Abstract
In skeletal muscle, mitochondria exist as two subcellular populations known as subsarcolemmal (SS) and intermyofibrillar (IMF) mitochondria. SS mitochondria preferentially respond to exercise training, suggesting divergent transcriptional control of the mitochondrial genomes. The transcriptional co-activator peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) and mitochondrial transcription factor A (Tfam) have been implicated in the direct regulation of the mitochondrial genome in mice, although SS and IMF differences may exist, and the potential signalling events regulating the mitochondrial content of these proteins have not been elucidated. Therefore, we examined the potential for PGC-1α and Tfam to translocate to SS and IMF mitochondria in human subjects, and performed experiments in rodents to identify signalling mechanisms regulating these translocation events. Acute exercise in humans and rats increased PGC-1α content in SS but not IMF mitochondria. Acute exposure to 5-aminoimidazole-4-carboxamide-1-β-ribofuranoside in rats recapitulated the exercise effect of increased PGC-1α protein within SS mitochondria only, suggesting that AMP-activated protein kinase (AMPK) signalling is involved. In addition, rendering AMPK inactive (AMPK kinase dead mice) prevented exercise-induced PGC-1α translocation to SS mitochondria, further suggesting that AMPK plays an integral role in these translocation events. In contrast to the conserved PGC-1α translocation to SS mitochondria across species (humans, rats and mice), acute exercise only increased mitochondrial Tfam in rats. Nevertheless, in rat resting muscle PGC-1α and Tfam co-immunoprecipate with α-tubulin, suggesting a common cytosolic localization. These data suggest that exercise causes translocation of PGC-1α preferentially to SS mitochondria in an AMPK-dependent manner.
Key points
The transcriptional co-activator peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α), in concert with mitochondrial transcription factor A (Tfam), has been implicated in the direct regulation of the mitochondrial genome.
In humans, rats and mice, acute exercise was found to promote PGC-1α translocation to subsarcolemmal (SS) mitochondria.
In rats, treatment with 5-aminoimidazole-4-carboxamide-1-β-ribofuranoside induced both PGC-1α and Tfam translocation and in addition PGC-1α and Tfam were found to co-locolize with α-tubulin.
In mice, rendering AMP-activated protein kinase (AMPK) inactive prevented PGC-1α translocation.
These data suggest that exercise causes translocation of PGC-1α preferentially to SS mitochondria in an AMPK-dependent manner.
Introduction
Peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) has recently been implicated in the direct regulation of the mitochondrial genome, as PGC-1α appears to be present within the mitochondria of HeLa, HEK293 and SH-SY5Y cells, as well as in human platelets, in which PGC-1α co-localizes within the D-loop of mtDNA with mitochondrial transcription factor A (Tfam; Aquilano et al. 2010). Additionally, following an acute bout of exercise in mice, PGC-1α has been shown to translocate to mitochondria and form a complex with Tfam in the mtDNA D-loop region (Safdar et al. 2011). Combined, these elegant studies suggest a novel hypothesis whereby PGC-1α can directly regulate the mitochondrial genome independent of a change in protein content via translocation events; however, it remains to be determined if this process occurs in human skeletal muscle.
Previous work has highlighted numerous signals provoked by environmental stimuli and/or cellular stress that induce mitochondrial biogenesis and recent work has shown that the molecular mechanisms stimulating mitochondrial biogenesis are activated immediately by a single bout of exercise (Pilegaard et al. 2003; Norrbom et al. 2004; Wright et al. 2007b). This process appears to be regulated in part by AMP-activated protein kinase (AMPK)-induced phosphorylation of PGC-1α (Jager et al. 2007), in addition to calcium/calmodulin dependent protein kinase (CaMK) activated signalling and p-38 mitogen-activated protein kinase (p38 MAPK) (Akimoto et al. 2005; Wright et al. 2007a; Pogozelski et al. 2009; Little et al. 2010). These signalling events have been implicated in both the activation and the nuclear translocation of PGC-1α, but whether these signalling events can influence PGC-1α translocation to mitochondria has yet to be investigated.
In addition to PGC-1α, exercise-induced increases in Tfam expression are thought to be necessary to coordinate the nuclear and mitochondrial genomes during the initiation of mitochondrial biogenesis. However, this is not consistently supported by experimental evidence as exercise-induced mitochondrial biogenesis can occur in the absence of an increase in whole-muscle Tfam expression (Gordon et al. 2001; Perry et al. 2010). Alternatively, similar to PGC-1α (Safdar et al. 2011), Tfam may exist in subcellular compartment(s) from which it can translocate to mitochondria in response to exercise, negating the necessity for an absolute increase in Tfam protein expression. Indeed, the possibility exists that both PGC-1α and Tfam may be induced by exercise to translocate to the mitochondria in an acute fashion to directly regulate the mitochondrial genome.
Clearly the subcellular localization of proteins is an important consideration in the context of mitochondrial biogenesis. In addition, mitochondria exist as two subcellular populations and based on their spatial locations have been termed subsarcolemmal (SS) and intermyofibrillar (IMF) mitochondria, creating another level of cellular compartmentalization. SS and IMF mitochondria have different characteristics in resting muscle (Palmer et al. 1985; Hoppel et al. 2002) and these mitochondrial subpopulations respond differently to various physiological perturbations, as SS mitochondria appear to be more malleable in response to exercise (Krieger et al. 1980), pharmacological interventions (Benton et al. 2008a) and PGC-1α over-expression (Benton et al. 2008b). It is therefore possible that SS mitochondria possess more sensitive transcriptional control of their mitochondrial genome, although this concept has yet to be investigated.
