Abstract
Polycystic ovary syndrome (PCOS) is associated with cardiovascular disease. The contribution of the nitric oxide (NO) dilator system to cutaneous endothelial dysfunction is currently unknown in PCOS. Our aim was to examine whether women with PCOS demonstrate impaired cutaneous microvascular NO function and whether exercise training can ameliorate any impairment. Eleven women with PCOS (age, 29 ± 7 years; body mass index, 34 ± 6 kg m−2) were compared with six healthy obese control women (age, 29 ± 7 years; body mass index, 34 ± 5 kg m−2). Six women with PCOS (30 ± 7 years; 31 ± 6 kg m−2) then completed 16 weeks of exercise training. Laser Doppler flowmetry, combined with intradermal microdialysis of l-NG-monomethyl-l-arginine, a nitric oxide antagonist, in response to incremental local heating of the forearm was assessed in women with PCOS and control women, and again in women with PCOS following exercise training. Cardiorespiratory fitness, homeostasis model assessment for insulin resistance, hormone and lipid profiles were also assessed. Differences between women with PCOS and control women and changes with exercise were analysed using Student's unpaired t tests. Differences in the contribution of NO to cutaneous blood flow [expressed as a percentage of maximal cutaneous vasodilatation (CVCmax)] were analysed using general linear models. At 42°C heating, cutaneous NO-mediated vasodilatation was attenuated by 17.5%CVCmax (95% confidence interval, 33.3, 1.7; P = 0.03) in women with PCOS vs. control women. Exercise training improved cardiorespiratory fitness by 5.0 ml kg−1 min−1 (95% confidence interval, 0.9, 9.2; P = 0.03) and NO-mediated cutaneous vasodilatation at 42°C heating by 19.6% CVCmax (95% confidence interval, 4.3, 34.9; P = 0.02). Cutaneous microvascular NO function is impaired in women with PCOS compared with obese matched control women but can be improved with exercise training.
Key points
Polycystic ovary syndrome (PCOS) is associated with cardiovascular disease.
Nitric oxide (NO) is a naturally occurring molecule that possesses anti-atherogenic properties.
The contribution of NO to the dilatation of microvessels in the skin is currently unknown in women with PCOS.
In this study, it was found that women with PCOS display impaired NO bioavailability compared with obese matched control women.
Exercise training improves the microvascular dysfunction displayed in women with PCOS, via the upregulation of NO.
These findings suggest exercise training can be a preventive strategy in women with PCOS.
Introduction
Polycystic ovary syndrome (PCOS) is a highly complex, heterogeneous phenotype, consisting of clinical or biochemical hyperandrogenism, oligo-/amenorrhoea and a polycystic appearance of the ovaries (Goodarzi et al. 2011). Polycystic ovary syndrome affects up to 10% of women according to the National Institutes of Health (NIH) criteria (Azziz et al. 2004) and up to 20% of women using the broader Rotterdam criteria (Broekmans et al. 2006). Polycystic ovary syndrome is therefore the most common endocrinopathy in women of reproductive age. Polycystic ovary syndrome is associated with several cardiometabolic abnormalities, including obesity, insulin resistance, impaired glucose tolerance or type 2 diabetes, dyslipidaemia and hypertension, all of which are associated with endothelial dysfunction (Moran et al. 2010).
Women with PCOS exhibit inherent endothelial dysfunction in large conduit arteries (Sprung et al. 2012), a prognostic indicator of future cardiovascular disease (Green et al. 2011), but studies of microvascular function in this patient population are scant. Microvessel endothelial dysfunction can impact upon both peripheral vascular resistance and glucose disposal, therefore potentially contributing to hypertension and insulin resistance (Wenner et al. 2011). Cutaneous microvessel vasodilator function can be used as a surrogate measure of small vessel function and provides a translational model to investigate preclinical cardiovascular disease (CVD) risk (Holowatz et al. 2008). A small number of studies have investigated cutaneous endothelial function in women with PCOS and have reported dysfunction compared with healthy women (Lakhani et al. 2005; Alexandraki et al. 2006). Nevertheless, one recent study suggested that microvascular dysfunction was explained by obesity rather than PCOS per se, because microvessel vasodilator dysfunction was apparent in both obese women with PCOS and control women (Ketel et al. 2008).
Previous studies on microvascular function in women with PCOS have utilized iontophoresis-mediated infusion of ACh (Lakhani et al. 2005; Ketel et al. 2008); a technique with technical limitations because the delivery method across the dermal layer of the skin is inconsistent and the vasodilator response to ACh may not be exclusive to NO (Holowatz et al. 2005; Cracowski et al. 2006). In contrast, intradermal administration via microdialysis of a competitive NO inhibitor allows for accurate assessment of the contribution of NO to cutaneous vasodilatation (Cracowski et al. 2006). Nitric oxide is a signalling molecule that can influence blood flow and distribution through the dilatation of blood vessels, and NO-related arterial wall function reflects aggregate atherogenic risk (Green, 2009). It has been proposed that skin vasodilator function, specifically mediated by NO, may likewise reflect generalized microvascular function (Holowatz et al. 2008). Despite the anti-atherogenic impacts of endothelium-derived NO and the reduction in CVD that this can confer (Green et al. 2011), the contribution of NO to cutaneous microvessel dysfunction has not yet been studied in women with PCOS. The first aim of the present study, therefore, was to examine NO-mediated cutaneous microvascular function in obese women with PCOS and obese control women. We hypothesized that the NO-mediated vasodilator response to gradual local heating would be impaired in women with PCOS compared with obese control women.
Lifestyle modification, including increasing levels of physical activity, is recommended as a primary prevention strategy of CVD in PCOS (Wild et al. 2010). It has recently been established that NO-mediated vasodilatation in the cutaneous microvessels is influenced by fitness in healthy individuals (Black et al. 2008) and that exercise training improves NO-mediated cutaneous microvascular function in healthy young and older individuals (Black et al. 2008; Simmons et al. 2011). Nevertheless, the impact of exercise training on cutaneous microvascular function has not yet been studied in women with PCOS. Therefore, we sought to examine the effect of a 16 week moderate-intensity aerobic exercise training programme on NO-mediated cutaneous microvascular function in obese women with PCOS, who are at increased risk of CVD. We hypothesized that supervised exercise training would improve NO-mediated microvascular function in women with PCOS.
