Abstract
The aim of the present study was to examine fibre type-specific Na+–K+ pump subunit expression and exercise-induced alterations in phospholemman (FXYD1) phosphorylation in humans. Segments of human skeletal muscle fibres were dissected and fibre typed, and protein expression was determined by Western blotting. The protein expression of the Na+–K+ pump α2 isoform was lower in type I than in type II fibres (0.63 ± 0.04 a.u. vs. 1.00 ± 0.07 a.u., P < 0.001), while protein expression of the Na+–K+ pump α1 and β1 isoforms was not different. Protein expression of the ATP-dependent potassium channel Kir6.2 was higher in type I compared with type II fibres. In both type I (P < 0.01) and type II fibres (P < 0.001) the AB_FXYD1 signal was lower after exercise compared with rest, indicating an increase in unspecific FXYD1 phosphorylation. The FXYD1 serine 68 phosphorylation was higher (P < 0.001) after exercise compared with rest in type II fibres (1.90 ± 0.25 vs. 1.00 ± 0.08) and not changed in type I fibres. Total FXYD1 was not expressed in a fibre type-specific manner. Expression of phosphofructokinase was lower (P < 0.001) in type I than in type II fibres, whereas citrate synthase and 3-hydroxyacyl-CoA dehydrogenase were more abundant (P < 0.001) in type I fibres. In conclusion, FXYD1 phosphorylation at serine 68 increased after an acute bout of intense exercise in human type II fibres, while AB_FXYD1 signal intensity was lower in both type I and type II fibres, indicating fibre type-specific differences in FXYD1 phosphorylation on serine 63, serine 68 and threonine 69. This, together with the observation of a higher abundance of the Na+–K+ pump α2 isoform protein in type II fibres, is likely to have importance for the exercise-induced human Na+–K+ pump activity in the different fibre types.
Key points
Most human experiments examine proteins at the whole muscle level, and knowledge of fibre type specificity is mainly obtained in animal muscles. We used a new methodology and provide novel findings at the single fibre level.
Potassium changes across muscle cell membranes may be important for development of fatigue during exercise. The Na+–K+ pump with the regulatory phospholemman (FXYD1) subunit and the ATP-dependent K+ channel Kir6.2 play crucial roles in potassium regulation.
We show that in humans Na+–K+ pump α2 is expressed to a greater extent in type II than in type I fibres and Kir6.2 is expressed primarily in type I fibres. During exercise, phosphorylation of FXYD1 serine 68 is increased only in type II fibres, indicating differences in Na+–K+ pump activity.
These results show that human Kir6.2 and Na+–K+ pump subunit expression and FXYD1 phosphorylation are fibre type specific, which may influence exercise-induced potassium regulation and fatigue development.
Introduction
Skeletal muscle is heterogeneous in nature, being comprised of fast-twitch (type II) fibres and slow-twitch (type I) fibres, which show different contractile and metabolic properties. The most commonly utilised muscle in human studies is the vastus lateralis, and the proportion of type I and type II fibres in this muscle in a given individual is dictated by activity status, genetic composition, health status and age (Saltin et al. 1977). Consequently, when examining a protein with a fibre type-specific profile (Murphy, 2011), biochemical analyses of mixed human skeletal muscle samples are often confounded by the heterogeneous nature of the muscle at the cellular level. Added to this complexity is the possibility that specific proteins will respond to exercise in both a contraction-type- and a fibre type-dependent manner (Tannerstedt et al. 2009). Thus, it is fundamental to undertake studies examining human skeletal muscle at the level of individual muscle fibres.
Accumulation of interstitial potassium during exercise impairs muscle cell function and is likely to contribute to the complex mechanisms involved in muscular fatigue development during intense exercise in humans (Sejersted & Sjøgaard, 2000; McKenna et al. 2008). In skeletal muscle, the ion-transporting sodium–potassium ATPase (Na+–K+ pump), consisting of an α-subunit and a β-subunit as well as an associated and regulative third subunit named phospholemman (FXYD1) in skeletal muscles, plays a crucial role in the regulation of potassium homeostasis during exercise (Nielsen & Clausen, 2000). Thus, the expression of the Na+–K+ pump subunits and activity of the pump in human skeletal muscles may be important for the complex regulation of muscle cell excitability during exercise. In rodent skeletal muscles, Na+–K+ pump α1 and β1 isoforms are more highly expressed in the type I-dominated soleus muscles than in the type II-dominated extensor digitorum longus (EDL) and white gastrocnemius muscles, while Na+–K+ pump α2 isoforms are more evenly distributed or slightly more highly expressed in type II-dominated muscles (Fowles et al. 2004; Kristensen & Juel, 2010; Ingwersen et al. 2011). In light of the rodent data, a likely, but currently unexplored, fibre type dependence of the various Na+–K+ pump subunits in human skeletal muscle might contribute to discrepancies in training adaptations between various human studies. Furthermore, the ATP-sensitive inwardly rectifying potassium channel 11 (Kir6.2) may be important for potassium regulation (Nielsen et al. 2003) and is primarily expressed in sarcolemmal giant vesicles prepared from rat glycolytic compared with oxidative muscles (Kristensen & Juel, 2010).
The degree of FXYD1 phosphorylation has been proposed to regulate Na+–K+ pump activity in muscles (Ingwersen et al. 2011), and phosphorylation at FXYD1 Ser63 and Ser68 has been shown to increase during acute exercise in human skeletal muscle (Benziane et al. 2011). However, electrical stimulation of mouse muscle resulted in an increase in FXYD1 Ser68 phosphorylation in predominantly slow-twitch soleus muscles but a decrease in fast-twitch EDL muscles (Thomassen et al. 2011), indicating differences between type I and type II fibres. Furthermore, treadmill running induced fibre type-specific changes in both Na+ affinity and maximal in vitro Na+–K+ pump activity in rat skeletal muscles (Juel, 2009). Thus, these findings suggest that the Na+–K+ pump activity is regulated differently in type I and type II muscle fibres. Given that the effect of acute exercise on alterations in FXYD1 phosphorylation has never been examined in individual fibres collected from human skeletal muscle samples, it is unclear whether exercise-induced FXYD1 phosphorylation is fibre type specific in human skeletal muscles.
Thus, the aim of the present study was to examine the expression of Kir6.2 and the Na+–K+ pump subunits as well as the exercise-induced fibre type-specific alterations in FXYD1 phosphorylation determined in human skeletal type I and type II muscle fibres. Protein expression of Na+–K+ pump α1 and β1 isoforms is hypothesized to be higher in type I than in type II fibres, while the α2 isoform and Kir6.2 are expressed to a greater extent in type II fibres. In addition, unspecific FXYD1 phosphorylation is hypothesized to increase in both fibre types during intense exercise, whereas FXYD1 Ser68 phosphorylation increases in type I and decreases in type II muscle fibres.