Therefore, in the present study, we examined whether PGC-1α and Tfam can be induced to translocate to SS and/or IMF mitochondria by exercise in human, rat and mouse skeletal muscle. Additionally, we investigated the role of AMPK in regulating these translocation events. We provide evidence that PGC-1α preferentially translocates to the SS mitochondrial fraction across a range of species, including humans, while Tfam translocation only occurred in rats. Furthermore, our data suggest that exercise causes the translocation of PGC-1α to mitochondria in an AMPK-dependent manner.
Methods
Ethical approval
The experimental procedures on humans were approved by the Research Ethics Boards at the University of Guelph in Guelph, ON, and McMaster University in Hamilton, ON. Informed consent was obtained in writing and all procedures conformed to the standards described in the Declaration of Helsinki. All facets of this study were approved by the University of Guelph Animal Care Committee, and conform to the guide for the care and use of laboratory animals published by the US National Institutes of Health.
Human subjects
Eight healthy, recreationally active men were recruited, and their mean (±SEM) age, height, weight, body mass index and 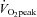 peak were 21.4 ± 0.6 years, 1.78 ± 0.03 m, 74.1 ± 3.7 kg, 23.4 ± 0.9 kg m−2 and 53.5 ± 3.5 ml kg−1 min−1, respectively. Subjects were given both oral and written information about the experimental procedures before giving their informed consent. Skeletal muscle samples were obtained from the lateral aspect of vastus lateralis by percutaneous needle biopsy under local subcutaneous anaesthesia (2% lidocaine without noradrenaline) before (rest), immediately after cycling for 2 h at 60%
peak were 21.4 ± 0.6 years, 1.78 ± 0.03 m, 74.1 ± 3.7 kg, 23.4 ± 0.9 kg m−2 and 53.5 ± 3.5 ml kg−1 min−1, respectively. Subjects were given both oral and written information about the experimental procedures before giving their informed consent. Skeletal muscle samples were obtained from the lateral aspect of vastus lateralis by percutaneous needle biopsy under local subcutaneous anaesthesia (2% lidocaine without noradrenaline) before (rest), immediately after cycling for 2 h at 60%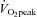 (post-exercise) and 3 h later (exercise + recovery). SS and IMF mitochondria were immediately isolated and used for Western blotting as described below.
(post-exercise) and 3 h later (exercise + recovery). SS and IMF mitochondria were immediately isolated and used for Western blotting as described below.
Animals
Female Sprague–Dawley rats (∼300 g) from our breeding colony were used, while male wild-type (WT) and AMPK kinase dead (KD) mice were generated by Dr Birnbaum (Zong et al. 2002) and bred onsite. Animals were housed in a climate- and temperature-controlled room, on a 12:12 h light–dark cycle, with chow and water provided ad libitum. For the exercise studies, muscle was harvested from different animals prior to exercise (rest), immediately after exercise (post-exercise) and 3 h after completing exercise (exercise + recovery). In rats, exercise consisted of treadmill running for 2 h at 15 m min−1, and thereafter the speed was increased by 5 m min−1 every 15 min until volitional termination of running (0% grade throughout), while mice were run at 15 m min−1 until volitional fatigue. 5-Aminoimidazole-4-carboxamide-1-β-ribofuranoside (AICAR) was administered subcutaneously (1 mg g−1 body weight in 0.45% saline) while control rats were injected with the same volume of 0.45% saline. Red gastrocnmeius was sampled 1 h following the injection of both AICAR and saline. In all experiments animals were anaesthetized with an intraperitoneal injection of sodium pentobarbital (60 mg kg−1) before muscle tissue was harvested.
Isolation of mitochondria from skeletal muscle
Differential centrifugation was used to obtain both SS and IMF mitochondrial fractions (starting tissue weight = 300–400 mg) and all procedures were identical to those that we have previously published (Holloway et al. 2009; Smith et al. 2011).
Western blotting
All samples were analysed for total protein (bicinchoninic acid protein assay), and samples were separated by electrophoresis on SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes as previously reported (Lally et al. 2012). Commercially available antibodies were used to detect PGC-1α (Calbiochem (516557), La Jolla, CA, USA, and Millipore (AB 3242), Billerica, MA, USA), Tfam (Santa Cruz Biotechnology, Santa Cruz, CA, USA), cytochrome c oxidase complex IV (COXIV; Invitrogen, Burlington, ON, Canada), LKB1 (Santa Cruz Biotechnology), phospho-LKB1 (Ser431; Santa Cruz Biotechnology), AMPKα (Cell Signaling Technology, Danvers, MA, USA), phospho-AMPKα (Thr172; Cell Signaling Technology), p38 MAPK (Cell Signaling Technology) and phospho-p38 MAPK (Thr180/Tyr182; Cell Signaling Technology), histone H2B (H2B; Abcam, Cambridge, MA, USA) SERCA2 (Sigma, St Louis, MO, USA), calnexin (Sigma) complex III subunit core I (Mitosciences, Eugene, OR, USA) and lactate dehydrogenase (LDH; Abcam). We have previously performed PGC-1α transient transfection experiments in rat soleus muscles to ensure over-expression was detectable (Benton et al. 2008b; Holloway et al. 2010), providing some validity for these antibodies. In addition, others have validated the PGC-1α Millipore antibody by small interfering RNA (Uguccioni & Hood, 2011). Given that both PGC-1α antibodies easily detected SS and IMF mitochondrial PGC-1α protein in resting muscle (data not shown) we elected to use the Calbiochem antibody for all exercise and AICAR experiments. All samples for a given protein were transferred and developed on the same membrane to limit variation and a loading control was used to ensure equal loading. Statistical analysis was the same when the data were analysed per microgram protein or normalized to the loading control, indicating that our loading control was appropriate and our Western blotting technique was consistent. Therefore we have chosen to express the protein content as per microgram protein. Blots were quantified using chemiluminescence and the ChemiGenius 2 Bioimaging system (SynGene, Cambridge, UK).
