Abstract
Solar energy is potentially the largest source of renewable energy at our disposal, but significant advances are required to make photovoltaic technologies economically viable and, from a life-cycle perspective, environmentally friendly, and consequently scalable. Cellulose nanomaterials are emerging high-value nanoparticles extracted from plants that are abundant, renewable, and sustainable. Here, we report on the first demonstration of efficient polymer solar cells fabricated on optically transparent cellulose nanocrystal (CNC) substrates. The solar cells fabricated on the CNC substrates display good rectification in the dark and reach a power conversion efficiency of 2.7%. In addition, we demonstrate that these solar cells can be easily separated and recycled into their major components using low-energy processes at room temperature, opening the door for a truly recyclable solar cell technology. Efficient and easily recyclable organic solar cells on CNC substrates are expected to be an attractive technology for sustainable, scalable, and environmentally-friendly energy production.
Organic solar cells are an attractive technology because of their potential for low-cost fabrication, light weight, and good mechanical flexibility1,2,3,4,5. Over the last decade, the power conversion efficiency (PCE) of champion small-area organic solar cells has improved from values around 3.5% up to 10.6%6. Despite having low PCEs and short lifetimes, recent cost-analysis studies suggest that organic solar cells could become competitive with other solar cell technologies if modules with PCE of 5% and a 5 year lifetime could be produced7,8. Polyethylene terephthalate (PET)9,10,11, polyethylene naphthalate (PEN)12, or polyethersulfone (PES)1, have been used for the demonstration of flexible organic solar cells. However, from a life-cycle perspective, these petroleum-based substrates are expensive and environmentally less attractive than easily recyclable or biodegradable substrates. Substrate materials which could be synthesized from renewable feedstocks, such as wood, at a low cost, are particularly attractive for the realization of a sustainable solar cell technology.
Paper is considered an interesting substrate for organic solar cells, because it is inexpensive, low-weight, flexible and it can be recycled. Solution-processed polymer solar cells have been fabricated recently on paper substrates13,14,15, but have shown limited performance because of the high surface roughness and porosity of paper substrates13. Even with the use of thick (several to tens of μm) planarization layers, solar cells on paper showed low performance with maximum PCE values in the range between 0.4–1.4%14,15. Furthermore, the use of thick planarization layers increases the device complexity, cost and may further compromise the recyclability and biodegradability of such devices.
Cellulose nanomaterials (CN) are cellulose-based nanoparticles that have good mechanical properties, high aspect ratio, low density, low thermal expansion, surfaces that can be readily chemically functionalized, low toxicity, are inherently renewable/sustainable, and have the potential to be produced in industrial-size quantities16,17,18,19,20,21,22,23. CNs have been studied for a wide variety of potential applications, including reinforcement phases in polymer composites, protective coatings, barrier/filter membrane systems, antimicrobial films, network structures for tissue engineering, and substrates for flexible electronics. Two general classes of CNs that can be extracted from plants, are cellulose nanocrystals (CNC, 3–10 nm wide by 50–500 nm in length, Fig. 1a) and cellulose nanofibers (CNF, 4–20 nm wide by > 1 μm in length). Neat and polymer composite films produced from CNCs and CNFs are attractive as substrates for organic electronic devices, and organic solar cells in particular, because they combine low density (1–1.5 g/cm3) with high tensile strength (30–240 MPa), high elastic modulus (6–30 GPa) and low coefficient of thermal expansion (CTE, 2–25 ppm/K)20,24,25,26. CNCs are also found to be thermally stable up to 210°C, and after processing optimization, up to 350°C; hence, they are compatible with the processing of organic semiconductors27. Recently, polymer solar cells have been fabricated on CNF substrates. However, these devices exhibited poor performance (with a maximum PCE of 0.4%) and poor rectification, mainly because of the relatively rough surface of the CNF substrates (with a surface height variation of 40 nm)22.
Figure 1.