Methods
Subjects
Six healthy recreationally active male subjects with an age, height, weight and maximal pulmonary oxygen uptake of 26.8 ± 5.4 years, 186.5 ± 8.0 cm, 77.6 ± 5.8 kg and 56.7 ± 5.9 ml O2 min−1 kg−1 (means ± SD), respectively, participated in the experiment after giving informed consent. The study conformed to the World Medical Association code of Ethics (Declaration of Helsinki), and the study protocol was approved by the Ethics Committee of Copenhagen and Frederiksberg communities.
Exercise protocol
The subjects were instructed to consume a standardized breakfast meal 1.5 h before arrival at the laboratory in the morning at 10.00 h. After resting for 30 min, the subjects performed a 5 min intense bout of exercise (295 ± 17 W, corresponding to 95 ± 2% of maximal pulmonary oxygen uptake) on a cycle ergometer. Muscle samples were collected at rest and immediately after exercise from the vastus lateralis muscle under local anaesthesia (xylocaine 20 mg ml−1; AstraZeneca, Copenhagen, Denmark) using the needle biopsy technique modified with suction. The muscle tissues were immediately frozen in liquid nitrogen and stored at −80°C until further analysis.
Analysis
Segments of human single muscle fibres
After freeze drying the muscle tissue samples (30–40 mg wet weight, n = 12) for 48 h, segments of single fibres were dissected under a microscope and stored in single microfuge tubes. The average size of the segments collected was roughly determined by measuring the length of the fibres under a microscope (1.5 ± 0.4 mm, mean ± SD, n = 342). Before SDS-PAGE, 13–20 μl of 6× Laemmli buffer (0.7 ml of 0.5 m Tris base, 3 ml glycerol, 0.93 g DTT, 1 g SDS and 1.2 mg Bromophenol Blue) diluted (1:3, v:v) in double distilled H2O (ddH2O) was added to each fibre and incubated for 1 h at room temperature.
Mixed muscle homogenate for standard curves
In order to prepare a sample of mixed fibre type for use as a standard curve, 25–50 mg wet weight of muscle from different samples was freeze dried and homogenized as previously described (Thomassen et al. 2010). Lysates from the different samples were then pooled before the total protein concentration was determined using bovine serum albumin (BSA) standards (Pierce, Rockford, IL, USA). The pooled lysate was diluted in 6× Laemmli buffer and ddH2O. For each gel loaded, a standard curve in the range 0.25–3 μg of total protein was included (Mollica et al. 2009).
Western blotting
Individual fibre segments were loaded onto 26-well Criterion gels (either 4–15% Tris-HCL or 16.5% Tris-Tricine; Bio-Rad Laboratories, Hercules, CA, USA). Proteins were separated by SDS-PAGE (55 mA per gel and a maximum of 150 V) for ∼120 min and then semi-dry transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore A/S, Copenhagen, Denmark) for 120 min at 70 mA per gel and a maximum of 25 V. After protein transfer, gels were incubated for 2 h in Coomassie stain, including 0.3% Coomassie Brilliant Blue R (Sigma-Aldrich), 40% ethanol (96%), 10% acetic acid (Merck) and 49.7% ddH2O. Gels were then destained in ddH2O overnight and imaged using a Kodak Image Station 2000MM (Kodak, Copenhagen, Denmark) or a ChemiDoc MP Imaging System (Bio-Rad Laboratories). These Coomassie-stained post-transferred gels were used to obtain an indication of the amount of protein in each lane, based on the myosin heavy chain band (MHC; ∼200 kDa) and the actin band (∼42 kDa; Murphy et al. 2006). Membranes were blocked in Tris-buffered saline including 0.1% Tween-20 (TBST) with either 2% skimmed milk or 3% BSA for 1 h and then incubated with primary antibodies overnight. After two washes in TBST, horseradish peroxidase-conjugated secondary antibody (DAKO, Copenhagen, Denmark) diluted 1:5000 in TBST with either 2% skimmed milk or 3% BSA was added, following which the membrane was washed in TBST (three times, for 15 min each). Bands were then visualized using chemiluminescent detection (Super Signal West Femto Maximum Sensitivity Substrate; Thermo Scientific, Rockford, IL, USA), and images were collected on a Kodak Image Station 2000MM or a ChemiDoc MP Imaging System. For further analyses, the membrane was kept in TBST and re-incubated in a new primary antibody overnight, giving the opportunity to determine the expression of several proteins with different molecular weights on the same segment of fibres (Murphy, 2011).
For determination of FXYD1 Ser68 phosphorylation by AB_FXYD1ser68 (∼12 kDa) and citrate synthase (CS; ∼48 kDa), the samples were loaded onto the 16.5% gels. It was necessary to use these gels to obtain the best separation of 5–20 kDa proteins, specifically because AB_FXYD1ser68 detected a non-specific band at <10 kDa, probably recognizing phospholamban (James et al. 1989). These gels were not ideal for detection of the larger molecular protein, MHC, required for fibre type identification and so a subset of longer fibre segments were selected (1.9 ± 0.3 mm, n = 90). Of this subset of fibres about one-third (7 μl) of a given fibre segment was loaded on a 4–15% gradient gel for fibre typing of the samples using antibodies targeting MHCI, MHCII and sarco(endo)plasmic reticulum Ca2+-ATPase isoform 1 (SERCA1) proteins. The remaining approximately two-thirds (13 μl) of the given fibre segments were then loaded onto 16.5% gels for FXYD1 Ser68 and CS detection.