Co-immunoprecipitation
Whole muscle (rat) was homogenized (10 s using a Polytron), and co-immunoprecipitation experiments were performed using the Pierce Classic IP Kit (Thermo Scientific, Nepean, Ontario, Canada) according to the manufacturer's directions as previously described (Smith et al. 2011). Pilot experiments were performed using 100–600 μg of muscle, which identified 400 μg of initial muscle as optimal. All other experiments were performed using 400 μg of muscle. The interaction between Tfam and PGC-1α appears to be tenuous, as excessive homogenization (1 min) is sufficient to prevent co-immunoprecipitation of these proteins (supplementary Fig. S1).
Statistics
All data are presented as the mean ± standard error of the mean (SEM). A one-way analysis of variance was used to examine the effects of exercise and AICAR within SS and IMF mitochondrial fractions, and when significance was obtained a Scheffe's post hoc analysis was performed. Statistical significance was accepted at P < 0.05.
Results
Cleanliness of isolated SS and IMF mitochondrial fractions in resting rat skeletal muscle
Given the tissue limitations of human skeletal muscle we first performed Western blots on mitochondria isolated from resting rat skeletal muscle to validate our isolation protocol, as well as to verify the presence of PGC-1α within mitochondria. The major concern was the presence of nuclear contamination, and therefore we performed titrations of up to 30 μg of isolated SS and IMF mitochondria to determine the presence/absence of H2B. Importantly, H2B was not detected while the positive control (COXIV) increased with increasing amounts of protein loaded (Fig. 1A). In highly coupled (respiratory control ratio, 8.8 ± 0.4; ratio of ADP consumed to oxygen utilized (P/O), 2.47 ± 0.04 with pyruvate and malate as substrates) and purified mitochondria devoid of contamination of other subcellular components (Fig. 1B), we confirmed (using two different antibodies) that PGC-1α exists in both SS and IMF mitochondria in resting skeletal muscle.
Figure 1. Mitochondrial isolation cleanliness checks.
A, to ensure that our mitochondrial isolation procedures yielded sample devoid of nuclear contamination we loaded 5, 10, 20 30 μg of isolated mitochondrial protein (subsarcolemmal (SS) and intermyofibrillar (IMF)) and probed for H2B (nuclear protein) and COXIV (mitochondrial protein). This check was performed on the same membrane and indicates that our isolation procedure yields highly purified mitochondria. B, to confirm that PGC-1α exists within both the SS and the IMF fraction, 10 μg of mitochondrial protein was loaded and detected in concert with other mitochondrial proteins and the absence of non-mitochondrial proteins. Thirty micrograms of muscle homogenate protein was loaded.
PGC-1α and Tfam subcellular location in human and rat skeletal muscle
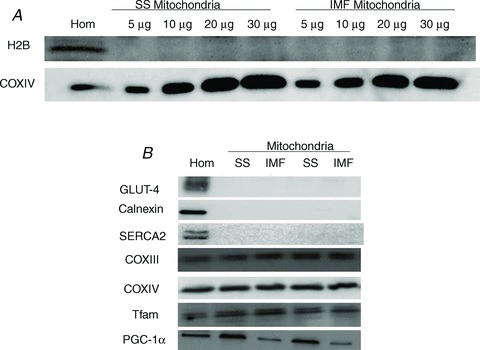
Given the previous work showing PGC-1α translocation to mitochondria in mice (Safdar et al. 2011) we performed experiments in humans to determine if either PGC-1α or Tfam translocate to SS and/or IMF mitochondria following an acute bout of exercise. In mitochondrial samples devoid of H2B and LDH contamination (Fig. 2A), PGC-1α increased in SS mitochondria following 3 h of recovery from exercise (+100%; Fig. 2B). In contrast to PGC-1α, Tfam did not translocate to either SS or IMF mitochondria following exercise in humans (Fig. 2C).
Figure 2. In human skeletal muscle, exercise promotes PGC-1α translocation to subsarcolemmal mitochondria.
A, representative control blots to ensure purified mitochondrial samples. B, following an acute bout of exercise at 60%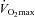 for 120 min, PGC-1α is increased in subsarcolemmal (SS) mitochondria 3 h post-exercise. C, in contrast, Tfam is not increased in either mitochondrial population; n = 8 for all independent experiments. Data are expressed as mean ± SEM. *P < 0.05, significantly different from rest. Ten micrograms of mitochondrial protein was loaded for all conditions. IMF, intermyofibrillar mitochondria.
for 120 min, PGC-1α is increased in subsarcolemmal (SS) mitochondria 3 h post-exercise. C, in contrast, Tfam is not increased in either mitochondrial population; n = 8 for all independent experiments. Data are expressed as mean ± SEM. *P < 0.05, significantly different from rest. Ten micrograms of mitochondrial protein was loaded for all conditions. IMF, intermyofibrillar mitochondria.