Methods
Participants
Eleven women with PCOS (aged 29 ± 7 years; body mass index, 34 ± 6 kg m−2) and six obese matched control women (aged 29 ± 7 years; body mass index, 34 ± 5 kg m−2) were recruited (see statistical power estimation, Statistical Analysis). Patients were recruited from a gynaecology clinic specifically for women with PCOS at Liverpool Women's Foundation Trust, and control subjects were recruited via university and local advertisement. In line with current clinical practice in the UK, PCOS was identified by means of the Rotterdam criteria for diagnosing PCOS (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004), based on the presence of two of the following three criteria: (i) clinical or biochemical hyperandrogenism; (ii) oligomenorrhoea or amenorrhoea; (iii) characteristic polycystic ovaries on ultrasonography, having excluded other possible causes by appropriate biochemical assessments. All control women experienced normal menses, had no history of menstrual dysfunction and demonstrated normal biochemical profiles with no evidence of hyperandrogenism. Only sedentary individuals were recruited, defined as <2 h low-intensity physical activity per week; none of the participants performed any structured or vigorous physical activity. All participants were over 18 years of age, normotensive and had no history of impaired glucose tolerance or type 2 diabetes, cardiovascular, liver, kidney or respiratory disease. Current use or use within the last 3 months of oral contraceptive pill, anti-androgens or fertility treatment or other medication which could alter vascular structure and/or function, such as insulin-sensitizing agents (e.g. metformin) or weight-loss medication (e.g. orlistat), resulted in exclusion. All participants were non-smokers and drank ≤14 units of alcohol per week. In control women, data was collected during the early follicular phase of their menstrual cycle (days 1–7 following the onset of menstruation). This was not feasible in PCOS women, owing to the menstrual cycle irregularities. Participants were asked to fast for 12 h, abstain from alcohol and caffeine for 24 h and refrain from exercise for 48 h prior to testing sessions. The study was approved by the local research ethics committee and conformed to the Declaration of Helsinki. All participants were informed of the methods before providing written informed consent.
Research design
Participants reported to the laboratory on two separate occasions. On visit 1, anthropometric measurements were made and a fasting blood sample was collected, followed by the assessment of cutaneous NO-mediated vasodilator function. On visit 2, less than 7 days later, cardiorespiratory fitness was assessed. All participants were studied at the same time of day (beginning at 09.00 h) to control for the impact of circadian variation. Following baseline assessment, six women with PCOS (aged 30 ± 7 years; body mass index, 31 ± 6 kg m−2) enrolled in a 16 week supervised moderate-intensity aerobic exercise-training programme. Upon completion, physiological assessments were repeated.
Anthropometric and biochemical evaluation
After obtaining a full medical history, a single person (V.S.S.) recorded all anthropometric measurements (weight, height, waist and hip circumference). Following an overnight fast, a blood sample was taken.
Plasma samples were analysed using the Olympus AU2700 analyser (Beckman Coulter Ltd, High Wycombe, UK) with standard proprietary reagents as follows: glucose with hexokinase, total cholesterol and high-density lipoprotein (HDL) with cholesterol esterase/oxidase, triglyceride with glycerol kinase and alanine transaminase (ALT) with International federation of clinical chemistry (IFCC) kinetic UV (without pyridoxal phosphate activation). The intra- and interassay coefficients of variation were ≤10%. Low-density lipoprotein (LDL) was calculated according to the Friedwald formula. Luteinizing hormone (LH), follicle stimulating hormone (FSH), oestradiol, progesterone, total testosterone and sex hormone binding globulin (SHBG) concentrations were measured by a chemiluminescence method (Siemens Centaur). Free androgen index was calculated as 100 × [testosterone concentration (in nanomoles per litre)/SHBG concentration (in nanomoles per litre)] (normal, <7%; Mathur et al. 1981). Insulin was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Invitrogen, UK). The intra- and interassay coefficients of variation were ≤9%. Using fasting baseline glucose and insulin concentrations, insulin resistance was calculated by the homeostasis model assessment for insulin resistance (HOMA-IR; Matthews et al. 1985). All laboratory assays were performed in the clinical biochemistry laboratory at University Hospital Aintree.
Peak oxygen consumption test
A fitness test (peak oxygen uptake) was performed on a treadmill ergometer following the Bruce protocol (Bruce et al. 1973). Following a 2 min warm-up at 2.2 km h−1 on a flat gradient, the initial workload was set at 2.7 km h−1 with a 5 deg gradient. Thereafter, stepwise increments in speed and gradient were made every minute. Heart rate (HR; Polar Electro Oy, Kempele, Finland) and rate of perceived exertion were monitored throughout (Burkhalter, 1996; Borg, 1998). Peak oxygen uptake was calculated from expired gas fractions (Oxycon Pro, Jaegar, Hochberg, Germany) as the highest consecutive 15 s period of data in the last minute before volitional exhaustion.
Exercise intervention
Initially, eight women with PCOS enrolled into the exercise-training programme, but two dropped out prior to the start of the intervention for personal reasons. The remaining six women with PCOS attended a thorough familiarization and induction session. Participants were required to attend the university gymnasium on a weekly basis and were provided with full supervision and guidance by a trained exercise physiologist. Based upon their individual basal fitness level, participants underwent 30 min moderate-intensity aerobic exercise three times per week at 30% of heart rate reserve (HRR), which progressed weekly based on HR responses. At week 12, participants were exercising five times a week for 45 min at 60% HRR. Heart rate reserve was calculated as (maximal HR – resting HR) and training intensity was calculated as follows: [(maximal HR – resting HR) × intensity]+ resting HR. The calculation used HR measures calculated during the maximal oxygen consumption test completed prior to exercise training. To ensure maximal compliance throughout the 16 week period, all participants were closely monitored via heart rate and the Technogym Wellness Key system. The Technogym Wellness Key system is a software program that remotely and accurately tracks the exercise activity of participants. No dietary modifications were made throughout the course of the exercise intervention, confirmed by use of a standard food diary.
Microdialysis fibre instrumentation
All intradermal microdialysis assessments were performed in a quiet, temperature-controlled laboratory. Upon arrival, participants were instrumented and cannulated for microdialysis probe insertion (∼15 min). Once seated comfortably in a custom-designed bed, the right arm was supinated and supported for insertion of microdialysis fibres. The insertion sites were marked on the skin, and cold packs were applied as local anaesthesia. Two 21-gauge needles were inserted ∼5 cm apart and ∼0.3–1.0 mm beneath the epidermal surface to enable threading and placement of two microdialysis fibres (Linear 30; CMA Microdialysis Ltd, Stockholm, Sweden), containing 10-mm-long 6 kDa membranes. The needles were then removed and the embedded fibres perfused with saline solution at a rate of 5 μl min−1 using a microinfusion pump (model 11 plus; Harvard Apparatus, Massachusetts, USA).
Following this, integrated laser Doppler probes (Perimed 413, Periflux 5001 System; Stockholm, Sweden) combined with local heating discs (Perimed 455; Stockholm, Sweden) set at 33°C were placed above both embedded microdialysis fibres sites. Laser Doppler flowmetry uses a seven-laser array emitted monochrome light at a penetrative depth of 1 mm. The incident monochrome light reflects off moving blood cells, causing a shift in the returning wavelength (Doppler effect), from which estimates of cutaneous blood flux (the concentration of red blood cells multiplied by their velocity) can be made. Consequently, laser Doppler flowmetry enables sensitive and quantifiable detection of relative changes in cutaneous red cell flux in response to a given stimulus.