Antibody details
The single fibre segments were characterized by Western blotting as either slow-twitch type I or fast-twitch type II by use of antibodies specific for MHCI and MHCII as well as the type II-specific SERCA1 protein (Murphy, 2011). Antibodies used were as follows: MHC type I, 0.5 μg ml−1, mouse monoclonal IgM, A4.840 (Developmental Studies Hybridoma Bank (DSHB), University of Iowa, USA); MHC type II, 2 μg ml−1, mouse monoclonal IgG, A4.74 (DSHB), both developed by Dr Blau; and SERCA1, 0.1 μg ml−1, mouse monoclonal MA3-912 (Thermo Scientific). Other antibodies used for determination of protein expression were as follows: Na+–K+ pump α1 isoform, 0.47 μg ml−1, mouse monoclonal, α6F, (DSHB), developed by Dr D. Fambrough; Na+–K+ pump α2 isoform, ∼5 μg ml−1, rabbit polyclonal, 07-674 (Millipore); Na+–K+ pump β1 isoform, 1 μg ml−1, mouse monoclonal, MA3-930 (Thermo Scientific); Kir6.2, 0.4 μg ml−1, goat polyclonal, Sc11228 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); phosphofructokinase (PFK), 0.2 μg ml−1, mouse monoclonal, Sc166722 (Santa Cruz Biotechnology,); CS, 1 μg ml−1, rabbit polyclonal, ab96600 (Abcam, Cambridge, UK); 3-hydroxyacyl-CoA dehydrogenase (HAD), 0.5 μg ml−1, rabbit polyclonal, ab54477 (Abcam); total FXYD1, 0.14 μg ml−1, rabbit polyclonal, 13721-1-AP (Proteintech Group, Chicago, IL, USA); unphosphorylated FXYD1, rabbit polyclonal, AB_FXYD1 (originally denoted C2), kindly donated by Dr. J. Randall Moorman (University of Virginia, USA); FXYD1 phosphorylated at ser68, rabbit polyclonal, AB_FXYD1ser68 (originally denoted CP68), kindly donated by Dr D. Bers (Loyola University, Chicago, IL, USA) (Rembold et al. 2005; Thomassen et al. 2011).
Data treatment
In total, 342 segments of human skeletal muscle single fibres were analysed. On each gel, nine fibres from a resting muscle and nine fibres from an exercised muscle from the same individual were loaded, but with unknown fibre type, leading to an unequal number of fibres in each group. Given the small size of segments of individual fibres, it was not possible also to determine the total protein concentration in each sample prior to sample loading. Consequently, different amounts of protein were loaded into each well. In order to compare the specific protein expression between fibre samples, densities of the proteins of interest were normalized to MHC and/or actin on the post-transferred gel (Fig. 1). The standard curve regression on the post-transferred Coomassie-stained gel was not different from that for the same standard curve on the membrane (Fig. 1), and it was deemed that an equal proportion of total protein was transferred to the membrane independent of the total amount loaded. Thus, the signal on the post-transferred Coomassie-stained gel was used for normalization, as previously demonstrated to be a reliable measure (Murphy et al. 2006; Murphy, 2011).
Figure 1. Equal protein transfer documented.
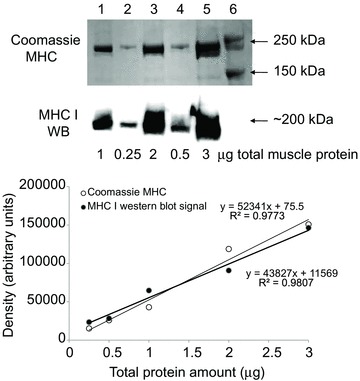
In order to compare the slopes of a standard curve based on Coomassie-stained myosin heavy chain (MHC) signals on the post-transferred gel (mean value of the five bands = 70,736 a.u.; upper image) and a standard curve based on MHCI signals on the Western blot membrane (mean value of the five bands = 784,843 a.u.; lower image), the standard curve is transferred by dividing the individual values with the ratio between the two mean values. Lanes 1–5 contain 1, 0.25, 2, 0.5 and 3 μg total protein of mixed human skeletal muscles, respectively, and lane 6 contains a protein marker (Precision Plus Protein Standards Dual Color, Bio-Rad). The thin tendency line and equation represent the post-transferred gel Coomassie MHC signals (open circles). The tendency line and equation in bold represent the transferred MHCI signals on the Western blot membrane (filled circles).
The Coomassie signal from each sample was first normalized to the gel mean and then used for normalization for the protein of interest. Fibres with a Coomassie signal level less than 0.4 of the gel mean (n = 8) were excluded owing to concerns about linearity in the lower end of the standard curves. Further fibres were excluded from the data set if a clear band was found when both MHCI and SERCA1 antibodies were used, indicating a fibre type coexpression or several fibres in the same tube (n = 19), or if fibres did not show any signal with either the MHCI or MHCII antibody but there was a clear band with SERCA1 (probably type IIx fibres; n = 15). On occasion (n = 9), the fibre visualized under the dissecting microscope was not detected by Western blotting, presumably indicating that the fibre was not successfully placed into the microfuge tube during collection (see lane 5 of Fig. 2A).
Figure 2. Fibre typing of segments of individual human skeletal muscle fibres.
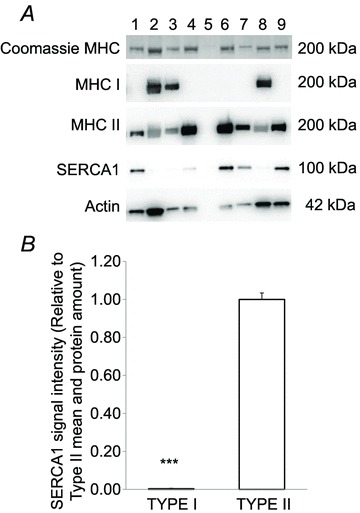
In A, lanes 2, 3 and 8 contain type I fibres; lanes 1, 4, 6, 7 and 9 contain type II fibres; and lane 5 is an example of an empty sample. B, fibre type-specific differences in sarco(endo)plasmic reticulum Ca2+-ATPase isoform 1 (SERCA1) protein expression (type I, n = 49 and type II, n = 52) were determined by Western blotting on segments of human single muscle fibres. Student's unpaired t test was performed to detect the differences. ***Significant difference (P < 0.001) between protein expression in type I and type II fibres. Data are expressed as means + SEM.
In total, segments from 144 type I fibres and 147 type II fibres were analysed, and 51 of 342 fibres were excluded. Overall, the fibres collected comprised the following: 48 type I at rest; 88 type II at rest; 96 type I after exercise and 59 type II after exercise.
The signal intensity for each protein of interest was first normalized to the mean intensity of all single human fibre bands for that protein on the gel. Afterwards, data were normalized to the total amount of protein in each sample determined by Coomassie staining of the remaining MHC or actin, in the case of AB_FXYD1ser68 and CS, which were determined on 16.5% gels. In order to compare fibres loaded on different gels, single values were finally normalized to either the mean of type II at rest (FXYD1 phosphorylation changes) or the mean of all type II fibres (differences in total protein expression).
Statistics
Changes in total protein expression were not expected to occur in response to the acute bout of exercise; thus, for determination of total protein expression, all type I and all type II fibres, respectively, were pooled independently of exercise. Fibre type-specific differences in total protein expression were determined by Student's unpaired t test (Murphy, 2011).