Similar to the human results, acute exercise in rats promoted PGC-1α translocation to the SS mitochondrial fraction 3 h post-exercise (+20%; Fig. 3A). In contrast to the human data, Tfam translocated to both the SS and the IMF mitochondrial fraction immediately post-exercise, although this response appears to be transient as the increase in mitochondrial Tfam post-exercise is lost following 3 h of recovery (Fig. 3B). Nonetheless, the fact that PGC-1α translocation occurs in humans (present study), rats (present study) and mice (Safdar et al. 2011) suggests that SS mitochondrial PGC-1α translocation is conserved across species.
Figure 3. Changes in rat skeletal muscle PGC-1α and Tfam subcellular localization following exhaustive exercise.
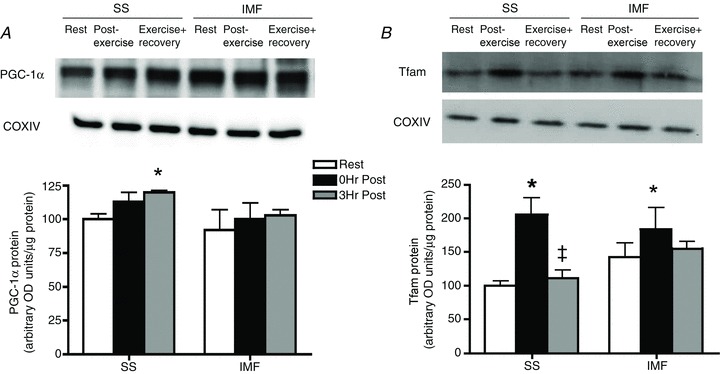
A, following 3 h of recovery PGC-1α is increased in subsarcolemmal (SS) mitochondria. B, immediately post-exercise, Tfam is transiently increased in both the SS and the intermyofibrillar (IMF) mitochondria; n = 6 for all independent experiments. Data are expressed as mean ± SEM. *P < 0.05, significantly different from rest. Ten micrograms of mitochondrial protein was loaded for all conditions.
AMPK signalling in regulating PGC-1α and Tfam translocation
We next examined the potential for AMPK to regulate mitochondrial translocation of PGC-1α and Tfam to SS and IMF mitochondria in rats. AICAR increased the phosphorylation of LKB1 (Ser431) and AMPK (Thr172) but not p38 MAPK (Thr180/Tyr182) (supplementary Fig. S2). Acute AICAR exposure increased PGC-1α protein in SS mitochondria (+25%; Fig. 4A), and also increased SS and IMF mitochondrial Tfam protein (+50%; Fig. 4B). To further examine the role of AMPK in mediating PGC-1α and Tfam mitochondrial translocation we exercised WT and AMPK KD mice and isolated SS and IMF mitochondria. Similar to humans and rats, exercise increased SS mitochondrial PGC-1α (+60%; Fig. 5A) in WT mice, but this response was completely ablated in AMPK KD mice (Fig. 5B), suggesting that AMPK activity is required for mitochondrial PGC-1α translocation. Due to the nature of the genetic alteration, the AMPK KD animals were only able to run for ∼52 min while the WT mice ran for ∼2 h before exhaustion. Therefore, both genotypes ran to exhaustion at the same relative intensity, but only the WT mice showed PGC-1α translocation. To ensure that the variable run time (duration) did not explain the observed differences, we exercised WT mice at an intensity that resulted in fatigue at ∼52 min, similar to the AMPK KD mice. In these animals PGC-1α was increased in SS mitochondria (+20%), suggesting that the shorter run time in the AMPK KD mice did not explain the absence of PGC-1α mitochondrial translocation, further supporting the interpretation that AMPK participates in mediating mitochondrial PGC-1α content with exercise. In addition, similar to the human data, but in contrast to the rat, exercise did not alter mitochondrial Tfam content in either WT or AMPK KD mice (Fig. 5C and D).
Figure 4. Acute 5-aminoimidazole-4-carboxamide-1-β-ribofuranoside (AICAR) treatment increases PGC-1α and Tfam in a mitochondrial population-specific manner.
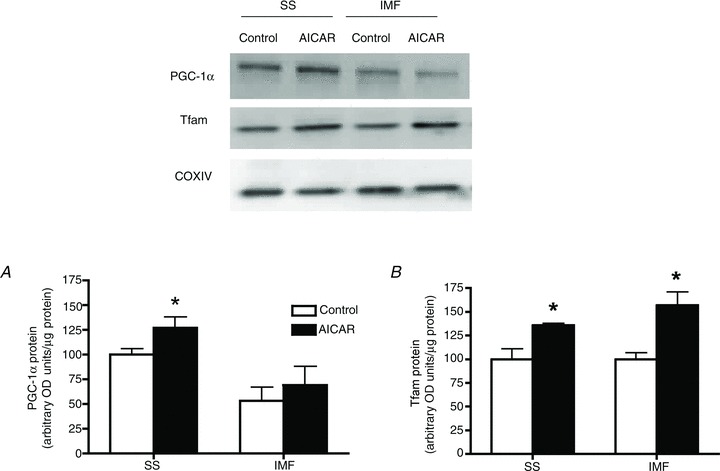
Rat skeletal muscle was harvested 60 min after an intraperitoneal injection (1 mg g body weight−1) of AICAR. A, acute AICAR treatment increases PGC-1α in SS mitochondria. B, acute AICAR treatment increases Tfam in both SS and intermyofibrillar (IMF) mitochondria; n = 4 for all independent experiments. Data are expressed as mean ± SEM. *P < 0.05, significantly different from rest. Ten micrograms of mitochondrial protein was loaded for all conditions.