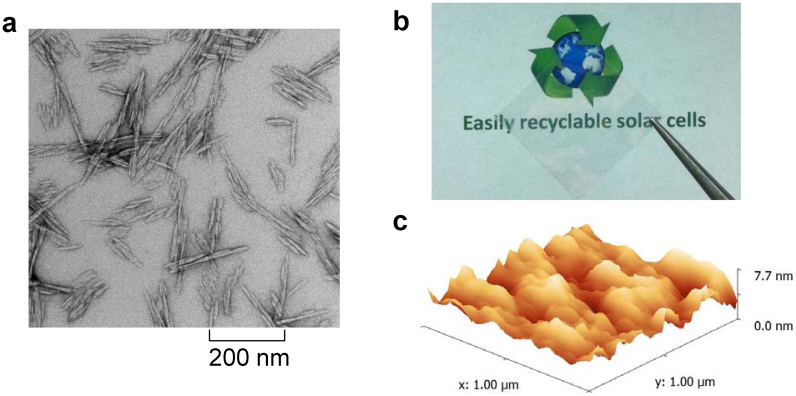
(a) A transmission electron microscopy (TEM) image of isolated CNCs which comprise the film used as the substrate for solar cells; (b) a picture of the CNC film on top of paper to demonstrate its transparency; (c) an atomic force microscopy image of the surface of a CNC film.
In this work, we report on polymer solar cells on free-standing transparent CNC substrates with much lower surface roughness compared with the CNF-based films. The solar cells are fabricated with Ag/polymer surface modification as the bottom electrode and MoO3/Ag as the top electrode without the need of aqueous solution. The solar cells show a large rectification in the dark and an average PCE of 2.7% and an average fill factor of 0.54 under illumination. The performance of these polymer solar cells is shown to be limited primarily by the transmittance of the thin Ag layer used as the semitransparent bottom electrode. Importantly, the polymer solar cells fabricated on CNC substrates are found to be easily recycled at room temperature by simply immersing them in water, where the CNC substrate is redispersed. The dissolution of the substrate in water leads to a separation of the rest of the components of the solar cell in the form of a thin polymer solar cell membrane comprised of the synthetic organic photoactive layer and the metal layers. These membranes can be easily filtered out of the water solution, and the organic and metal components can then be separated by immersing the membrane into an organic solvent in which the photoactive layer can be dissolved, leaving behind the metal and oxide electrode that can be filtered out of the solution.
Results
Properties of CNC substrates
Fig. 1b illustrates the high optical transparency of the CNC film that is necessary for incident sunlight to pass through the substrate. The optical transparency is greatly improved in thinner films as shown in Fig. S1 in the supplementary information (SI). The limited transmittance of CNC films is believed to be due to scattering, not absorption, caused by the random distribution of CNCs (a few hundred nanometers long, Fig. 1a) in the film, which causes refractive index inhomogeneities over areas with dimensions that are of the same order of magnitude of the wavelength of visible light. Scattering spreads the incident light into a large solid angle, reducing the intensity (energy per solid angle) reaching a detector and thus resulting in a reduced transmittance. However, when a solar cell is fabricated on a CNC substrate, after light passes through the substrate, even those components that are scattered far away from the sample normal can reach the active layer, where they can be absorbed and contribute to the current generated by the solar cell. Fig. 1c shows the surface morphology of a CNC substrate. Averaged over three locations, the root-mean-square (RMS) value of the surface roughness is 1.8 ± 0.6 nm. The very smooth surface eliminates the need for any surface planarization. To fabricate polymer solar cells on the CNC substrates, a transparent or semitransparent electrode is needed for light to reach the photoactive layer. For the bottom electrode (i.e. in contact with the CNC substrate), a semitransparent 20-nm thick Ag layer was deposited by vacuum thermal evaporation on a CNC substrate and was found to be conductive (i.e. above percolation). Ag films deposited simultaneously on bare glass were found to be below the percolation threshold due to wetting limitations, and consequently nonconductive. Based on our recent discovery1, we modified the Ag film using a thin layer of ethoxylated polyethylenimine (PEIE) to turn silver into an efficient electron-collecting electrode. For the top electrode, MoO3/Ag was evaporated onto the photoactive layer of [poly[(4,8-bis-(2-ethylhexyloxy)-benzo[1,2-b:4,5-b′]dithiophene)-2,6-diyl-alt-(4-(2-ethylhexanoyl)-thieno[3,4-b]thiophene)-2,6-diyl]: phenyl-C61-butyric acid methyl ester] (PBDTTT-C:PCBM, Fig. 2b) to collect holes. The device geometry is shown in Fig. 2a. It should be noted that the spin-coating of PEIE from a 2-methoxyethanol solution did not damage the CNC substrates. The latter were also found to allow the spin-coating of the PBDTTT-C:PCBM photoactive layer from a chlorobenzene: 1,8-diiodooctane (97:3, v/v) solution. A fabricated solar cell is shown in Fig. 2c. The high and specular reflectivity of the Ag top electrode further demonstrates the surface smoothness of the CNC substrates and the uniformity of the active layer on the CNC substrates.