To determine changes in FXYD1 phosphorylation in the segments of human single fibres, a two-way ANOVA general linear model (SigmaPlot version 11.0, Systat Software, Inc, Chicago, IL, USA) was used, with fibre type and exercise as factors. If significant main effects were observed, then a Bonferroni t test post hoc analysis was performed to identify the specific differences in protein expression within fibre type and exercise. Data are expressed as means ± SEM unless otherwise stated. The level of significance was set at P < 0.05.
Results
Fibre type-specific protein expression
The fibre type of each fibre segment was ascertained by the expression of either MHCI or MHCII (Fig. 2). In addition, SERCA1 expression was almost undetectable in fibres assigned as type I according to MHC determination, and overall SERCA1 protein was lower (P < 0.001) in type I compared with type II fibres (0.004 ± 0.003 a.u., n = 134 vs. 1.00 ± 0.03 a.u., n = 135; Fig. 2). Protein expression of PFK was lower (P < 0.001) in type I (0.35 ± 0.03, n = 39) than in type II fibres (1.00 ± 0.03, n = 65; Fig. 3A and B), whereas CS and HAD were more abundant (P < 0.001) in type I fibres than in type II fibres (CS, 2.04 ± 0.14, n = 34 vs. 1.00 ± 0.07, n = 59; and HAD, 2.26 ± 0.09, n = 44 vs. 1.00 ± 0.04, n = 40; Fig. 3C–F).
Figure 3. Fibre type-specific differences in protein expression of metabolic enzymes determined by Western blotting on segments of human single muscle fibres.
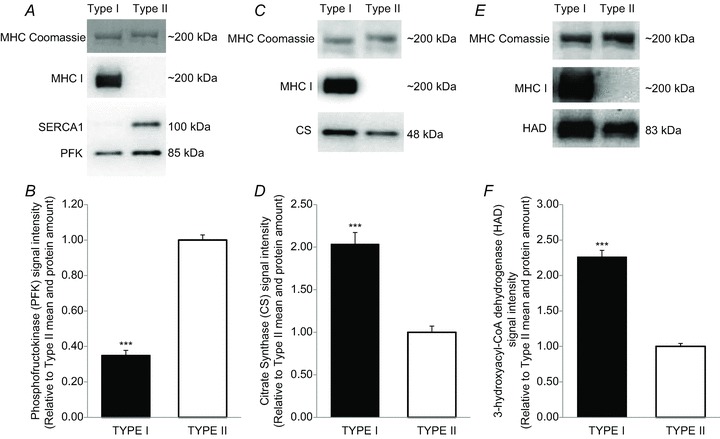
Representative Western blot and bar graphs denoting the amount of each protein expressed relative to MHC or actin. A and B, phosphofructokinase (PFK), 4–15% gel (type I, n = 39 and type II, n = 65). C and D, citrate synthase (CS), 4–15% gel (type I, n = 34 and type II, n = 59). E and F, 3-hydroxyacyl-CoA dehydrogenase (HAD), 4–15% gel (type I, n = 44 and type II, n = 40). For all data, Student's unpaired t tests were performed to detect the differences. ***Significant difference (P < 0.001) between protein expression in type I and type II fibres. Data are expressed as means + SEM.
The protein expression of the Na+–K+ pump α1 isoform was not different (P = 0.15) between type I and type II fibres (1.29 ± 0.14, n = 51 vs. 1.00 ± 0.09, n = 27; Fig. 4A and B), while the Na+–K+ pump α2 isoform expression was lower (P < 0.001) in type I than in type II fibres (0.63 ± 0.04, n = 44 vs. 1.00 ± 0.07, n = 40; Fig. 4C and D). Protein expression of the Na+–K+ pump β1 isoform was not different in type I and type II fibres (1.07 ± 0.05, n = 53 vs. 1.00 ± 0.03, n = 38; Fig. 4D and E), while Kir6.2 protein expression was higher (P < 0.001) in type I compared with type II fibres (3.80 ± 0.39, n = 49 vs. 1.00 ± 0.09, n = 41; Fig. 4F and G).
Figure 4. Fibre type-specific differences in potassium-regulating protein expression determined by Western blotting on segments of human single muscle fibres.
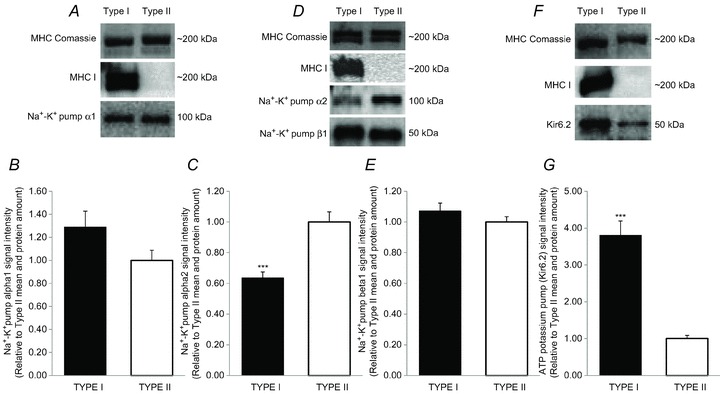
Representative Western blot and bar graphs denoting the amount of each protein expressed relative to MHC. A and B, Na+–K+ pump α1 subunit, 4–15% gel (type I, n = 51 and type II, n = 27). C and D, Na+–K+ pump α2 subunit, 4–15% gel (type I, n = 44 and type II, n = 40). D and E, Na+–K+ pump β1 subunit, 4–15% gel (type I, n = 53 and type II, n = 38). F and G, the ATP-sensitive potassium channel Kir6.2, 4–15% gel (type I, n = 49 and type II, n = 41). For all data, Student's unpaired t tests were performed to detect the differences. ***Significant difference (P < 0.001) between protein expression in type I and type II fibres. Data are expressed as means + SEM.
Phosphorylation of FXYD1: effects of exercise and fibre type
Total FXYD1 protein expression was not different between type I and type II fibres, but increased overall (P < 0.05) after the intense exercise bout compared with rest (1.23 ± 0.05, n = 73 vs. 1.03 ± 0.04, n = 63; Fig. 5A and B). The AB_FXYD1 signal was lower (P < 0.01) after exercise compared with rest in both type I (0.73 ± 0.05, n = 46 vs. 1.01 ± 0.08, n = 24) and type II fibres (0.54 ± 0.05, n = 16 vs. 1.00 ± 0.06, n = 35), indicating an increase in unspecific FXYD1 phosphorylation (Fig. 5C and D). The AB_FXYD1ser68 signal intensity was higher (P < 0.001) in type II fibres after exercise than at rest (1.90 ± 0.25, n = 25 vs. 1.00 ± 0.08, n = 35) but was not changed in type I fibres (1.91 ± 0.16, n = 26 vs. 1.33 ± 0.19, n = 7; Fig. 5E and F). There were no differences in AB_FXYD1ser68 signal intensity between type I and type II fibres.