Figure 5. Differences in exercise-induced PGC-1α translocation between AMPK KD and WT mice.
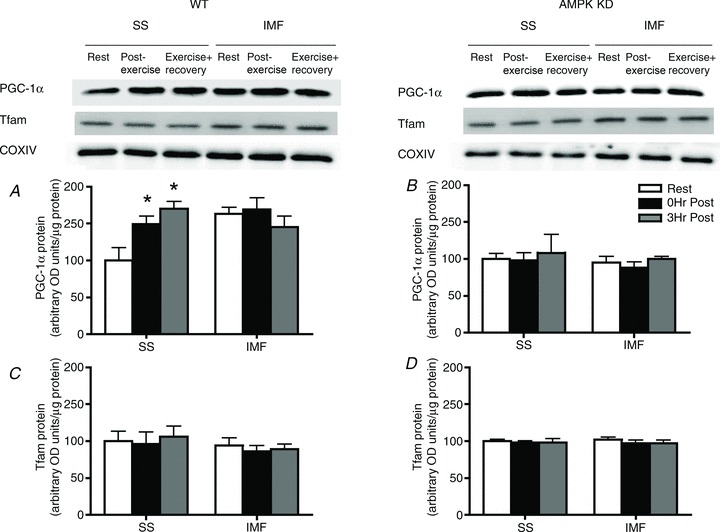
A, following exhaustive exercise, PGC-1α is increased in the subsarcolemmal (SS) mitochondria of WT mice immediately post and 3 h post exercise. B, in contrast, in AMPK KD mice, exercise did not induce PGC-1α translocation to either mitochondrial population. C and D, Tfam is not increased in either mitochondrial population following exhaustive exercise; n = 4 for all independent experiments. Data are expressed as mean ± SEM. *P < 0.05, significantly different from rest. Ten micrograms of mitochondrial protein was loaded for all conditions. IMF, intermyofibrillar mitochondria.
Cytosolic PGC-1α and Tfam subcellular location
We next performed co-immunoprecipitation experiments in rats to determine if PGC-1α and Tfam co-localize in the cytosol. To accomplish this, we immunoprecipitated Tfam (Fig. 6), as well as the reverse experiment immunoprecipitating PGC-1α (Fig. 6), to determine the presence of various proteins. In both experiments we provide evidence that PGC-1α and Tfam co-localize in the cytosol, where they are both bound to α-tubulin (Fig. 6). The immunoprecipitation samples were devoid of mitochondrial membranes and LDH, suggesting only local proteins were immunoprecipated with PGC-1α and Tfam. The α-tubulin band is in close proximity to a potential IgG artifact, and therefore we also performed negative control experiments devoid of muscle. The absence of a band at ∼55 kDa in these experiments (Fig. 6) suggests the α-tubulin band observed in the immunoprecipitation experiments does not represent an artifact. This interpretation is further supported by the observation that performing immunoprecipitation experiments in muscle that is excessively homogenized results in the absence of interactions between Tfam, PGC-1α and α-tubulin (supplementary Fig. S1). Together, these data indicate that both PGC-1α and Tfam proteins may be bound to α-tubulin in close proximity to one another, suggesting that they are available for intracellular trafficking.
Figure 6. PGC-1α and Tfam co-localize with α-tubulin in the cytosol.
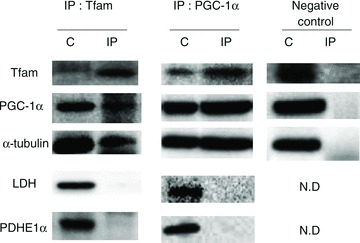
PGC-1α and Tfam co-immunoprecipitate (IP) with each other and with α-tubulin. In contrast, lactate dehydrogenase (LDH), cytochrome c oxidase complex IV (COXIV) and pyruvate dehydrogenase (PDHE1α) do not co-immunoprecipitate with Tfam. Gels were cut simultaneously; n = 4 for all independent experiments. Two independent representative blots shown. C, control elutant wash-through.
Discussion
In the present study we provide evidence that PGC-1α can be induced by exercise to translocate to the SS mitochondria in humans, rat and mice and that this translocation event is potentially mediated by AMPK. Additionally, the role of Tfam translocation is somewhat contentious and species specific, but a cellular mechanism may exist as we show that Tfam and PGC-1α co-localize with α-tubulin in the cytosol. As a functional constituent of cellular trafficking processes, α-tubulin plays an important role in regulating translocation events within the cell. The present experiments illustrate the concept of a higher order organization within the cell where proteins are housed in the cytosol and can be induced to translocate along the cellular highways (microtubules) via the activation of specific signalling events (AMPK).