Physiological NO-mediated vasodilatation
Following a ∼90 min equilibration period, the skin surrounding both microdialysis probes was gradually heated, using local heating discs, from 33 to 42°C at a rate of 0.5°C per 2.5 min (45 min). Thereafter, both sites were continuously heated at 42°C for a further 30 min. This gradual heating protocol was used to minimize the impact of heating on axon reflexes, which are less NO mediated than slow heating component responses (Minson et al. 2001; Houghton et al. 2006). Saline solution was infused throughout the protocol in one site and NG-monomethyl-l-arginine (l-NMMA; 10 mm, 5 μl min−1; Clinalfa, CalBiochem, Weil am Rhein, Germany) infused through the second, from 30 min prior to the onset of heating. Sodium nitroprusside (SNP; 56 mm; Mayne Pharma, Royal Leamington Spa, UK), a potent NO donor, was infused at the end of the protocol for 30 min (Minson et al. 2002; Cracowski et al. 2006) to initiate peak vasodilatation (Fig. 1).
Figure 1.
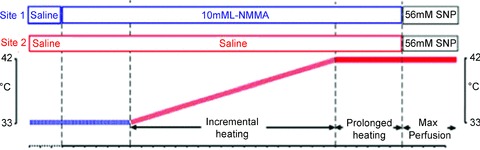
Schematic diagram of the physiological (localized heating) NO-mediated vasodilatation protocol
Assessment of forearm skin blood flow
To obtain an index of cutaneous blood flow, cutaneous red cell flux was measured by placing integrated laser Doppler probes. The laser Doppler probe signals were continuously monitored via an online software chart recorder (PSW; Perimed AB). At each designated study time point (2.5 min intervals), cutaneous blood flow was assessed by averaging laser Doppler flux, measured in perfusion units (PU), over a stable 30 s period. These data were subsequently converted to cutaneous vascular conductance (CVC), calculated as the laser Doppler flux divided by mean arterial pressure, where mean arterial pressure was derived from contemporaneous automated blood pressure measures (GE Dinamap Pro 300V2, Freiburg, Germany) in the contralateral arm. Values were expressed relative to the maximal CVC achieved during infusion of 56 mm SNP at 42°C, i.e. ‘%CVCmax’. This is the preferred method of data expression used in the literature (Cracowski et al. 2006).
Data reduction
Data during the incremental heating were calculated and presented at each temperature (every 0.5°C from 33 to 42°C) for both the saline and l-NMMA microdialysis sites. The contribution of NO was calculated by subtracting individual l-NMMA data from saline data collected simultaneously. Data are therefore presented as the NO contribution to microvascular red cell flux or cutaneous blood flow. Data were also calculated for blood flow following 5 min at 42°C and following 30 min continuous heating at 42°C.
Statistical analysis
There has been only one previous study in which our primary outcome (NO contribution to endothelial dysfunction) was measured (Black et al. 2008). In that study, the difference between fit (26.9 ± 3.9%CVCmax) and sedentary individuals (46.2 ± 7.0%CVCmax) translated to a very large standardized effect size of over 3.5. In a related study conducted by Lakhani et al. (2005), the difference between women with PCOS (125.1 ± 21.7 erythrocyte flux) and healthy women (200.8 ± 28.5 erythrocyte flux) also translated to a large effect size of over 3.0. Therefore, we predicted that our primary outcome would be sensitive for detection of differences between samples. Using the N-Query software (Statistical Solutions, Cork, Ireland) we estimated that a Student's unpaired t test with a 0.05 two-sided significance level would have 90% power to detect a standardized effect size of at least 1.8 when the sample sizes in the two groups are 11 and 6, respectively (a total sample size of 17). We also estimated that when the sample size is 6, a Student's paired t test with a 0.05 two-sided significance level would have 90% power to detect a standardized effect size of at least 1.7.
Analyses were performed using the Statistics Package for Social Sciences for windows, version 17.0 (SPSS Inc., Chicago, IL, USA). Initially, all data were explored for underlying distribution and transformed if appropriate. All differences in baseline characteristics between groups (PCOS vs. control women) were compared using Student's unpaired t tests, and changes following exercise training (pre- vs. postexercise) were examined using Student's paired t tests. Cutaneous blood flow data are presented as the %CVCmax, and analysis was performed on these normalized data (Cracowski et al. 2006). The underlying relationship between measurements of blood flow and CVCmax conformed to a ratio, rendering use of the %CVCmax appropriate (Atkinson & Batterham, 2012). Data were logarithmically transformed if the residuals from the statistical analysis were skewed, which is common with ratio variables (Atkinson et al. 2010). Logarithmically transformed data were back transformed to the original units and presented in the text as mean [95% confidence intervals (CIs)], unless otherwise stated. Statistical significance was delimited at P < 0.05, and exact P values are cited (P values of ‘0.000’ provided by the statistics package are reported as ‘<0.001’).
To ensure a successful increase in NO production with the local heat stimulus and successful blockade of NO production, saline and l-NMMA data were individually compared using a two-factor (site vs. temperature) ANOVA. A two-factor (group vs. temperature) repeated-measures ANOVA was also employed to compare the contribution of NO (saline %CVCmax minus l-NMMA %CVCmax at equivalent time points) with the %CVCmax response at baseline (PCOS vs. control women) and following exercise training (pre- vs. postexercise). Statistically significant interactions between these two factors were followed up with the least significant difference (LSD) approach to multiple comparisons (Perneger, 1998).
Results
Women with PCOS vs. control women: clinical characteristics
Women with PCOS and control women were similar in terms of age, body mass index and cardiorespiratory fitness (Table 1). Women with PCOS displayed elevated testosterone [0.8 mmol l−1 (95% CI, 0.3, 1.4); P = 0.006] and free androgen index [4.0 mmol l−1 (95% CI, 0.1, 7.9); P = 0.05] compared with control women.
Table 1.
