M. drafted the manuscript.
References
- Abraham DJ, Mehanna AS, Wireko FC, Whitney J, Thomas RP, Orringer EP. Vanillin, a potential agent for the treatment of sickle cell anemia. Blood. 1991;77:1334–1341. [PubMed] [Google Scholar]
- Ataga KI, Reid M, Ballas SK, Yasin Z, Bigelow C, St. James L, Smith WR, Galacteros F, Kutlar A, Hull JH, Stockler JW. Improvements in haemolysis and indicators of erythrocyte survival do not correlate with acute vaso-occlusive crises in patients with sickle cell disease: a phase III randomised, placebo-controlled, double-blind study of the gardos channel blocker senicapoc (ICA-17043) Br J Haematol. 2011;153:92–104. doi: 10.1111/j.1365-2141.2010.08520.x. [DOI] [PubMed] [Google Scholar]
- Bernhardt I, Hall AC, Ellory JC. Effects of low ionic strength media on passive human red cell monovalent cation transport. J Physiol. 1991;434:489–506. doi: 10.1113/jphysiol.1991.sp018482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt I, Kummerow D, Weiss E. K+(Na+)/H+ exchange in human erythrocytes activated under low ionic strength conditions. Blood Cells Mol Dis. 2001;27:108–111. doi: 10.1006/bcmd.2000.0360. [DOI] [PubMed] [Google Scholar]
- Blackstock EJ, Stewart GW. The dependence on external cation of sodium and potassium fluxes across the human red cell membrane at low temperatures. J Physiol. 1986;375:403–420. doi: 10.1113/jphysiol.1986.sp016124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookchin RM, Lew VL. Effect of a ‘sickling pulse’ on calcium and potassium transport in sickle red cells. J Physiol. 1981;312:265–280. doi: 10.1113/jphysiol.1981.sp013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges KR, Barabino GA, Brugnara C, Cho MR, Dover CG, Ewenstein BM, Golan DE, Guttmann CRG, Hofrichter J, Mulkern RV, Zhang B, Eaton WA. A multiparameter analysis of sickle erythrocytes in patients undergoing hydroxyurea therapy. Blood. 1996;88:4701–4710. [PubMed] [Google Scholar]
- Browning JA, Robinson HC, Ellory JC, Gibson JS. Deoxygenation-induced non-electrolyte pathway in red cells from sickle cell patients. Cell Physiol Biochem. 2007;19:165–174. doi: 10.1159/000099204. [DOI] [PubMed] [Google Scholar]
- Brugnara C, De Franceschi L, Armsby CC, Saadane N, Trudel M, Beuzard Y, Rittenhouse A, Rifai N, Platt O, Alper SL. A new therapeutic approach for sickle cell disease. Blockade of the red cell Ca2+-activated K+ channel by clotrimazole. Ann N Y Acad Sci. 1995;763:262–271. doi: 10.1111/j.1749-6632.1995.tb32411.x. [DOI] [PubMed] [Google Scholar]
- Bunn HF, Noguchi CT, Hofrichter J, Schechter GP, Schechter AN, Eaton WA. Molecular and cellular pathogenesis of hemoglobin SC disease. Proc Natl Acad Sci U S A. 1982;79:7527–7531. doi: 10.1073/pnas.79.23.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning DM, Huntsman RG. An assessment of sickledex as an alternative to the sickling test. J Clin Pathol. 1970;23:736–737. doi: 10.1136/jcp.23.8.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charache S, Dover GJ, Moyer MA, Moore JW. Hydroxyurea-induced augmentation of fetal haemoglobin production in patients with sickle cell anemia. Blood. 1987;69:109–116. [PubMed] [Google Scholar]
- Clark MR, Mohandas N, Shohet SB. Hydration of sickle cells using the sodium ionophore monensin. J Clin Invest. 1982;70:1074–1080. doi: 10.1172/JCI110695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MR, Rossi ME. Permeability characteristics of deoxygenated sickle cells. Blood. 1990;76:2139–2145. [PubMed] [Google Scholar]
- Cohen CM, Jackson PL, Branton D. Actin–membrane interactions: association of G-actin with the red cell. J Supramol Struct. 1978;9:113–124. doi: 10.1002/jss.400090111. [DOI] [PubMed] [Google Scholar]
- Dalibalta S, Ellory JC, Browning JA, Wilkins RJ, Rees DC, Gibson JS. Novel permeability characteristics of red blood cells from sickle cell patients. Blood Cells Mol Dis. 2010;45:46–52. doi: 10.1016/j.bcmd.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Dunham PB, Ellory JC. Passive potassium transport in low potassium sheep red cells: dependence upon cell volume and chloride. J Physiol. 1981;318:511–530. doi: 10.1113/jphysiol.1981.sp013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JW, Hofrichter J. Hemoglobin S gelation and sickle cell disease. Blood. 1987;70:1245–1266. [PubMed] [Google Scholar]
- Ellory JC, Sequeira R, Constantine A, Wilkins RJ, Gibson JS. Non-electrolyte permeability of deoxygenated sickle cells compared. Blood Cells Mol Dis. 2008;41:44–49. doi: 10.1016/j.bcmd.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Estensen RD, Plageman PGW. Cytochalasin B: inhibition of glucose and glucosamine transport. Proc Natl Acad Sci U S A. 1972;69:1430–1434. doi: 10.1073/pnas.69.6.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkin O, Nagel RL, Vekilov PG. The kinetics of nucleation and growth of sickle cell hemoglobin fibres. J Mol Biol. 2007;365:425–439. doi: 10.1016/j.jmb.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Gardos G. The function of calcium and potassium permeability of human erythrocytes. Biochim Biophys Acta. 1958;30:653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- Gibson JS, Ellory JC. Membrane transport in sickle cell disease. Blood Cells Mol Dis. 2002;28:1–12. doi: 10.1006/bcmd.2002.0515. [DOI] [PubMed] [Google Scholar]