PGC-1α translocation to the mitochondria
The exercise-induced translocation of PGC-1α to mitochondria appears to be conserved across cell-culture lines (Aquilano et al. 2010), rats (current study), mice (current study; Safdar et al. 2011) and humans (current study). While this appears to be conserved across species, it is not conserved between mitochondrial subpopulations, as PGC-1α only increased in SS mitochondria following exercise in all species. Given that SS mitochondria display greater malleability, the exclusive increase in PGC-1α within this mitochondrial subpopulation may partially explain the preferential biogenesis in these mitochondria following exercise training (Krieger et al. 1980). The mechanism responsible for the selective increase in SS mitochondrial PGC-1α protein following exercise remains unknown but may be mediated by greater rates of protein import (Takahashi & Hood, 1996). In addition, based on the concept of cellular compartmentalization, cytosolic pools of PGC-1α are probably organized in a well-ordered manner throughout the cell. The SS mitochondrial fraction is located in close proximity to the nucleus and given that PGC-1α has previously been shown to translocate to the nucleus following exercise (Wright et al. 2007b) combined with the present data showing PGC-1α translocation to SS mitochondria, we hypothesize that PGC-1α may be concentrated within this region and be more readily available for translocation to the SS fraction. While this hypothesis has yet to be investigated, if correct it would suggest that PGC-1α translocation to IMF mitochondria would be delayed following exercise, as opposed to improbable. Therefore, future research should also focus on determining when, and if, IMF mitochondrial PGC-1α translocation occurs. Despite the lack of PGC-1α translocation to IMF mitochondria, IMF mitochondria have been shown to respond to training (Bizeau et al. 1998). Therefore, it is unclear if the resting content of PGC-1α protein within IMF mitochondria is sufficient to regulate exercise-induced adaptations, or if PGC-1α translocation to IMF mitochondria occurs at a later time point in recovery (i.e. >3 h).
Nevertheless, evidence derived from the present study in humans, rats and mice, in concert with previous studies (Aquilano et al. 2010; Safdar et al. 2011), lends itself to support the hypothesis that PGC-1α may be important in the regulation of exercise-induced transcription of the mitochondrial genome.
Translocation of Tfam to the mitochondria
The translocation of Tfam to the mitochondria following exercise is an attractive hypothesis given the significant role Tfam plays in mitochondrial biogenesis (Virbasius & Scarpulla, 1994). Tfam regulates the expression of the mitochondrial genome by binding upstream of the light-strand promoter, where it is believed to assist with either the assembly of the initiation complex or the melting of the promoter (Dairaghi et al. 1995; Litonin et al. 2010). In the current study, despite the observation that Tfam is induced to translocate to the SS and IMF mitochondria following exercise (and AICAR) in rats, Tfam translocation was not found to be present in humans or mice following exercise. It is unclear why this species variation exists. Although we attempted to match exercise intensity and duration between species, the rats ran for a longer duration and at a higher intensity, suggesting the observed species differences may be related to the intensity of the exercise. However, both rats and mice were exercised to exhaustion, and WT mice and humans exercised for longer than the exposure time following AICAR in rats (60 min), suggesting this is an unlikely explanation. Given the apparent transient response of mitochondrial Tfam translocation in rats it is also possible that the timing is different across species.
An alternative explanation for these disparate results between species may be related to species-dependent requirements for mtDNA stabilization. The yeast homologue of Tfam, ABF2, while not obligatory for mtDNA transcriptional events, is required for mtDNA maintenance (Xu & Clayton, 1992). Evidence is also available suggesting that Tfam, and its homologues, has a primary role in stabilizing mtDNA by binding, wrapping and packaging mtDNA (Wang & Bogenhagen, 2006; Kang et al. 2007). Therefore, Tfam may translocate to mitochondria in rats during exercise to stabilize mtDNA, as opposed to regulating transcription, a response not required in mice or humans. This concept of mtDNA stabilization may also account for the difference in response time between Tfam and PGC-1α translocation within the rat model. To speculate, during exercise there may be a requirement for mtDNA stabilization such that Tfam translocates to perform this function. Once exercise ceases, the requirement for stabilization is no longer present and Tfam is exported out of the mitochondria.
While these proposals remain highly speculative, given that Tfam does not appear to translocate to mitochondria in response to exercise in mice (present study; Safdar et al. 2011), nor, more importantly, in human skeletal muscle, the relevance of mitochondrial Tfam translocation remains ambiguous.
The role of AMPK in exercise-induced translocation
In the current study, we attempted to determine if AMPK signalling is involved in the redistribution of PGC-1α to mitochondria utilizing two independent approaches: (1) acute AICAR treatment to activate AMPK in rats, and (2) exercise in WT and AMPK KD mice. Both approaches implicate AMPK as a primary mediator of PGC-1α mitochondrial translocation. Specifically, AICAR increased mitochondrial PGC-1α protein in SS mitochondria, as well as AMPK phosphorylation without affecting p38 MAPK phosphorylation. In addition, while exercise increased SS mitochondrial PGC-1α content in WT mice, this response was completely ablated in AMPK KD mice. Considering the hypothesized role of PGC-1α regarding the direct regulation of the mitochondrial genome (Aquilano et al. 2010; Safdar et al. 2011), the finding that AMPK is required for PGC-1α mitochondrial translocation may partially account for why AICAR administration in AMPK α2 knockout (KO) mice is unsuccessful at increasing the expression of COX1, a mitochondrial encoded protein (Jorgensen et al. 2007), and why β-guanidinopropionic acid (which can activate AMPK) does not induce mtDNA increases in AMPK KD mice (Zong et al. 2002). However, chronic exercise can still induce mitochondrial biogenesis in mice lacking either PGC-1α or AMPK α2 (Jorgensen et al. 2005; Leick et al. 2008), suggesting that translocation of PGC-1α to the mitochondria is probably not essential for regulating the mitochondrial genome. Additionally, a recent report in muscle-specific PGC-1α KO mice has concluded that PGC-1α is not required for exercise-induced skeletal muscle mitochondrial biogenesis, indicating that redundant mechanisms to promote mitochondrial biogenesis probably exist (Rowe et al. 2012). Therefore, mitochondrial PGC-1α may augment the regulatory function of Tfam at the D-loop of the mtDNA and act as a putative mitochondrial genomic cofactor, although redundant mechanisms to promote the transcription of mtDNA are also likely to be present. This proposed cofactor function of PGC-1α in skeletal muscle mitochondrial gene expression would mirror the regulatory role of PGC-1α within the nucleus (Wu et al. 1999; Safdar et al. 2011).