Baseline characteristics of women with polycystic ovary syndrome (PCOS; n = 11) and control women (n = 6)
| Characteristic | Women with PCOS | Control women | P value |
|---|---|---|---|
| Age (years) | 29 (25, 34) | 29 (21, 37) | 0.98 |
| Body mass index (kg m−2) | 34 (30, 38) | 34 (28, 39) | 0.84 |
| Weight (kg) | 91.2 (79.3, 103.0) | 91.0 (75.7, 106.3) | 0.98 |
| Waist circumference (cm) | 106 (97, 115) | 106 (89, 123) | 0.98 |
| Systolic blood pressure (mmHg) | 119 (112, 125) | 126 (114, 139) | 0.16 |
| Diastolic blood pressure (mmHg) | 71 (66, 76) | 78 (65, 90) | 0.16 |
| Heart rate (beats min−1) | 73 (67, 78) | 73 (65, 77) | 0.70 |
| Peak oxygen uptake (ml kg−1 min−1) | 26.1 (23.0, 29.2) | 25.3 (17.4, 36.3) | 0.44 |
| FSH (IU l−1) | 4.9 (3.8, 6.0) | 5.7 (4.0, 7.3) | 0.37 |
| LH (IU l−1) | 11.0 (6.2, 15.8) | 7.6 (3.5, 11.7) | 0.31 |
| Progesterone (nmol l−1)† | 3.0 (2.1, 4.2) | 2.2 (0.8, 3.1) | 0.41 |
| Oestradiol (pmol l−1)† | 253 (188, 340) | 244 (127, 467) | 0.89 |
| Testosterone (nmol l−1) | 2.8 (2.4, 3.2) | 1.9 (1.5, 2.4) | 0.006* |
| Free androgen index (%) | 9.7 (7.0, 12.5) | 5.7 (3.8, 7.7) | 0.05* |
| SHBG (nmol l−1)† | 31.3 (21.5, 45.7) | 40.0 (26.6, 48.7) | 0.59 |
| ALT (IU l−1)† | 22.3 (14.5, 34.5) | 24.5 (10.4, 57.7) | 0.80 |
| Glucose (mmol l−1) | 4.6 (4.4, 4.8) | 4.6 (4.4, 4.9) | 0.97 |
| Insulin (pmol l−1)† | 17.5 (13.2, 23.2) | 18.8 (12.0, 29.5) | 0.75 |
| HOMA-IR† | 3.6 (2.6, 4.9) | 3.9 (2.5, 6.3) | 0.71 |
| Cholesterol (mmol l−1)† | 5.0 (4.5, 5.5) | 5.1 (4.5, 5.7) | 0.89 |
| Triglyceride (mmol l−1)† | 2.0 (1.2, 3.2) | 2.2 (1.3, 3.7) | 0.74 |
| HDL (mmol l−1)† | 1.4 (1.2, 1.7) | 1.4 (1.1, 1.7) | 0.79 |
| LDL (mmol l−1) | 3.0 (2.6, 3.4) | 3.1 (2.6, 3.6) | 0.74 |
Abbreviations: ALT, alanine transaminase; FSH, follicle stimulating hormone; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment for insulin resistance; LDL, low-density lipoprotein; LH, luteinizing hormone; and SHBG, sex hormone binding globulin. Data is presented as mean (95% CI).
Significantly different (P < 0.05).
Variables analysed following logarithmic transformation.
Women with PCOS vs. control women: incremental heating
In response to local heating, the %CVCmax increased steadily at microdialysis sites infused with both saline and l-NMMA in all participants (P < 0.001; Fig. 2). However, the site infused with l-NMMA displayed a reduced %CVCmax in both women with PCOS and control women (P = 0.001; Fig. 2), suggesting that the cutaneous response to local heating in both groups was, at least in part, mediated by the NO dilator system. A significant microdialysis site × temperature interaction was evident (P < 0.001; Fig. 2), and pairwise comparisons revealed significant differences between the saline and l-NMMA site from 39 to 42°C in women with PCOS and from 40 to 42°C in control women (P < 0.05).
Figure 2. Cutaneous blood flow during incremental heating in PCOS (n= 11; A) and control women (n= 6; B) and the contribution of NO in women with PCOS and control women (C).
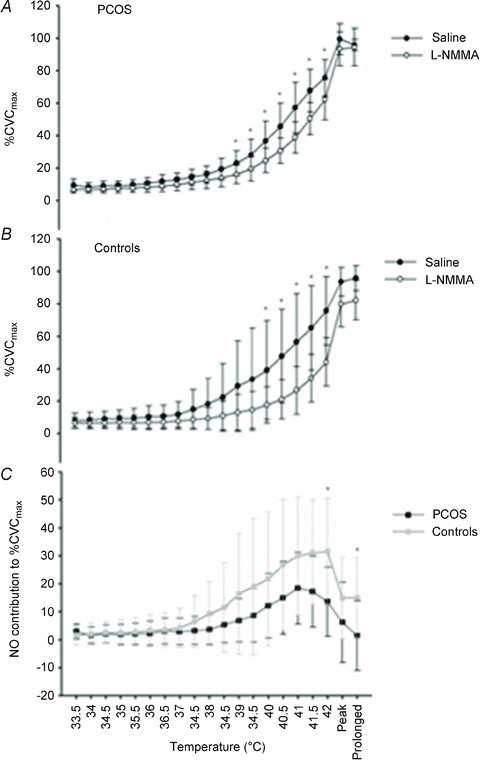
Data are presented as means ± SD. *Significant difference (P < 0.05). The effect of blockade of NO with NG-monomethyl-l-arginine (l-NMMA) on cutaneous blood flow during incremental heating, at ‘peak’ (5 min at 42°C) and ‘prolonged’ heating (30 min at 42°C) in women with polycystic ovary syndrome (PCOS; n = 11; A) and control women (n = 6; B), analysed using a repeated-measures two-factor (site × temperature) general linear model, and the contribution of NO [saline percentage of maximal cutaneous vascular conductance (%CVCmax) minus l-NMMA %CVCmax] in women with PCOS and control women (C), analysed using a repeated-measures two-factor (group × temperature) general linear model. Data are presented as means ± SD. *Significant difference (P < 0.05) between l-NMMA and saline sites (A and B) and between women with PCOS and control women (C), identified using pairwise comparisons.
Women with PCOS vs. control women: contribution of NO to incremental heating
There was a significant group × temperature interaction between women with PCOS and control women (P = 0.01, Fig. 2). Subsequent pairwise comparisons revealed that cutaneous NO-mediated vasodilatation was attenuated at 42°C [17.5%CVCmax (95% CI, 33.3, 1.7); P = 0.03] and subsequently when the heating stimulus remained at 42°C for both 5 min [16.0%CVCmax (95% CI, 32.5, –0.6); P = 0.07] and 30 min [15.4%CVCmax (95% CI, 29.6, 1.3); P = 0.04; Fig. 2] in women with PCOS vs. control women.
Impact of exercise training in women with PCOS: clinical characteristics
Women with PCOS demonstrated 90% compliance to exercise sessions. Body mass index and biochemical parameters were similar following the exercise intervention (P > 0.05; Table 2). The exercise intervention increased cardiorespiratory capacity by 5.0 ml kg−1 min−1 (95% CI, 0.9, 9.2; P = 0.03; Table 2).
Table 2.