Figure 5. Exercise-induced and fibre type-specific differences in FXYD1 phosphorylation determined by Western blotting on segments of human single muscle fibres.
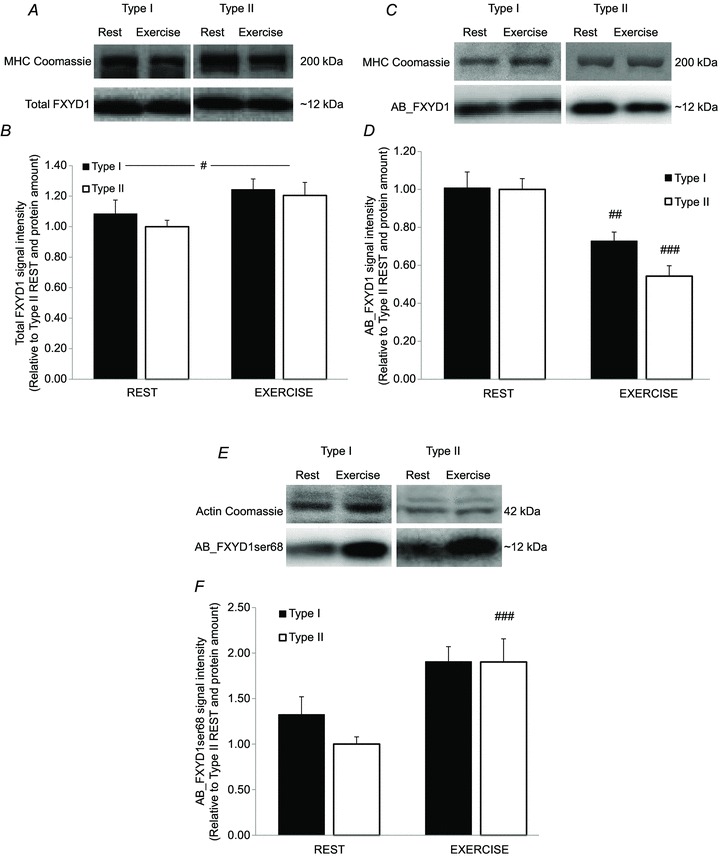
Representative Western blot and bar graphs denoting the amount of each protein expressed relative to MHC or actin. A and B, total FXYD1 protein determined by 13721-1-AP, Proteintech Group (type I rest, n = 22; type II rest, n = 41; type I exercise, n = 43; and type II exercise, n = 30) C and D, unphosphorylated FXYD1 protein determined by AB_FXYD1 (type I rest, n = 24; type II rest, n = 35; type I exercise, n = 46; and type II exercise, n = 16). E and F, FXYD1 proteins phosphorylated at Ser68 determined by AB_FXYD1ser68 (type I rest, n = 7; type II rest, n = 35; type I exercise, n = 26; and type II exercise, n = 25). A two-way ANOVA was performed to detect the differences. #P < 0.05, ##P < 0.01 and ###P < 0.001 significant difference between rest and exercise in the same fibre type. Data are expressed as means + SEM.
For some of the subjects (n = 3), both AB_FXYD1ser68 and total FXYD1 were determined on the same fibre segments (n = 46). In these samples, the AB_FXYD1ser68/FXYD1 ratio was higher (P < 0.05) in type I fibres than in type II fibres at rest (2.22 ± 0.27, n = 4 vs. 1.08 ± 0.11, n = 18) and was increased (P < 0.01) by exercise in type II fibres (1.08 ± 0.11, n = 18 vs. 1.92 ± 0.28, n = 12), while the ratio was not changed in type I fibres (2.22 ± 0.27, n = 4 vs. 1.87 ± 0.15, n = 12).
Discussion
The main findings of the present study were that FXYD1 Ser68 phosphorylation was increased by an acute bout of intense exercise in type II fibres only, and that the AB_FXYD1 signal intensity was reduced by exercise in both type I and type II fibres. In addition, the protein expression of PFK and Na+–K+ pump α2 isoform was higher in type II than type I fibres, whereas the opposite was the case for Kir6.2, HAD and CS protein expression. The total FXYD1 and Na+–K+ pump α1 and β1 isoform protein expression was not fibre type dependent.
This is the first study to determine exercise-induced fibre type-specific alterations in protein phosphorylation in single segments of human skeletal muscle fibres. The present study showed that intense exercise (∼100% peak pulmonary O2 uptake for 5 min) decreased the signal of AB_FXYD1 in both type I and type II fibres in human skeletal muscles concomitant with an unchanged total FXYD1, indicating an increased unspecific FXYD1 phosphorylation, because the antibody is phosphosensitive and primarily recognizes unphosphorylated proteins (Rembold et al. 2005; Thomassen et al. 2011). In contrast, AB_FXYD1ser68 was significantly increased only in type II fibres, suggesting that the FXYD1 Ser68 phosphorylation was fibre type specific. This observation was supported by the finding that the AB_FXYD1ser68/total FXYD1 ratio increased only in type II fibres. Given that unspecific FXYD1 phosphorylation was increased in both fibre types, and Ser68 phosphorylation was increased only in type II fibres, it is likely that FXYD1 phosphorylation of Ser63 and/or Thr69 was responsible for the increase in unspecific phosphorylation in type I fibres. It is not possible to evaluate whether the increase in Ser68 phosphorylation caused the entire increase in unspecific phosphorylation in the type II fibres or if Ser63 and/or Thr69 phosphorylation were contributing to the overall increased phosphorylation.
The present observation of an exercise-induced increase in unspecific FXYD1 phosphorylation is in agreement with findings in human muscle homogenates, where acute exercise has been shown to increase FXYD1 phosphorylation at multiple sites, including phosphorylation at Ser68 (Benziane et al. 2011; Thomassen et al. 2011), but in contrast to findings in rat muscles, where FXYD1 Ser68 phosphorylation was not affected by exercise (Rasmussen et al. 2008). One explanation may be phosphospecificity of the AB_FXYD1ser68 antibody. Based on a recent study with electrically stimulated soleus and EDL mouse muscles, it was expected that FXYD1 Ser68 phosphorylation would increase in type I fibres and decrease in type II fibres (Thomassen et al. 2011). However, the present study showed an exercise-induced increase in FXYD1 Ser68 phosphorylation in type II fibres. The discrepancy might be explained by species differences in amino acid sequence for the protein (Sweadner & Rael, 2000), which could influence antibody binding affinity (Fuller et al. 2009), differences in type of contraction or true differences in FXYD1 phosphorylation between species.