Figure 2.
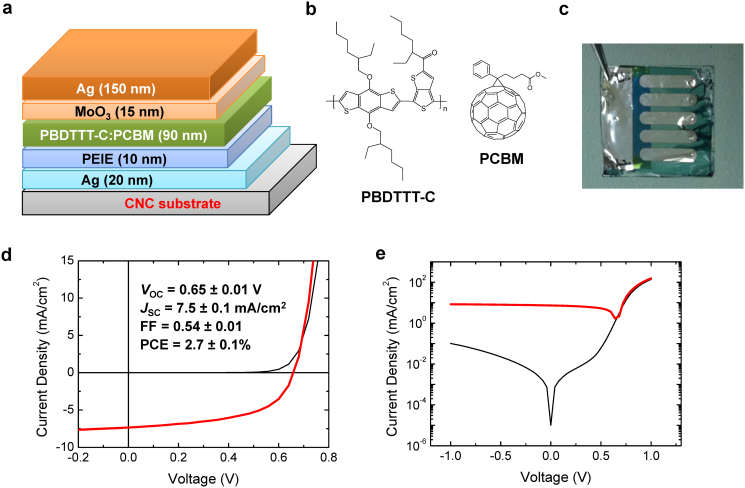
(a) Device structure of solar cells on CNC substrates: CNC/Ag/PEIE/PBDTTT-C:PCBM/MoO3/Ag; (b) Chemical structure of PBDTTT-C and PCBM; (c) a picture of a fabricated solar cell; (d) J–V characteristics of the solar cell on CNC substrate in the dark (thin black line) and under 95 mW/cm2 of AM1.5 illumination (thick red line); (e) the J–V characteristics on a semi-logarithmic scale in the dark (thin black line) and under illumination (thick red line).
Performance of solar cells on CNC substrates
Fig. 2d shows the current density-voltage (J–V) characteristic of a solar cell fabricated on a CNC substrate in the dark and under illumination. In the dark, the device shows low reverse saturation current and large rectification ratio of 103 at ±1 V (Fig. 2e). This indicates few pin holes and a large work function contrast between Ag/PEIE and MoO3/Ag. Under 95 mW/cm2 of AM 1.5G illumination, the devices show VOC = 0.65 ± 0.01 V, JSC = 7.5 ± 0.1 mA/cm2, and FF = 0.54 ± 0.01, yielding PCE = 2.7 ± 0.1%, averaged over 3 devices. Although this is still modest performance compared to state-of-the-art devices, it represents a significant improvement over previously demonstrated organic solar cells on paper-like or CNF substrates14,22. Furthermore, we recently reported that devices with a structure: Glass/ITO/PEIE/PBDTTT-C:PCBM/MoO3/Ag yield values of VOC = 0.68 ± 0.01 V, JSC = 16.1 ± 0.4 mA/cm2, FF = 0.61 ± 0.01, and PCE = 6.6 ± 0.2%, averaged over 5 devices1. Remarkably, the VOC and FF of the solar cells on a CNC substrate are not that different to the ones obtained on a glass/ITO substrate. This is in contrast to previous realizations of polymer solar cells on paper-like substrates, wherein the electrical performance of the solar cells, namely the VOC and FF values, were found to be significantly lower than those on devices fabricated on glass or plastic substrates13,14,22. Hence, the lower PCE value obtained, is mainly attributed to the smaller JSC value on solar cells processed on CNC/Ag substrates as compared to the JSC value obtained on glass/ITO substrates. This is caused by the lower transmittance of both the CNC substrates compared to glass, and of the 20-nm-thick Ag electrode compared to ITO28. The ability to tune CNC substrates (composition, orientation, interfaces, etc.) should allow further optimization of its optical and mechanical properties. Likewise, if the Ag film (bottom electrode) can be replaced with a higher transmittance material (e.g. metal-oxide or conducting polymer) further improvements in the performance level of these polymer solar cells fabricated on CNC substrates can be achieved, and possibly comparable to devices fabricated on glass or petroleum-based flexible substrates.