Changes in the characteristics of women with PCOS following supervised exercise training (n = 6)
| Characteristic | Pre-exercise | Postexercise | Change | P value |
|---|---|---|---|---|
| Body mass index (kg m−2) | 31 (25, 37) | 30 (24, 37) | −0.3 (−1.6, 1.0) | 0.54 |
| Weight (kg) | 79.6 (65.5, 93.7) | 78.8 (64.3, 93.2) | −0.8 (−4.2, 2.6) | 0.56 |
| Waist circumference (cm) | 100 (84, 117) | 96 (77, 116) | −3.8 (−10.4, 2.8) | 0.20 |
| Systolic blood pressure (mmHg) | 118 (105, 130) | 113 (104, 121) | 5.8 (−14.9, 3.2) | 0.16 |
| Diastolic blood pressure (mmHg) | 72 (62, 82) | 67 (60, 74) | −5.3 (−15.5, 4.8) | 0.24 |
| Heart rate (beats min−1) | 71 (63, 79) | 66 (60, 71) | −5 (−13, 2) | 0.14 |
| Peak oxygen uptake (ml kg−1 min−1) | 27.1 (21.6, 32.5) | 32.1 (24.0, 40.2) | 5.0 (0.9, 9.2) | 0.03* |
| FSH (IU l−1)† | 5.6 (2.8, 8.4) | 5.3 (1.8, 8.8) | −0.3 (−3.1, 2.5) | 0.75 |
| LH (IU l−1)† | 11.9 (2.2, 21.6) | 9.6 (2.2, 17.0) | −0.4 (−6.9, 2.2) | 0.22 |
| Progesterone (nmol l−1) | 2.5 (1.6, 3.4) | 1.3 (0.5, 2.0) | −1.3 (−2.8, 0.3) | 0.08 |
| Oestradiol (pmol l−1) | 232 (126, 337) | 197 (129, 264) | −35 (−133, 63) | 0.34 |
| Testosterone (nmol l−1) | 2.5 (1.7, 3.3) | 2.4 (1.6, 3.2) | −0.1 (−0.7, 0.5) | 0.64 |
| Free androgen index (%)† | 8.5 (6.2, 10.7) | 9.2 (7.0, 11.5) | 0.7 (−1.2, 2.8) | 0.15 |
| SHBG (nmol l−1) | 29.0 (21.7, 36.3) | 24.5 (17.4, 33.6) | −4.4 (12.3, 7.5) | 0.51 |
| ALT (IU l−1)† | 38.5 (10.9, 67.9) | 41.8 (17.9, 61.4) | 3.3 (−18.1, 24.6) | 0.66 |
| Glucose (mmol l−1) | 4.5 (4.1, 4.9) | 4.8 (4.4, 5.1) | 0.3 (−0.4, 0.9) | 0.32 |
| Insulin (pmol l−1)† | 17.1 (8.7, 25.5) | 23.9 (12.8, 35.1) | 6.8 (−6.4, 19.9) | 0.24 |
| HOMA-IR† | 3.5 (0.6, 5.5) | 4.0 (0.3, 7.7) | 0.6 (−0.6, 4.8) | 0.80 |
| Cholesterol (mmol l−1) | 4.8 (4.4, 5.3) | 4.6 (4.1, 5.1) | −0.2 (−1.0, 0.6) | 0.43 |
| Triglyceride (mmol l−1) | 1.3 (0.1, 2.5) | 1.5 (0.1, 2.8) | 0.2 (−0.4, 0.7) | 0.44 |
| HDL (mmol l−1) | 1.3 (0.8, 1.8) | 1.2 (0.4, 1.0) | −0.1 (−0.4, 0.2) | 0.35 |
| LDL (mmol l−1) | 3.1 (2.5, 3.6) | 2.7 (2.4, 3.0) | −0.3 (−1.0, 0.4) | 0.23 |
Abbreviations are as for Table 1. Data are presented as mean (95% CI).
Significantly different (P < 0.05).
Variables analysed following logarithmic transformation.
Impact of exercise training in women with PCOS: incremental heating
Prior to and following exercise training, cutaneous blood flow increased in response to local heating in both the microdialysis site infused with saline and the site infused with l-NMMA (P < 0.001; Fig. 3), suggesting that the cutaneous response to local heating was, at least in part, mediated by the NO dilator system. There was a significant interaction (microdialysis site × temperature) pre- and postexercise training (P < 0.001; Fig. 3). Pairwise comparisons revealed differences between sites from 36 to 42°C pre-exercise and from 36.5 to 42°C postexercise (P < 0.05).
Figure 3. Cutaneous blood flow during incremental heating in PCOS pre (n= 6; A) and post-exercise training (n= 6; B) and the contribution of NO pre- and post-exercise training (C).
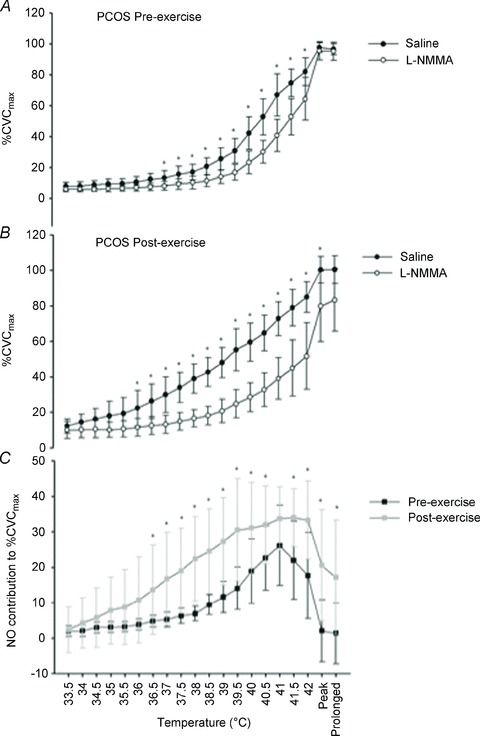
Data are presented as means ± SD. *Significant difference (P < 0.05). The effect of blockade of NO with l-NMMA on cutaneous blood flow during incremental heating, at ‘peak’ (5 min at 42°C) and ‘prolonged’ heating (30 min at 42°C) in women with PCOS pre- (n = 6; A) and postexercise training (n = 6; B), analysed using a repeated-measures two-factor (site × temperature) general linear model. C, the contribution of NO (saline %CVCmax minus l-NMMA %CVCmax) pre- and postexercise training, analysed using a repeated-measures two-factor (training × temperature) general linear model. Data are presented as means ± SD. *Significant difference (P < 0.05) between l-NMMA and saline sites (A and B) and between women with PCOS and control women (C), identified using pairwise comparisons.
Impact of exercise training in women with PCOS: contribution of NO to incremental heating
A significant intervention × temperature interaction was evident in the contribution of NO to the %CVCmax with exercise training (P < 0.001; Fig. 3). The NO contribution to incremental heating was greater postexercise when incremental heating reached 36.5°C up to 42°C [19.4%CVCmax (95% CI, 0.4, 38.4); P = 0.05] and subsequently, when the heating stimulus remained at 42°C for both 5 min [19.6%CVCmax (95% CI, 4.3, 39.4); P = 0.02] and 30 min [17.1%CVCmax (95% CI, 2.2, 32.1); P = 0.03; Fig. 3].
Maximal cutaneous vascular responses during infusion of 56 mm SNP at 42°C
The 30 min infusion of 56 mm SNP combined with local heating at 42°C was similar between the l-NMMA and saline microdialysis sites in women with PCOS [−0.2CVC (95% CI, −0.7, 0.3); P = 0.44] and control women [0.4CVC (95% CI, −1.4, 2.2); P = 0.60] at baseline. Likewise, exercise training did not alter maximal cutaneous conductance in either the l-NMMA [0.4CVC (95% CI, −0.8, 1.6); P = 0.42] or the saline site [0.02CVC (95% CI, −1.1, 1.1); P = 0.97].