- Joiner CH. Cation transport and volume regulation in sickle red blood cells. Am J Physiol. 1993;264:C251–C270. doi: 10.1152/ajpcell.1993.264.2.C251. [DOI] [PubMed] [Google Scholar]
- Joiner CH, Dew A, Ge DL. Deoxygenation-induced fluxes in sickle cells. I. Relationship between net potassium efflux and net sodium influx. Blood Cells. 1988;13:339–348. [PubMed] [Google Scholar]
- Joiner CH, Morris CL, Cooper ES. Deoxygenation-induced cation fluxes in sickle cells. III. Cation selectivity and response to pH and membrane potential. Am J Physiol. 1993;264:C734–C744. doi: 10.1152/ajpcell.1993.264.3.C734. [DOI] [PubMed] [Google Scholar]
- Lew VL, Bookchin RM. Ion transport pathology in the mechanism of sickle cell dehydration. Physiol Rev. 2005;85:179–200. doi: 10.1152/physrev.00052.2003. [DOI] [PubMed] [Google Scholar]
- Lew VL, Ortiz OE, Bookchin RM. Stochastic nature and red cell population distribution of the sickling-induced Ca2+ permeability. J Clin Invest. 1997;99:2727–2735. doi: 10.1172/JCI119462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y-L, Rees DC, Gibson JS, Ellory JC. The conductance of red blood cells from sickle cell patients: ion selectivity and inhibitors. J Physiol. 2012;590:2095–2105. doi: 10.1113/jphysiol.2012.229609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhatadze G, Privalov PL. Protein interactions with urea and guanidinium chloride. J Mol Biol. 1992;33:491–497. doi: 10.1016/0022-2836(92)90963-k. [DOI] [PubMed] [Google Scholar]
- Mohandas N, Rossi ME, Clark MR. Association between morphologic distortion of sickle cells and deoxygenation-induced cation permeability increases. Blood. 1986;68:450–454. [PubMed] [Google Scholar]
- Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, Wethers DL, Pegelow CH, Gill FM. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–294. [PubMed] [Google Scholar]
- Pedersen SF, Hoffmann EK, Mills JW. The cytoskeleton and cell volume regulation. Comp Biochem Physiol A. 2001;130:385–399. doi: 10.1016/s1095-6433(01)00429-9. [DOI] [PubMed] [Google Scholar]
- Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease: life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- Platt OS, Thorington BD, Brambilla DJ, Milner PFA, Rosse WF, Vichinsky E, Kinney TR. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325:11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376:2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- Rodriguez GI, Kuhn JG, Weiss GR, Hilsenbeck SG, Eckardt JR, Thurman A, Rinaldi DA, Hodges S, Von Hoff DD, Rowinsky EK. A bioavailability and pharmacokinetic study of oral and intravenous hydroxyurea. Blood. 1998;91:1533–1541. [PubMed] [Google Scholar]
- Rodgers GP, Dover GJ, Noguchi CT, Schechter AN, Nienhuis AW. Hematologic responses of patients with sickle cell disease to treatment with hydroxyurea. N Engl J Med. 1990;322:1037–1045. doi: 10.1056/NEJM199004123221504. [DOI] [PubMed] [Google Scholar]
- Rosa R, Bierer BA, Thomas R, Stoff JS, Kruskall M, Robinson S, Bunn HF, Epstein FH. A study of hyponatremia in the prevention and treatment of sickle-cell crisis. N Engl J Med. 1980;303:1138–1143. doi: 10.1056/NEJM198011133032002. [DOI] [PubMed] [Google Scholar]
- Speake PF, Roberts CA, Gibson JS. Effect of changes in respiratory blood parameters on equine red blood cell K–Cl cotransporter. Am J Physiol. 1997;273:C1811–C1818. doi: 10.1152/ajpcell.1997.273.6.C1811. [DOI] [PubMed] [Google Scholar]
- Steinberg MH, Long Z-H, Barton FB, Terrin ML, Charache S, Dover GJ. Fetal hemoglobin in sickle cell anemia: determinants of response to hydroxyurea. Blood. 1997;89:1078–1088. [PubMed] [Google Scholar]
- Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- Stocker JW, De Franceschi LD, McNaughton-Smith GA, Corrocher R, Beuzard Y, Brugnara C. ICA-17043, a novel Gardos channel blocker, prevents sickled red blood cell dehydration in vitro and in vivo in SAD mice. Blood. 2003;101:2412–2418. doi: 10.1182/blood-2002-05-1433. [DOI] [PubMed] [Google Scholar]
- Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364:1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- Theodoropoulos PA, Gravanis A, Tsapara A, Margioris AN, Papadogiorgaki E, Galanopoulos V, Stournaras C. Cytochalasin B may shorten actin filaments by a mechanism independent of barbed end capping. Biochem Pharmacol. 1994;47:1875–1881. doi: 10.1016/0006-2952(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Vichinsky EP, Neumayr LD, Earles AN, Williams R, Lennette ET, Dean D, Nickerson B, Orringer E, McKie V, Bellevue R, Daeschner C, Manci EA. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000;342:1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- Zaugg RH, Walder JA, Klotz IM. Schiff base adducts of hemoglobin. Modifications that inhibit erythrocyte sickling. J Biol Chem. 1977;252:8542–8548. [PubMed] [Google Scholar]