Conclusion
In summary, we provide evidence in humans, rats and mice that PGC-1α can be induced to translocate to the SS mitochondria in response to acute exercise. In addition, mitochondrial PGC-1α translocation appears to require AMPK signalling. This AMPK-dependent preferential translocation of PGC-1α to the SS mitochondria may help to explain the heightened malleability of this mitochondrial population in response to physiological stimuli. In contrast, the translocation of Tfam to mitochondria appears to be species dependent and additional work examining mitochondrial Tfam translocation is warranted.
Acknowledgments
This work was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC), and infrastructure was purchased with assistance from NSERC and the Canadian Foundation for Innovation.
Glossary
- AICAR
5-aminoimidazole-4-carboxamide-1-β-ribofuranoside
- AMPK
AMP-activated protein kinase
- CaMK
calcium/calmodulin dependent protein kinase
- COXIV
cytochrome c oxidase complex IV
- H2B
histone H2B
- IMF
intermyofibrillar mitochondria
- KD
kinase dead
- LDH
lactate dehydrogenase
- PDHE1α
pyruvate dehydrogease
- PGC-1α
peroxisome proliferator-activated receptor γ co-activator 1α
- p38 MAPK
p-38 mitogen-activated protein kinase
- SS
subsarcolemmal mitochondria
- Tfam
mitochondrial transcription factor A
- WT
wild-type
Author contributions
B.K.S. and G.P.H. designed and performed the experiments, analysed and interpreted the data and wrote the manuscript. K.M., B.J.G., J.L. and A.C.M. performed the experiments, analysed and interpreted the data and edited the manuscript. G.J.F.H. and L.L.S. designed and performed the experiments, interpreted the data and edited the manuscript.
Supplementary material
Supplementary Materials
References
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1α transcription in skeletal muscle through activation of the p38 MAPK pathway 1. J Biol Chem. 2005;280:19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- Aquilano K, Vigilanza P, Baldelli S, Pagliei B, Rotilio G, Ciriolo MR. Peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) and sirtuin 1 (SIRT1) reside in mitochondria: possible direct function in mitochondrial biogenesis. J Biol Chem. 2010;285:21590–21599. doi: 10.1074/jbc.M109.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton CR, Holloway GP, Campbell SE, Yoshida Y, Tandon NN, Glatz JF, Luiken JJ, Spriet LL, Bonen A. Rosiglitazone increases fatty acid oxidation and fatty acid translocase (FAT/CD36) but not carnitine palmitoyltransferase I in rat muscle mitochondria. J Physiol. 2008a;586:1755–1766. doi: 10.1113/jphysiol.2007.146563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton CR, Nickerson JG, Lally J, Han XX, Holloway GP, Glatz JF, Luiken JJ, Graham TE, Heikkila JJ, Bonen A. Modest PGC-1α overexpression in muscle in vivo is sufficient to increase insulin sensitivity and palmitate oxidation in subsarcolemmal, not intermyofibrillar, mitochondria. J Biol Chem. 2008b;283:4228–4240. doi: 10.1074/jbc.M704332200. [DOI] [PubMed] [Google Scholar]
- Bizeau ME, Willis WT, Hazel JR. Differential responses to endurance training in subsarcolemmal and intermyofibrillar mitochondria. J Appl Physiol. 1998;85:1279–1284. doi: 10.1152/jappl.1998.85.4.1279. [DOI] [PubMed] [Google Scholar]
- Dairaghi DJ, Shadel GS, Clayton DA. Human mitochondrial transcription factor A and promoter spacing integrity are required for transcription initiation 2. Biochim Biophys Acta. 1995;1271:127–134. doi: 10.1016/0925-4439(95)00019-z. [DOI] [PubMed] [Google Scholar]
- Gordon JW, Rungi AA, Inagaki H, Hood DA. Effects of contractile activity on mitochondrial transcription factor A expression in skeletal muscle 3. J Appl Physiol. 2001;90:389–396. doi: 10.1152/jappl.2001.90.1.389. [DOI] [PubMed] [Google Scholar]
- Holloway GP, Gurd BJ, Snook LA, Lally J, Bonen A. Compensatory increases in nuclear PGC1α protein are primarily associated with subsarcolemmal mitochondrial adaptations in ZDF rats. Diabetes. 2010;59:819–828. doi: 10.2337/db09-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway GP, Jain SS, Bezaire VS, Han XX, Glatz JF, Luiken JJ, Harper ME, Bonen A. FAT/CD36 null mice reveal that mitochondrial FAT/CD36 is required to up-regulate mitochondrial fatty acid oxidation in contracting muscle. Am J Physiol Regul Integr Comp Physiol. 2009;297:R960–R967. doi: 10.1152/ajpregu.91021.2008. [DOI] [PubMed] [Google Scholar]
- Hoppel CL, Moghaddas S, Lesnefsky EJ. Interfibrillar cardiac mitochondrial comples [sic] III defects in the aging rat heart. Biogerontology. 2002;3:41–44. doi: 10.1023/a:1015251212039. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF, Richter EA. Role of AMPKα2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am J Physiol Endocrinol Metab. 2007;292:E331–E339. doi: 10.1152/ajpendo.00243.2006. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H. Effects of α-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005;19:1146–1148. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]