Discussion
The novel findings of the present study are that cutaneous NO-mediated microvascular function (assessed in response to local heating) is defective in obese women with PCOS compared with obese control women, and that this is improved by moderate-intensity exercise training. These data suggest that NO-mediated cutaneous microvessel endothelial dysfunction, which potentially contributes to microvessel CVD risk, is evident in obese women with PCOS. These findings also provide evidence for the beneficial effects of exercise training in ameliorating NO-mediated microvascular dysfunction in women with PCOS.
Surprisingly, given the anti-atherogenic roles of NO in the cardiovascular system (Green, 2009), this is the first study to examine the contribution of NO, using a specific antagonist, to microvessel endothelial function in obese women with PCOS. The NO-mediated vasodilator pathway plays a crucial role in the control of cutaneous endothelial function (Giles et al. 2012), and the stimulus of localized heating at 42°C has been reported to elicit the largest contribution of NO (up to 70%) to the vasodilator response (Minson et al. 2001). The optimal approach to assessing microvascular NO function involves the intradermal infusion of a potent NO blocker (Goldsmith et al. 1996), during gradual heating (Cracowski et al. 2006). The present study, using this technique, identified a specific defect, which is that obese women with PCOS display reduced bioavailability of NO, resulting in impaired cutaneous microvessel vasodilatation compared with obese control women. Given that cutaneous microvessel dysfunction is correlated with coronary endothelial dysfunction (Khan et al. 2008) and CVD risk factors such as hypercholesterolaemia (Khan et al. 1999), hypertension (Rizzoni et al. 2003) and type 2 diabetes (Sokolnicki et al. 2007b), it is plausible to suggest that such NO-mediated microvessel dysfunction in PCOS may contribute to increased microvascular disease risk in women with PCOS.
Alexandraki et al. (2006) found no difference in forearm blood flow between overweight women with PCOS and matched control women, measured using strain-gauge venous occlusion plethysmography. This measure of microvascular function relates to whole limb blood flow as opposed to NO-mediated vasodilatation specifically, which may account for the discrepancy with the findings described in the present study. Other previous studies have shown cutaneous microvessel dysfunction in obese women with PCOS in comparison to healthy control women (Lakhani et al. (2005), but these studies have investigated cutaneous function in response to iontophoresis-mediated infusion of ACh. Although the response to iontophoresis of ACh is thought to reflect NO bioavailability, it is also mediated by prostanoids (Khan et al. 1997), with minimal contribution from NO (Holowatz et al. 2005). Moreover, the most recent study using iontophoresis of ACh suggested that microvascular dysfunction in PCOS was explained by obesity rather than PCOS per se, because microvessel vasodilator dysfunction was apparent in both obese women with PCOS and obese control women (Ketel et al. 2008). In the present study, we compared obese women with PCOS and obese control women, and while we observed no differences between them in response to heating stimuli at the saline sites, our NO blockade studies revealed specific impairment in the contribution of NO to increases in skin blood flow in the PCOS group.
Testosterone and free androgen index were elevated in the women with PCOS, and could be proposed as a potential explanation for microvessel dysfunction. Previous research has suggested that testosterone impairs endothelium-dependent vasodilatation in conduit vessels (Herman et al. 1997). In contrast, the impact of testosterone on the cutaneous vessels is unclear. Lakhani et al. (2005) observed microvessel dysfunction in hyperandrogenic women with PCOS, but the impairment remained following statistical adjustment for testosterone. Furthermore, Sokolnicki et al. (2007a) reported that administration of exogenous testosterone did not alter the response of cutaneous microvessels to gradual local heating in older men. We found very little difference in the NO contribution data between the seven women who fulfilled all three criteria for PCOS and the four women who fulfilled two of the three criteria (data not shown). Nevertheless, we acknowledge that the chronic impact of clinical and biochemical hyperandrogenism on cutaneous microvessel function, specifically SHBG and free testosterone, warrants further investigation. An alternative mechanism influencing microvessel dysfunction, not tested in the present study, could involve excess deposition of visceral adipose tissue in PCOS (Jones et al. 2012). Accumulation of visceral adipose tissue is responsible for the upregulation of β-oxidation, leading to oxidative stress and the release of inflammatory cytokines and adipokines, all of which could contribute to NO-mediated endothelial dysfunction (Carmina et al. 2006, 2009). Thus, the influence of low-grade inflammation in NO-mediated dysfunction also warrants further exploration.
This is also the first study to demonstrate that exercise training in PCOS can improve cutaneous NO-mediated microvessel vasodilatation. Our data demonstrate that 16 weeks of moderate-intensity aerobic exercise induced an increase in the contribution of NO to local heating, indicative of an improvement in cutaneous endothelium-dependent function. Our findings also indicate that the exercise-induced upregulation of NO reflects changes in cutaneous microvascular function, independent of changes in maximal vasodilator capacity. These findings may be of clinical relevance, given that NO-mediated microvessel vasodilatation improved (or normalized) following exercise training to a level similar to that of the control obese women, and microvessel function is correlated with coronary endothelial dysfunction (Khan et al. 2008) and a number of microvascular CVD risk factors (Khan et al. 1999; Rizzoni et al. 2003; Sokolnicki et al. 2007b). Specifically, this finding provides support for the utilization of exercise-based lifestyle modification as a first-line preventive strategy in PCOS.
Exercise has previously been reported as an effective intervention to improve cutaneous microvessel function in aged sedentary individuals, in that exercise training induced an increase in NO-mediated cutaneous vasodilatation measured using intradermal microdialysis of l-NAME during local heating (Black et al. 2008). The authors suggested that this upregulation of NO bioavailability may be a function of enhanced cardiorespiratory fitness. Likewise, in the present study the improved NO-mediated cutaneous vasodilatation in women with PCOS paralleled an improvement in cardiorespiratory fitness. An alternative, but not mutually exclusive, mechanistic explanation could be related to the increases in microvascular shear stress associated with repeated bouts of exercise (Green et al. 2010). Indeed, shear stress has been shown to upregulate endothelial nitric oxide synthase production in conduit arteries (Hambrecht et al. 2003), and limiting the increase in shear stress during repeated, localized heating (using cuff inflation on one arm) prevents increases in cutaneous microvascular function (Green et al. 2010) in humans.
The exercise stimulus in the present study did not elicit any changes in clinical characteristics or biochemical parameters, including insulin resistance and body mass index, of women with PCOS, supporting the hypothesis that exercise training had a direct therapeutic effect on the microvascular endothelium (Green et al. 2008). It might be suggested that an exercise-training stimulus of higher intensity might have produced changes in BMI and thus elicited greater improvements in microvascular function. However, Middlebrooke et al. (2006) administered 6 months of relatively high-intensity exercise training (70–80% maximal HR) in type 2 diabetic patients and observed no changes in cutaneous microvessel function in response to either iontophoretically applied ACh or localized heating to 42°C. Despite the different technique and population in that study, these results suggest that high-intensity exercise training may not be a suitable stimulus to enhance cutaneous microvessel function. Indeed, the inefficacy of high-intensity exercise in changing microvascular function may be due to increased oxidative stress during higher intensity exercise, resulting in a reduction in NO bioavailability (Goto et al. 2003).