Even though fibre type-dependent differences in protein expression and phosphorylation have been shown in the present study, methodological considerations need to be taken into account. In the present study, FXYD1 phosphorylation was determined using a total FXYD1 antibody and two distinct and well-known polyclonal antibodies (AB_FXYD1ser68 and AB_FXYD1) with an epitope located in the C-terminal end of the protein (Rembold et al. 2005), where all three known phosphorylation sites are located (Sweadner & Rael, 2000). The AB_FXYD1ser68 signal intensity is reported to be severely limited both by mutation of the adjacent Thr69 amino acid to alanine and in the presence of adjacent phosphorylation on the Thr69 site (Fuller et al. 2009), and it has been shown in different studies that AB_FXYD1ser68 underestimates the level of FXYD1 Ser68 phosphorylation (Fuller et al. 2009; Thomassen et al. 2011). Thus, the present exercise-induced increase in FXYD1 Ser68 phosphorylation in type II fibres determined by AB_FXYD1ser68 is likely to be negatively affected simultaneously by Ser63 and especially Thr69 phosphorylation (Benziane et al. 2011; Thomassen et al. 2011) and, if anything, to be more pronounced than reported. In type I fibres, the lack of change in Ser68 phosphorylation may also be due to phosphorylation on Ser63 and Thr69.
The AB_FXYD1 recognizes unphosphorylated FXYD1 proteins and has been reported to be phosphosensitive, so phosphorylation on FXYD1 Ser63, Ser68 and/or Thr69 in human skeletal muscles will decrease AB_FXYD1 signal intensity (Rembold et al. 2005; Fuller et al. 2009; Benziane et al. 2011; Thomassen et al. 2011). In the present study, both type I and type II fibres showed a higher degree of unspecific phosphorylation, determined by a decrease in AB_FXYD1 signal intensity, concomitant with a type II-specific increase in Ser68 phosphorylation and a minor overall increase in total FXYD1. Thus, in type I fibres it is a concomitant increase in FXYD1 Ser63, Ser68 and Thr69 phosphorylation that induces the lower AB_FXYD1 signal intensity together with no changes in AB_FXYD1ser68 signal intensity. In mouse muscles, where AB_FXYD1 antibody binding is not affected by phosphorylation (Benziane et al. 2011), amino acid number 69 in the FXYD1 protein, with a marked influence on the AB_FXYD1ser68 binding capacity (Fuller et al. 2009), is serine, in contrast to the phosphorylated Thr69 site in humans (Sweadner & Rael, 2000). Thus, it seems most likely that phosphorylation on Thr69 is important for the phosphosensitivity of the AB_FXYD1 and the decrease in AB_FXYD1 signal intensity shown in human type I fibres. In contrast, in type II fibres with a concomitant decrease in AB_FXYD1 signal and increase in AB_FXYD1ser68 signal, it seems to be induced by a higher concomitant Ser63 and Ser68 phosphorylation rather than a higher Thr69 phosphorylation. Alterations in FXYD1 content and FXYD1 phosphorylation are probably more important for Na+–K+ pump activity than total Na+–K+ pump content, because there is a poor relationship between pump activity measured in vitro and total Na+–K+ pump as well as Na+–K+ pump α2 isoform content (Fowles et al. 2004). The FXYD1 protein is coexpressed with the Na+–K+ pump α-subunits (Reis et al. 2005), and dephosphorylated FXYD1 inhibits Na+–K+ pump activity, whereas phosphorylation of FXYD1 Ser68 increases Na+–K+ pump activity (Pavlovićet al. 2007). Thus, the present exercise-induced increase in unspecific FXYD1 phosphorylation may have increased Na+–K+ pump activity in both type I and type II human muscle fibres. Phosphorylation of FXYD1 is primarily regulated by activity of protein kinase A and protein kinase C (Bibert et al. 2008). Protein kinase A-induced phosphorylation of FXYD1 Ser68 increases the apparent Na+ affinity of Na+–K+ pump α1/β and α2/β isozymes, but has no effect on the maximal Na+–K+ pump activity (Bibert et al. 2008). In contrast, protein kinase C phosphorylation of FXYD1 Ser63 and Ser68 increases the maximal activity of the Na+–K+ pump α2/β isozyme but does not affect Na+ affinity (Bibert et al. 2008). In total membranes of glycolytic rat muscles, 30 min of treadmill running induced a significant decrease in Na+–K+ pump Km for Na+ (increased affinity) and abolished the fibre type differences between oxidative and glycolytic muscles in resting Na+ affinity (Juel, 2009). Thus, the increase in FXYD1 Ser68 phosphorylation, observed in type II fibres and not in type I fibres, may have altered Na+–K+ pump Na+ affinity. The present data are not able to distinguish between activities of protein kinase A and protein kinase C, because exercise activates both pathways (Williamson et al. 2006; Benziane et al. 2011), but in type I fibres phosphorylation on Ser63 and Thr69 was probably responsible for the increase in unspecific phosphorylation, and therefore the maximal pump activity rather than the Na+ affinity is likely to have increased (Juel, 2009). Given that the alterations in Ser63 and Thr69 are not determined directly, the effects on Na+–K+ pump activities in human type I and type II fibres needs further investigation.
This is the first study to determine the fibre type-specific expression of Na+–K+ pump subunits, the Kir6.2 channel and metabolic enzymes in segments of human skeletal muscle fibres. In contrast to the hypothesis, the protein expression of Na+–K+ pump α2 isoform was markedly higher in type II fibres than in type I fibres. The majority of studies in rat muscles did not find any differences in α2 isoform expression between EDL and soleus muscles, but a few observed a greater expression of the α2 isoform in type II fibres (Kristensen & Juel, 2010), indicating a species difference in the α2 isoform expression.
The protein expression of the Na+–K+ pump α1 and β1 isoforms in the human muscles were, in contrast to studies on rat muscles (Kristensen & Juel, 2010), not different between type I and type II fibres. In rat membrane fractions, the Na+–K+ pump α1 isoform content was 2.4-fold higher in oxidative muscles compared with glycolytic muscles (Juel et al. 2001). Furthermore, in rat muscle homogenates the amounts of the Na+–K+ pump α1 and β1 isoforms were, respectively, 1.5- to 4-fold and 1.5- to 2-fold greater in soleus than in EDL, red and white gastrocnemius muscles (Fowles et al. 2004). The discrepancies between human and rat muscle in the fibre type-specific expression of the α1 isoform may be explained by the observation that the isoform is almost exclusively located in the cell surface membrane and at a low level compared with the α2 isoform (Kristensen & Juel, 2010). Thus, possible differences in fibre diameter between individual fibres and the relatively low abundance of the α1 isoform, together with the unequal number of fibres in the type I and type II groups, may have affected the comparison of protein expression of the Na+–K+ pump α1 isoform in type I and type II fibres. However, it should be noted that previously, studies have compared protein content between fibre types only in the membrane fractions of the fibre (Juel et al. 2001) or by the non-quantitative immunostaining method (Zhang et al. 2006). In light of these issues and given our analysis of single muscle fibres without fractionation, it appears that expression of the Na+–K+ pump α1 and β1 isoforms is not different between type I and type II fibres in humans.