- Kang D, Kim SH, Hamasaki N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion. 2007;7:39–44. doi: 10.1016/j.mito.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Krieger DA, Tate CA, Millin-Wood J, Booth FW. Populations of rat skeletal muscle mitochondria after exercise and immobilization. J Appl Physiol. 1980;48:23–28. doi: 10.1152/jappl.1980.48.1.23. [DOI] [PubMed] [Google Scholar]
- Lally JS, Snook LA, Han XX, Chabowski A, Bonen A, Holloway GP. Subcellular lipid droplet distribution in red and white muscles in the obese Zucker rat. Diabetologia. 2012;55:479–488. doi: 10.1007/s00125-011-2367-2. [DOI] [PubMed] [Google Scholar]
- Leick L, Wojtaszewski JF, Johansen ST, Kiilerich K, Comes G, Hellsten Y, Hidalgo J, Pilegaard H. PGC-1α is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E463–E474. doi: 10.1152/ajpendo.00666.2007. [DOI] [PubMed] [Google Scholar]
- Litonin D, Sologub M, Shi Y, Savkina M, Anikin M, Falkenberg M, Gustafsson CM, Temiakov D. Human mitochondrial transcription revisited: only TFAM and TFB2M are required for transcription of the mitochondrial genes in vitro. J Biol Chem. 2010;285:18129–18133. doi: 10.1074/jbc.C110.128918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little JP, Safdar A, Cermak N, Tarnopolsky MA, Gibala MJ. Acute endurance exercise increases the nuclear abundance of PGC-1α in trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2010;298:R912–R917. doi: 10.1152/ajpregu.00409.2009. [DOI] [PubMed] [Google Scholar]
- Norrbom J, Sundberg CJ, Ameln H, Kraus WE, Jansson E, Gustafsson T. PGC-1α mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J Appl Physiol. 2004;96:189–194. doi: 10.1152/japplphysiol.00765.2003. [DOI] [PubMed] [Google Scholar]
- Palmer JW, Tandler B, Hoppel CL. Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: effects of procedural manipulations. Arch Biochem Biophys. 1985;236:691–702. doi: 10.1016/0003-9861(85)90675-7. [DOI] [PubMed] [Google Scholar]
- Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol. 2010;588:4795–4810. doi: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogozelski AR, Geng T, Li P, Yin X, Lira VA, Zhang M, Chi JT, Yan Z. p38γ Mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PLoS One. 2009;4:e7934. doi: 10.1371/journal.pone.0007934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe GC, El-Khoury R, Patten IS, Rustin P, Arany Z. PGC-1α is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle. PLoS One. 2012;7:e41817. doi: 10.1371/journal.pone.0041817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA. Exercise increases mitochondrial PGC-1α content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem. 2011;286:10605–10617. doi: 10.1074/jbc.M110.211466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Smith BK, Jain SS, Rimbaud S, Dam A, Quadrilatero J, Ventura-Clapier R, Bonen A, Holloway GP. FAT/CD36 is located on the outer mitochondrial membrane, upstream of long-chain acyl-CoA synthetase, and regulates palmitate oxidation. Biochem J. 2011;437:125–134. doi: 10.1042/BJ20101861. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Hood DA. Protein import into subsarcolemmal and intermyofibrillar skeletal muscle mitochondria. Differential import regulation in distinct subcellular regions. J Biol Chem. 1996;271:27285–27291. doi: 10.1074/jbc.271.44.27285. [DOI] [PubMed] [Google Scholar]
- Uguccioni G, Hood DA. The importance of PGC-1α in contractile activity-induced mitochondrial adaptations. Am J Physiol Endocrinol Metab. 2011;300:E361–E371. doi: 10.1152/ajpendo.00292.2010. [DOI] [PubMed] [Google Scholar]
- Virbasius JV, Scarpulla RC. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci U S A. 1994;91:1309–1313. doi: 10.1073/pnas.91.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Bogenhagen DF. Human mitochondrial DNA nucleoids are linked to protein folding machinery and metabolic enzymes at the mitochondrial inner membrane. J Biol Chem. 2006;281:25791–25802. doi: 10.1074/jbc.M604501200. [DOI] [PubMed] [Google Scholar]
- Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO. Calcium induces increases in peroxisome proliferator-activated receptor γ coactivator-1α and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem. 2007a;282:18793–18799. doi: 10.1074/jbc.M611252200. [DOI] [PubMed] [Google Scholar]
- Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J Biol Chem. 2007b;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Xu B, Clayton DA. Assignment of a yeast protein necessary for mitochondrial transcription initiation. Nucleic Acids Res. 1992;20:1053–1059. doi: 10.1093/nar/20.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