Recyclability of solar cells on CNC substrates
Recyclability of the CNC substrates and solar cells was tested by immersing them into distilled water. Fig. S2 displays time-lapse images that illustrate the dissolution of the CNC substrates in water. The CNC film quickly swells after being immersed into water and completely disintegrates within 30 min. The redispersed CNC turns into a solid residue on the petri dish after the water has evaporated. This residue can potentially be recovered and recycled. As for the solar cell, the CNC substrate also swells rapidly producing clear warping of the photoactive layer and electrodes (insets of Fig. S2) until they turn into a free-standing film or membrane. This allows for the full separation of the solar cell components (substrate, organic and inorganic materials) at room temperature by using a filter paper. A video illustrating the process whereby the materials of a solar cell can be easily recovered is available in the SI. Fig. 3a displays vials containing the solutions and the final residue on the filter paper to illustrate the final products of this recycling process. The process is described as follows: a solar cell was immersed into the vial containing distilled water until the CNC substrate disintegrated (shaking accelerates the disintegration to less than 10 min) and the solid residues were filtered from the liquid using a filter paper. The resulting distilled water waste appears as a milky dispersion of CNCs in water, shown in vial #1 in Fig. 3a. Distilled water is shown as a clear liquid in vial #0 in Fig. 3a as a visual reference. The photoactive layer was then separated from the electrodes by rinsing the solid residues on the filter paper with chlorobenzene. This process resulted in a green-colored solution of a mixture of chlorobenzene and PBDTTT-C:PCBM, as shown in vial #2 in Fig. 3a. A second rinse with chlorobenzene revealed that most of the active layer could be dissolved during the first rinse, as illustrated by the clear color of the vial #3 in Fig. 3a. The solid waste left in the filter paper shown in #4 in Fig. 3a, therefore, corresponds primarily to the Ag and MoO3 used as electrodes on the solar cell. In this way, organic solar cells fabricated on CNC substrates can be easily separated into their major components using a minimal amount of solvents and energy. Furthermore, solar cells on CNC substrates that were exposed to low temperature flame (to burn off the polymer components), produced ashes from which the metal components could be recovered. Fig. 3b displays images of solar cells burning and rapidly reducing into ashes. While burning may not be an ideal way to dispose of the devices, the ability to burn them, leaving a residue of ashes, is dramatically different than what could be obtained using glass or plastic substrates.
Figure 3.
(a) Vials (#0-3) and filter paper (#4) illustrating the separation of solar cells into their major components by immersion in water and chlorobenzene. Vial #0: distilled water; Vial #1: CNC redispersed in distilled water after solar cells were immersed into water; Vial #2: solution of photoactive layer in chlorobenzene obtained by rinsing the solid waste left after the immersion into water; Vial #3: solution generated by the second rinsing the solid waste with chlorobenzene; #4: solid residues left on the filter paper after the second rinsing with chlorobenzene. The inset is a close-up of the solid waste left on the filter paper showing residues of Ag and MoO3. (b) Time lapse sequence of three frames illustrating the ignition of solar cells on CNC substrates: #1: an image of a solar cell before burning; #2: while burning; #3: after burning. Burning lasted less than 2 s.