The amount of the ATP-sensitive inward rectifier Kir6.2 channel was expected to be highest in type II fibres, because the relative content in the cell surface membrane of mixed glycolytic rat muscles is ∼2.4-fold higher than in mixed oxidative muscles (Nielsen et al. 2003; Kristensen & Juel, 2010). Additionally, in humans the expression in vesicles is reported to be higher than in homogenates, and the channel was found by immunohistochemical analysis in all fibres, but without any quantitative indication of fibre type specificity (Nielsen et al. 2003). In the present study, the same antibody was used, and the Kir6.2 channel was ∼4-fold more expressed in type I fibres compared with type II fibres. This discrepancy can be explained by marked differences between rat muscles and human muscles or by the differences between the giant vesicles method and the present method of Western blot analyses on segments of single human muscle fibres.
Expression of PFK was ∼3-fold higher in human skeletal muscle type II fibres than in type I fibres, while protein expression of CS (∼2-fold) and HAD (∼2.3-fold) was higher in type I fibres compared with type II fibres. In agreement, a weak but significant relationship (r = 0.42, P = 0.023) was reported between the relative area of type II fibres and the PFK enzyme activity in human skeletal muscles (Jaworowski et al. 2002). Furthermore, when enzyme activities in pools of human muscle fibres were determined, it was shown that type I fibres had a higher HAD and CS but a lower PFK activity than type II fibres (Essén-Gustavsson & Henriksson, 1984; Borges & Essén-Gustavsson, 1989). These findings suggest that the fibre type-specific differences in PFK, CS and HAD enzyme activities are related to differences in total protein expression of the enzymes. Together with the obvious fibre type-specific expression of SERCA1, these data confirm that the methodology for determination of protein expression in segments of human skeletal muscle fibres by Western blotting allows detection of fibre type-specific expression of muscle proteins (Murphy, 2011).
Summary
The FXYD1 Ser68 phosphorylation increased following an acute bout of intense exercise in type II fibres, while the AB_FXYD1 signal intensity was reduced in both type I and type II fibres, indicating fibre type differences in FXYD1 phosphorylation on Ser63, Ser68 and Thr69. The Na+–K+ pump α2 isoform was more highly expressed in type II compared with type I fibres, whereas the expression of Kir6.2 was higher in type I fibres. Expression of the Na+–K+ pump α1 and β1 isoforms was not fibre type dependent.
Perspectives
The opportunity to evaluate the acute response and chronic adaptations of type I and type II human skeletal muscle fibres provides interesting perspectives. The advances made with the enhanced technique of the commonly used Western blotting (Murphy, 2011) will allow a greater understanding of skeletal muscle responses and adaptations at the level of single muscle fibres.
Acknowledgments
This experiment was supported by grants from the Ministry of Culture in Denmark (Kulturministeriets udvalg for Idrætsforskning) and Team Denmark.
Glossary
- CS
citrate synthase
- EDL
extensor digitorum longus
- FXYD1
phospholemman
- HAD
3-hydroxyacyl-CoA dehydrogenase
- Kir6.2
ATP-sensitive inwardly rectifying potassium channel 11
- MHC
myosin heavy chain
- Na+–K+ pump
sodium–potassium ATPase
- PFK
phosphofructokinase
- SERCA1
sarco(endo)plasmic reticulum Ca2+-ATPase isoform 1
Author contributions
Conception and design of the experiments: M.T., R.M.M. and J.B. Collection, analysis and interpretation of data: M.T., R.M.M. and J.B. Drafting the article or revising it critically for important intellectual content: M.T., R.M.M. and J.B. All authors approved the final version of the manuscript, and the experiments were conducted at the Department of Nutrition, Exercise and Sports, University of Copenhagen, DK-2100, Denmark.
References
- Benziane B, Widegren U, Pirkmajer S, Henriksson J, Stepto NK, Chibalin AV. Effect of exercise and training on phospholemman phosphorylation in human skeletal muscle. Am J Physiol Endocrinol Metab. 2011;301:E456–E466. doi: 10.1152/ajpendo.00533.2010. [DOI] [PubMed] [Google Scholar]
- Bibert S, Roy S, Schaer D, Horisberger JD, Geering K. Phosphorylation of phospholemman (FXYD1) by protein kinases A and C modulates distinct Na,K-ATPase isozymes. J Biol Chem. 2008;283:476–486. doi: 10.1074/jbc.M705830200. [DOI] [PubMed] [Google Scholar]
- Borges O, Essén-Gustavsson B. Enzyme activities in type I and II muscle fibres of human skeletal muscle in relation to age and torque development. Acta Physiol Scand. 1989;136:29–36. doi: 10.1111/j.1748-1716.1989.tb08626.x. [DOI] [PubMed] [Google Scholar]
- Essén-Gustavsson B, Henriksson J. Enzyme levels in pools of microdissected human muscle fibres of identified type. Adaptive response to exercise. Acta Physiol Scand. 1984;120:505–515. doi: 10.1111/j.1748-1716.1984.tb07414.x. [DOI] [PubMed] [Google Scholar]
- Fowles JR, Green HJ, Ouyang J. Na+-K+-ATPase in rat skeletal muscle: content, isoform, and activity characteristics. J Appl Physiol. 2004;96:316–326. doi: 10.1152/japplphysiol.00745.2002. [DOI] [PubMed] [Google Scholar]
- Fuller W, Howie J, McLatchie LM, Weber RJ, Hastie CJ, Burness K, Pavlovic D, Shattock MJ. FXYD1 phosphorylation in vitro and in adult rat cardiac myocytes: threonine 69 is a novel substrate for protein kinase C. Am J Physiol Cell Physiol. 2009;296:C1346–C1355. doi: 10.1152/ajpcell.00523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingwersen MS, Kristensen M, Pilegaard H, Wojtaszewski JF, Richter EA, Juel C. Na,K-ATPase activity in mouse muscle is regulated by AMPK and PGC-1α. J Membr Biol. 2011;242:1–10. doi: 10.1007/s00232-011-9365-7. [DOI] [PubMed] [Google Scholar]