Some methodological issues warrant discussion. First, previous studies using intradermal microdialysis have infused l-NAME, a more specific NO blocker (Black et al. 2008), rather than l-NMMA. Nevertheless, l-NMMA has been reported to elicit a comparable inhibitory effect of NO-mediated dilatation in a study employing whole body heating (Dietz et al. 1994). Second, the inclusion of a non-exercising PCOS control group, ideally via a randomized controlled allocation method, for comparison would have enabled a direct comparison to conventional clinical care. Third, inclusion of a lean PCOS group for comparison would have allowed extrapolation to lean women. Finally, we also acknowledge that our insulin sensitivity and body composition methods have limitations. An oral glucose tolerance test (Abdul-Ghani et al. 2007) or a two-stage hyperinsulinaemic–euglycaemic clamp, with infusion of deuterated glucose (Shojaee-Moradie et al. 2007), would have given a more comprehensive assessment of insulin sensitivity. Moreover, magnetic resonance imaging or dual-energy X-ray absorptiometry would have provided more detailed information on fat deposition.
Whilst we have shown a role for NO dysfunction in PCOS in the present study, we acknowledge that other pathways contribute to vasodilatation (e.g. prostanoid pathways; Frangos et al. 1985) and vasoconstriction (e.g. endothelin-1; Wenner et al. 2011) of the cutaneous microvasculature function in PCOS. We have focused on the NO pathway, due to its anti-atherogenic effect on the vasculature and prognostic value in the prediction of future cardiovascular events (Green et al. 2011). Moreover, we have found that NO function, and thus potentially CVD risk, can be directly improved with exercise training in this group of women at high risk of CVD. Our findings advocate exercise training as a primary CVD prevention strategy in PCOS and strongly support the guidelines of the Androgen Excess Society (Azziz et al. 2006).
In summary, these data indicate that women with PCOS exhibit reduced bioavailability of NO, compared with obese control women, evidenced by reduced NO-mediated cutaneous vasodilator responses to local heating, and that cutaneous vasodilator function can be improved via the upregulation of the anti-atherogenic molecule NO. These findings argue for the prescription of exercise training as a preventive strategy for NO-mediated microvascular dysfunction in women with PCOS.
Acknowledgments
We would like to express gratitude to Terry Owen, the Aintree Volunteers and the medical research charity, Weight Matters, for funding support.
Glossary
- ALT
alanine transaminase
- CVC
cutaneous vascular conductance
- CVD
cardiovascular disease
- FSH
follicle stimulating hormone
- HDL
high-density lipoprotein
- HOMA-IR
homeostasis model assessment for insulin resistance
- HR
heart rate
- HRR
heart rate reserve
- LDL
low-density lipoprotein
- LH
luteinizing hormone
- l-NMMA
NG-monomethyl-l-arginine
- LSD
least significant difference
- NO
nitric oxide
- PCOS
polycystic ovary syndrome
- PU
arbitrary perfusion units
- SHBG
sex hormone binding globulin
- SNP
sodium nitroprusside
Author contributions
V.S.S. and C.J.A.P. conducted all exercise training, data collection, and analysis and significantly contributed to writing/revising the manuscript. H.J. together with D.J.C. and N.T.C. contributed to the conception of the project and experimental design. H.J. contributed to all aspects of the research study and manuscript preparation. D.J.C. made critical revisions to the manuscript during preparation. N.T.C. contributed to the intellectual content of the manuscript specifically in relation to exercise and microvascular physiology. C.D. made critical revisions to the manuscript prior to submission. G.A. provided invaluable contributions to the study design and statistical analysis of data. N.A. led recruitment of patients by setting up contact during clinic hours and contributed to the intellectual content of the project and manuscript revision. G.J.K. provided significant contribution in relation to the interpretation of data and revision of the manuscript. D.J.G. introduced the microdialysis technique to the group and advised on the manner of data analysis and interpretation with respect to simultaneous NO blockade and saline infusion. All authors approved this version of the manuscript.
References
- Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care. 2007;30:89–94. doi: 10.2337/dc06-1519. [DOI] [PubMed] [Google Scholar]
- Alexandraki K, Protogerou AD, Papaioannou TG, Piperi C, Mastorakos G, Lekakis J, Panidis D, Diamanti-Kandarakis E. Early microvascular and macrovascular dysfunction is not accompanied by structural arterial injury in polycystic ovary syndrome. Hormones (Athens) 2006;5:126–136. doi: 10.14310/horm.2002.11176. [DOI] [PubMed] [Google Scholar]
- Atkinson G, Batterham AM. The use of ratios and percentage changes in sports medicine: time for a rethink. Int J Sports Med. 2012;33:505–506. doi: 10.1055/s-0032-1316355. [DOI] [PubMed] [Google Scholar]
- Atkinson G, Pugh C, Scott MA. Exploring data distribution prior to analysis: benefits and pitfalls. Int J Sports Med. 2010;31:841–842. doi: 10.1055/s-0030-1268491. [DOI] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF Androgen Excess Society. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- Black MA, Green DJ, Cable NT. Exercise prevents age-related decline in nitric-oxide-mediated vasodilator function in cutaneous microvessels. J Physiol. 2008;586:3511–3524. doi: 10.1113/jphysiol.2008.153742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G. Borg's Perceived Exertion and Pain Scales. Champaign, Illinois: Human Kinetics Publishers; 1998. [Google Scholar]
- Broekmans FJ, Knauff EA, Valkenburg O, Laven JS, Eijkemans MJ, Fauser BC. PCOS according to the Rotterdam consensus criteria: change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG. 2006;113:1210–1217. doi: 10.1111/j.1471-0528.2006.01008.x. [DOI] [PubMed] [Google Scholar]
- Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- Burkhalter N. Evaluation of Borg's perceived exertion scale in cardiac rehabilitation. [Article in Portuguese.] Rev Lat Am Enfermagem. 1996;4:65–73. doi: 10.1590/s0104-11691996000300006. [DOI] [PubMed] [Google Scholar]
- Carmina E, Bucchieri S, Mansueto P, Rini G, Ferin M, Lobo RA. Circulating levels of adipose products and differences in fat distribution in the ovulatory and anovulatory phenotypes of polycystic ovary syndrome. Fertil Steril. 2009;91:1332–1335. doi: 10.1016/j.fertnstert.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Carmina E, Orio F, Palomba S, Longo RA, Cascella T, Colao A, Lombardi G, Rini GB, Lobo RA. Endothelial dysfunction in PCOS: role of obesity and adipose hormones. Am J Med. 2006;119:356.e1–356.e6. doi: 10.1016/j.amjmed.2005.10.059. [DOI] [PubMed] [Google Scholar]
- Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci. 2006;27:503–508. doi: 10.1016/j.tips.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Dietz NM, Rivera JM, Warner DO, Joyner MJ. Is nitric oxide involved in cutaneous vasodilation during body heating in humans. J Appl Physiol. 1994;76:2047–2053. doi: 10.1152/jappl.1994.76.5.2047. [DOI] [PubMed] [Google Scholar]
- Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- Giles TD, Sander GE, Nossaman BD, Kadowitz PJ. Impaired vasodilation in the pathogenesis of hypertension: focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins. J Clin Hypertens (Greenwich) 2012;14:198–205. doi: 10.1111/j.1751-7176.2012.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith PC, Leslie TA, Hayes NA, Levell NJ, Dowd PM, Foreman JC. Inhibitors of nitric oxide synthase in human skin. J Invest Dermatol. 1996;106:113–118. doi: 10.1111/1523-1747.ep12328204. [DOI] [PubMed] [Google Scholar]
- Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 2003;108:530–535. doi: 10.1161/01.CIR.0000080893.55729.28. [DOI] [PubMed] [Google Scholar]
- Green DJ. Exercise training as vascular medicine: direct impacts on the vasculature in humans. Exerc Sport Sci Rev. 2009;37:196–202. doi: 10.1097/JES.0b013e3181b7b6e3. [DOI] [PubMed] [Google Scholar]
- Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DH, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol. 2010;588:1571–1577. doi: 10.1113/jphysiol.2010.186965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter. Hypertension. 2011;57:363–369. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
- Green DJ, O’Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise. J Appl Physiol. 2008;105:766–768. doi: 10.1152/japplphysiol.01028.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- Herman SM, Robinson JT, McCredie RJ, Adams MR, Boyer MJ, Celermajer DS. Androgen deprivation is associated with enhanced endothelium-dependent dilatation in adult men. Arterioscler Thromb Vasc Biol. 1997;17:2004–2009. doi: 10.1161/01.atv.17.10.2004. [DOI] [PubMed] [Google Scholar]
- Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol. 2005;563:965–973. doi: 10.1113/jphysiol.2004.080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol. 2008;105:370–372. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol. 2006;572:811–820. doi: 10.1113/jphysiol.2005.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H, Sprung VS, Pugh CJ, Daousi C, Irwin A, Aziz N, Adams VL, Thomas EL, Bell JD, Kemp GJ, Cuthbertson DJ. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97:3709–3716. doi: 10.1210/jc.2012-1382. [DOI] [PubMed] [Google Scholar]
- Ketel IJ, Stehouwer CD, Serné EH, Korsen TJ, Hompes PG, Smulders YM, de Jongh RT, Homburg R, Lambalk CB. Obese but not normal-weight women with polycystic ovary syndrome are characterized by metabolic and microvascular insulin resistance. J Clin Endocrinol Metab. 2008;93:3365–3372. doi: 10.1210/jc.2008-0626. [DOI] [PubMed] [Google Scholar]
- Khan F, Davidson NC, Littleford RC, Litchfield SJ, Struthers AD, Belch JJ. Cutaneous vascular responses to acetylcholine are mediated by a prostanoid-dependent mechanism in man. Vasc Med. 1997;2:82–86. doi: 10.1177/1358863X9700200202. [DOI] [PubMed] [Google Scholar]
- Khan F, Litchfield SJ, Stonebridge PA, Belch JJ. Lipid-lowering and skin vascular responses in patients with hypercholesterolaemia and peripheral arterial obstructive disease. Vasc Med. 1999;4:233–238. doi: 10.1177/1358836X9900400405. [DOI] [PubMed] [Google Scholar]
- Khan F, Patterson D, Belch JJ, Hirata K, Lang CC. Relationship between peripheral and coronary function using laser Doppler imaging and transthoracic echocardiography. Clin Sci (Lond) 2008;115:295–300. doi: 10.1042/CS20070431. [DOI] [PubMed] [Google Scholar]
- Lakhani K, Leonard A, Seifalian AM, Hardiman P. Microvascular dysfunction in women with polycystic ovary syndrome. Hum Reprod. 2005;20:3219–3224. doi: 10.1093/humrep/dei199. [DOI] [PubMed] [Google Scholar]
- Mathur RS, Moody LO, Landgrebe S, Williamson HO. Plasma androgens and sex hormone-binding globulin in the evaluation of hirsute females. Fertil Steril. 1981;35:29–35. doi: 10.1016/s0015-0282(16)45254-4. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Middlebrooke AR, Elston LM, Macleod KM, Mawson DM, Ball CI, Shore AC, Tooke JE. Six months of aerobic exercise does not improve microvascular function in type 2 diabetes mellitus. Diabetologia. 2006;49:2263–2271. doi: 10.1007/s00125-006-0361-x. [DOI] [PubMed] [Google Scholar]
- Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93:1644–1649. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16:347–363. doi: 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Shojaee-Moradie F, Baynes KC, Pentecost C, Bell JD, Thomas EL, Jackson NC, Stolinski M, Whyte M, Lovell D, Bowes SB, Gibney J, Jones RH, Umpleby AM. Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose metabolism. Diabetologia. 2007;50:404–413. doi: 10.1007/s00125-006-0498-7. [DOI] [PubMed] [Google Scholar]
- Simmons GH, Wong BJ, Holowatz LA, Kenney WL. Changes in the control of skin blood flow with exercise training: where do cutaneous vascular adaptations fit in. Exp Physiol. 2011;96:822–828. doi: 10.1113/expphysiol.2010.056176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolnicki LA, Khosla S, Charkoudian N. Effects of testosterone and estradiol on cutaneous vasodilation during local warming in older men. Am J Physiol Endocrinol Metab. 2007a;293:E1426–E1429. doi: 10.1152/ajpendo.00535.2007. [DOI] [PubMed] [Google Scholar]
- Sokolnicki LA, Roberts SK, Wilkins BW, Basu A, Charkoudian N. Contribution of nitric oxide to cutaneous microvascular dilation in individuals with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2007b;292:E314–E318. doi: 10.1152/ajpendo.00365.2006. [DOI] [PubMed] [Google Scholar]
- Sprung VS, Atkinson G, Cuthbertson DJ, Pugh CJ, Aziz N, Green DJ, Cable NT, Jones H. Endothelial function measured using flow mediated dilation in polycystic ovarian syndrome: a meta-analysis of the observational studies. Clin Endocrinol (Oxf) 2013;78:438–446. doi: 10.1111/j.1365-2265.2012.04490.x. [DOI] [PubMed] [Google Scholar]
- Wenner MM, Taylor HS, Stachenfeld NS. Endothelin B receptor contribution to peripheral microvascular function in women with polycystic ovary syndrome. J Physiol. 2011;589:4671–4679. doi: 10.1113/jphysiol.2011.216218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95:2038–2049. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]