Discussion
Efficient and recyclable polymer solar cells fabricated on free-standing cellulose nanocrystal substrates have been demonstrated here. The cellulose nanocrystal substrates are optically transparent enabling light to go through, and have a low surface roughness (RMS value of 1.8 nm) which is critical for the thin polymer solar cells to work nicely with very low reverse leakage current and large rectification ratio in the dark. Furthermore, the cellulose nanocrystal substrates allow the deposition of polymers using coating techniques from non-aqueous solutions. The polymer solar cells on cellulose nanocrystal substrates reached a power conversion efficiency of 2.7%, an unprecedented level of performance for polymer solar cell fabricated on recyclable substrates derived from renewable feedstocks. The power conversion efficiency of these solar cells was found to be primarily limited by the low transmittance of the Ag bottom electrode. Optimization of the optical properties of the bottom electrode should lead to significant future improvements on the power conversion efficiency, for instance, by reducing the thickness of the Ag layer and by introducing a layer with a high reflective index material between the substrate and the Ag layer29 or by depositing a transparent metal-oxide electrode. If this is realized, polymer solar cells with a power conversion efficiency that is similar to that of solar cells fabricated on glass or petroleum-based plastic substrates should be achievable. We have shown that these solar cells can be easily separated into their major components using low-energy processes at room temperature, opening the door for a truly fully recyclable solar cell technology. Efficient and easily recyclable polymer solar cells on cellulose nanocrystal substrates could be an ideal technology for sustainable, scalable and environmentally-friendly energy production and could have an overreaching impact for the sustainability of printed electronics.
Methods
Preparation and characterization of CNC samples
CNCs were produced at USDA Forest Service-Forest Products Laboratory (Madison, WI) following procedures described by Beck-Candanedo et al.30 CNC suspensions were produced by sulfuric acid hydrolysis of softwood pulp (64% sulfuric acid, 8 to 1 acid to pulp weight ratio, 45°C, 60 minutes) followed by quenching with deionized water, centrifuge rinsing, washing, and then dialysis for about a week to remove remaining acid. The suspension was then ultrasonicated to disperse the CNCs via mechanical agitation and centrifuged a final time for macroparticle removal. Films were prepared by blending 1.65 wt.% CNC suspension (30 g) with 1 wt.% glycerol solution (4.95 g) for 24 hours. Glycerol (Aldrich) was added to make the films more flexible for handling. The homogeneous glycerol/CNC water suspension was then poured into plastic 80 mm diameter plastic petri dishes and allowed to dry at 23°C and 30%–40% relative humidity. The dried CNC/glycerol films were detached from petri dishes and cut into 2.5 cm × 2.5 cm glycerol/CNC substrates. Note that the addition glycerol is consistent with renewable and biodegrade theme of the CNC film and it is non-toxic and is a byproduct of biodiesel production. The optical transmittance of CNC substrates was measured using a spectroscopic ellipsometer apparatus (M-2000UI, J.A. Woollam Co.). The surface roughness of the CNC samples was measured under atmospheric conditions using atomic force microscopy (Dimension 3100, Veeco) equipped with a NanoScope III controller.
Fabrication and characterization of solar cells on CNC substrates
First, the CNC films were attached to rigid glass substrates with a piece of cured polydimethylsiloxane (PDMS). Then, a 20-nm thick Ag film was deposited on half of the CNC substrates through a shadow mask, using a vacuum thermal evaporation system (SPECTROS, Kurt J. Lesker). Then, the polymer modification layer, PEIE (423475, Mw = 70,000 g/mol, from Sigma-Aldrich) was deposited on Ag by spin-coating at a speed of 4000 rpm for 1 minute from a 0.4 wt.% 2-methoxyethanol (284467, 99.8% anhydrous, from Sigma-Aldrich Co.) solution and annealed on a hot plate at 80°C for 5 minutes. The average thickness of the PEIE is estimated to be 10 nm, from measurements by spectroscopic ellipsometry on independent films deposited on Si substrates1. After the substrates cooled down for 10 minutes, a layer of PBDTTT-C (Solarmer Materials Inc): PCBM (1:1.5 by weight, Nano-C Inc.) was spin-coated on the substrates as the photoactive layer from a mixture of chlorobenzene:1,8-diiodooctane (97:3, v/v) solution with a total concentration of 25 mg/ml at a speed of 1000 rpm and 10000 rpm/s acceleration for 1 minute. The thickness of the photoactive layer was 90 nm. All the processing was done in a N2-filled glove box. Samples were transferred into the vacuum thermal evaporation system (SPECTROS, Kurt J. Lesker) and the top electrode of MoO3/Ag (15 nm/150 nm) was deposited to finish the device fabrication. Current density-voltage (J–V) characteristics of the solar cells were measured inside the N2-filled glove box by using a source meter (2400, Keithley Instruments, Cleveland, OH) controlled by a LabVIEW program. To test the solar cell properties under illumination, a calibrated 300 W Oriel solar simulator (91160, Newport) with an intensity of 95 mW/cm2 was used as the light source.