- James P, Inui M, Tada M, Chiesi M, Carafoli E. Nature and site of phospholamban regulation of the Ca2+ pump of sarcoplasmic reticulum. Nature. 1989;342:90–92. doi: 10.1038/342090a0. [DOI] [PubMed] [Google Scholar]
- Jaworowski A, Porter MM, Holmback AM, Downham D, Lexell J. Enzyme activities in the tibialis anterior muscle of young moderately active men and women: relationship with body composition, muscle cross-sectional area and fibre type composition. Acta Physiol Scand. 2002;176:215–225. doi: 10.1046/j.1365-201X.2002.t01-2-01004.x. [DOI] [PubMed] [Google Scholar]
- Juel C. Na+-K+-ATPase in rat skeletal muscle: muscle fiber-specific differences in exercise-induced changes in ion affinity and maximal activity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R125–R132. doi: 10.1152/ajpregu.90760.2008. [DOI] [PubMed] [Google Scholar]
- Juel C, Grunnet L, Holse M, Kenworthy S, Sommer V, Wulff T. Reversibility of exercise-induced translocation of Na+-K+ pump subunits to the plasma membrane in rat skeletal muscle. Pflugers Arch. 2001;443:212–217. doi: 10.1007/s004240100674. [DOI] [PubMed] [Google Scholar]
- Kristensen M, Juel C. Potassium-transporting proteins in skeletal muscle: cellular location and fibre-type differences. Acta Physiol (Oxf) 2010;198:105–123. doi: 10.1111/j.1748-1716.2009.02043.x. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Bangsbo J, Renaud JM. Muscle K+, Na+, and Cl– disturbances and Na+-K+ pump inactivation: implications for fatigue. J Appl Physiol. 2008;104:288–295. doi: 10.1152/japplphysiol.01037.2007. [DOI] [PubMed] [Google Scholar]
- Mollica JP, Oakhill JS, Lamb GD, Murphy RM. Are genuine changes in protein expression being overlooked? Reassessing Western blotting. Anal Biochem. 2009;386:270–275. doi: 10.1016/j.ab.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Murphy RM. Enhanced technique to measure proteins in single segments of human skeletal muscle fibers: fiber-type dependence of AMPK-α1 and -β1. J Appl Physiol. 2011;110:820–825. doi: 10.1152/japplphysiol.01082.2010. [DOI] [PubMed] [Google Scholar]
- Murphy RM, Verburg E, Lamb GD. Ca2+ activation of diffusible and bound pools of μ-calpain in rat skeletal muscle. J Physiol. 2006;576:595–612. doi: 10.1113/jphysiol.2006.114090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JJ, Kristensen M, Hellsten Y, Bangsbo J, Juel C. Localization and function of ATP-sensitive potassium channels in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2003;284:R558–R563. doi: 10.1152/ajpregu.00303.2002. [DOI] [PubMed] [Google Scholar]
- Nielsen OB, Clausen T. The Na+/K+-pump protects muscle excitability and contractility during exercise. Exerc Sport Sci Rev. 2000;28:159–164. [PubMed] [Google Scholar]
- Pavlović D, Fuller W, Shattock MJ. The intracellular region of FXYD1 is sufficient to regulate cardiac Na/K ATPase. FASEB J. 2007;21:1539–1546. doi: 10.1096/fj.06-7269com. [DOI] [PubMed] [Google Scholar]
- Rasmussen MK, Kristensen M, Juel C. Exercise-induced regulation of phospholemman (FXYD1) in rat skeletal muscle: implications for Na+/K+-ATPase activity. Acta Physiol (Oxf) 2008;194:67–79. doi: 10.1111/j.1748-1716.2008.01857.x. [DOI] [PubMed] [Google Scholar]
- Reis J, Zhang L, Cala S, Jew KN, Mace LC, Chung L, Moore RL, Ng YC. Expression of phospholemman and its association with Na+-K+-ATPase in skeletal muscle: effects of aging and exercise training. J Appl Physiol. 2005;99:1508–1515. doi: 10.1152/japplphysiol.00375.2005. [DOI] [PubMed] [Google Scholar]
- Rembold CM, Ripley ML, Meeks MK, Geddis LM, Kutchai HC, Marassi FM, Cheung JY, Moorman JR. Serine 68 phospholemman phosphorylation during forskolin-induced swine carotid artery relaxation. J Vasc Res. 2005;42:483–491. doi: 10.1159/000088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Henriksson J, Nygaard E, Andersen P, Jansson E. Fiber types and metabolic potentials of skeletal muscles in sedentary man and endurance runners. Ann N Y Acad Sci. 1977;301:3–29. doi: 10.1111/j.1749-6632.1977.tb38182.x. [DOI] [PubMed] [Google Scholar]
- Sejersted OM, Sjøgaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. 2000;80:1411–1481. doi: 10.1152/physrev.2000.80.4.1411. [DOI] [PubMed] [Google Scholar]
- Sweadner KJ, Rael E. The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics. 2000;68:41–56. doi: 10.1006/geno.2000.6274. [DOI] [PubMed] [Google Scholar]
- Tannerstedt J, Apró W, Blomstrand E. Maximal lengthening contractions induce different signaling responses in the type I and type II fibers of human skeletal muscle. J Appl Physiol. 2009;106:1412–1418. doi: 10.1152/japplphysiol.91243.2008. [DOI] [PubMed] [Google Scholar]
- Thomassen M, Christensen PM, Gunnarsson TP, Nybo L, Bangsbo J. Effect of 2-wk intensified training and inactivity on muscle Na+-K+ pump expression, phospholemman (FXYD1) phosphorylation, and performance in soccer players. J Appl Physiol. 2010;108:898–905. doi: 10.1152/japplphysiol.01015.2009. [DOI] [PubMed] [Google Scholar]
- Thomassen M, Rose AJ, Jensen TE, Maarbjerg SJ, Bune L, Leitges M, Richter EA, Bangsbo J, Nordsborg NB. Protein kinase Cα activity is important for contraction-induced FXYD1 phosphorylation in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1808–R1814. doi: 10.1152/ajpregu.00066.2011. [DOI] [PubMed] [Google Scholar]
- Williamson DL, Kubica N, Kimball SR, Jefferson LS. Exercise-induced alterations in extracellular signal-regulated kinase 1/2 and mammalian target of rapamycin (mTOR) signalling to regulatory mechanisms of mRNA translation in mouse muscle. J Physiol. 2006;573:497–510. doi: 10.1113/jphysiol.2005.103481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Morris KJ, Ng YC. Fiber type-specific immunostaining of the Na+,K+-ATPase subunit isoforms in skeletal muscle: age-associated differential changes. Biochim Biophys Acta. 2006;1762:783–793. doi: 10.1016/j.bbadis.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