Recycling and combustion of solar cells
A piece of CNC sample and a piece of solar cell on CNC substrate were immersed into distilled water in a glass petri dish until the CNC were redispersed at room temperature. Another piece of solar cell on a CNC substrate was immersed in distilled water in a vial. The solution was filtered using a P5 Filter paper (Fisher Scientific). The solid waste in the filter was rinsed with chlorobenzene and the waste collected in a vial. Chlorobenzene rinsing was repeated for the second time. For the combustion test, a piece of CNC sample and a piece of solar cell on CNC were ignited using a commercial lighter and burned inside a fume hood.
Author Contributions
B.K., C.F., R.J.M. and J.P.Y. conceived this project. Y.Z., T.K. and J.S. designed and fabricated the solar cells. J.L. fabricated the CNC films. J.H. and A.D. collected the data of transmittance and surface morphology of the films. Y.Z. and C.F. performed the recycling experiments of solar cells. Y.Z. and C.F. wrote the draft of the manuscript, and all authors contributed to the edits of the manuscript.
Supplementary Material
Video of the demonstration of the recycling and the burning of the solar cells
Supplementary info_transmittance of CNC samples and dissolving test in water
Acknowledgments
This research was funded in part through the Center for Interface Science: Solar Electric Materials, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0001084 (Y.Z., J.S., C.F., A.D.), by the Air Force Office of Scientific Research (Grant No. FA9550-09-1-0418) (J. H.), by the Office of Naval Research (Grant No. N00014-04-1-0313) (T.K., B.K.), and the US Department of Agriculture –Forest Service (Grant No. 12-JV-11111122-098). Funding for CNC substrate processing was provided by USDA-Forest Service (Grant No. 11-JV-11111129-118) (R.J.M., J.P.Y., J.L.). The authors thank Rick Reiner and Alan Rudie from the U.S. Forest Service- Forest Products Laboratory (FPL) for providing CNC materials.
References
- Zhou Y. H. et al. A Universal Method to Produce Low-Work Function Electrodes for Organic Electronics. Science 336, 327–332 (2012). [DOI] [PubMed] [Google Scholar]
- Kippelen B. & Bredas J. L. Organic photovoltaics. Energy Environ. Sci. 2, 251–261 (2009). [Google Scholar]
- He Z. et al. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photon. 6, 593–597 (2012). [Google Scholar]
- Brabec C. J. et al. Polymer-fullerene bulk-heterojunction solar cells. Adv. Mater. 22, 3839–3856 (2010). [DOI] [PubMed] [Google Scholar]
- Sondergaard R., Hosel M., Angmo D., Larsen-Olsen T. T. & Krebs F. C. Roll-to-roll fabrication of polymer solar cells. Mater. Today 15, 36–49 (2012). [Google Scholar]
- You J. et al. A polymer tandem solar cell with 10.6% power conversion efficiency. Nat. Commun. 4, 1446 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzopardi B. et al. Economic assessment of solar electricity production from organic-based photovoltaic modules in a domestic environment. Energy Environ. Sci. 4, 3741–3753 (2011). [Google Scholar]
- Espinosa N., Garcia-Valverde R. & Krebs F. C. Life-cycle analysis of product integrated polymer solar cells. Energy Environ. Sci. 4, 1547–1557 (2011). [Google Scholar]
- Zhou Y. et al. Investigation on polymer anode design for flexible polymer solar cells. Appl. Phys. Lett. 92, 233308 (2008). [Google Scholar]
- Formica N. et al. Highly stable Ag–Ni based transparent electrodes on PET substrates for flexible organic solar cells. Sol. Energy Mater. Sol. Cells 107, 63–68 (2012). [Google Scholar]
- Krebs F. C. Polymer solar cell modules prepared using roll-to-roll methods: Knife-over-edge coating, slot-die coating and screen printing. Sol. Energy Mater. Sol. Cells 93, 465–475 (2009). [Google Scholar]
- Wang J.-C. et al. Highly efficient flexible inverted organic solar cells using atomic layer deposited ZnO as electron selective layer. J. Mater. Chem. 20, 862–866 (2010). [Google Scholar]
- Wang F., Chen Z., Xiao L., Qu B. & Gong Q. Papery solar cells based on dielectric/metal hybrid transparent cathode. Sol. Energy Mater. Sol. Cells 94, 1270–1274 (2010). [Google Scholar]
- Kim T.-S. et al. Solution-processible polymer solar cells fabricated on a papery substrate. Phys. Status Solidi RRL 6, 13–15 (2012). [Google Scholar]
- Hübler A. et al. Printed Paper Photovoltaic Cells. Adv. Energy Mater. 1, 1018–1022 (2011). [Google Scholar]
- Vartiainen J. et al. Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 18, 775–786 (2011). [Google Scholar]
- Lin N., Huang J. & Dufresne A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: a review. Nanoscale 4, 3274–3294 (2012). [DOI] [PubMed] [Google Scholar]
- Lavoine N., Desloges I., Dufresne A. & Bras J. Microfibrillated cellulose – Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 90, 735–764 (2012). [DOI] [PubMed] [Google Scholar]
- Habibi Y., Lucia L. A. & Rojas O. J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 110, 3479–3500 (2010). [DOI] [PubMed] [Google Scholar]
- Moon R. J., Martini A., Nairn J., Simonsen J. & Youngblood J. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem. Soc. Rev. 40, 3941–3994 (2011). [DOI] [PubMed] [Google Scholar]
- Klemm D. et al. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 50, 5438–5466 (2011). [DOI] [PubMed] [Google Scholar]
- Hu L. et al. Transparent and conductive paper from nanocellulose fibers. Energy Environ. Sci. 6, 513–518 (2013). [Google Scholar]
- Siró I. & Plackett D. Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose 17, 459–494 (2010). [Google Scholar]
- Nogi M., Iwamoto S., Nakagaito A. N. & Yano H. Optically Transparent Nanofiber Paper. Adv. Mater. 21, 1595–1598 (2009). [Google Scholar]
- Fukuzumi H., Saito T., Iwata T., Kumamoto Y. & Isogai A. Transparent and High Gas Barrier Films of Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation. Biomacromolecules 10, 162–165 (2008). [DOI] [PubMed] [Google Scholar]
- Kim J., Yun S. & Ounaies Z. Discovery of cellulose as a smart material. Macromolecules 39, 4202–4206 (2006). [Google Scholar]
- Yu H. et al. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A 1, 3938–3944 (2013). [Google Scholar]
- Hsu J. et al. Linear and nonlinear optical properties of Ag/Au bilayer thin films. Opt. Express 20, 8629–8640 (2012). [DOI] [PubMed] [Google Scholar]
- Salinas J.-F. et al. Optical Design of Transparent Thin Metal Electrodes to Enhance In-Coupling and Trapping of Light in Flexible Polymer Solar Cells. Adv. Mater. 24, 6362–6367 (2012). [DOI] [PubMed] [Google Scholar]
- Beck-Candanedo S., Roman M. & Gray D. G. Effect of Reaction Conditions on the Properties and Behavior of Wood Cellulose Nanocrystal Suspensions. Biomacromolecules 6, 1048–1054 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video of the demonstration of the recycling and the burning of the solar cells
Supplementary info_transmittance of CNC samples and dissolving test in water