Abstract
Staphylococcus aureus is one of the prominent Gram positive human pathogen secretes many surface and secretary proteins including various enzymes and pathogenic factors that favour the successful colonization and infection of host tissue. α-amylase is one of the enzymes secreted by S. aureus which catalyses the breakdown of complex sugars to monosaccharides, which are required for colonization and survival of this pathogen in any anatomical locales. In the present study we have cloned, sequenced, expressed and characterized α-amylase gene from S. aureus ATCC12600. The recombinant enzyme has a molecular weight of 58kDa and the kinetics showed Vmax 0.0208±0.033 (mg/ml)/mg/min and Km 10.633±0.737mg/ml. The multiple sequence analysis showed α- amylase of S. aureus exhibited large differences with Bacillus subtilis and Streptococcus bovis. As the crystal structure of S. aureus α- amylase was unavailable, we used homology modelling method to build the structure. The built structure was validated by Ramachandran plot which showed 90% of the residues in the allowed region while no residue was found in the disallowed region and the built structure was close to the crystal structure with Z-Score: -6.85. The structural superimposition studies with α- amylases of Bacillus subtilis and Streptococcus bovis showed distinct differences with RMSD values of 18.158Åand 7.091Å respectively which correlated with enzyme kinetics, indicating α-amylase is different among these bacteria.
Keywords: α-amylase, Km, Vmax, Z-score, RMSD
Background
Staphylococcus aureus can colonize and survive in a wide variety of environmental niches. It is capable of causing a range of mild to life-threatening diseases, including septicaemia, meningitis, toxic shock syndrome, food poisoning and skin abscesses [1]. It colonizes tissues using a repertoire of virulence determinants that includes cell surface-associated protein, as well as extracellular proteins. One of the extracellular proteins is α- amylase secreted by the bacteria. α-amylase is randomly cleaves the 1,4-α-D-glycosidic linkages between adjacent glucose units to yield smaller starches and ultimately Maltrotriose, maltose, amylose, glucose and limit dextrin from amylopectin. These sugars are further converted to glucose-6-phosphate; this very step is the basis for the synthesis of exopolysaccharide matrix, Polysaccharide intracellular adhesion (PIA), and thus finally leading to Biofilm formation. Biofilms are defined as communities of microorganisms that are encased in a selfsynthesized extracellular polymeric matrix (EPS) and grow attached to a biotic or abiotic surface [2]. Staphylococcal biofilms have a significant impact on human health [3] as they frequently exhibit an enhanced pathogenic capability relative to bacteria in solution by virtue of their sessile behaviour, increased resistance to antimicrobial agents, and the potential for detachment and distal embolization of large biofilm fragments [4].
α-amylases are classified as family 13 of the glycosyl hydrolases which have similar structures and catalytic sites and the same catalytic mechanisms [5]. In general they are composed of three domains. Domain A central barrel containing the active site residues and chloride ion binding site, domain B a long loop region inserted between third β strand and the α-helix of domain A, that contains calcium binding site and domain C a terminal β-sheet domain consisting of a motif which appears to show some variability in sequence and length between amylases according to the organism [6].
The kinetics of α-amylases varies distinctly different among bacteria for example in case of Psuedoalteromonas arctica and Bacillus subtilis the kinetics of α-amylase showed Km of 7.28 mg/ml; 2.68 mg/ml and Vmax 13.07 (mg/ml)mg/min; 1.773U/ml/min respectively [7, 8]. The α-amylase gene from Bacillus stearothermophilus was expressed in S. aureus and secreted in to the culture filtrate. All staphylococcal species were able to secrete α-amylase, since more than 80% of the enzyme activity was found in the culture supernatant [9] making very important role in this human pathogen, large amounts of monosaccharides which may have profound role in the pathogenicity of S. aureus therefore; the present study is focussed on characterization of α-amylase from Staphylococcus aureus ATCC 12600 and its comparison with Bacillus subtilis and Streptococcus bovis α-amylases.
Methodology
Staphylococcus aureus ATCC12600 was grown on modified Baird Parkar Agar media at 37°C. After overnight incubation single black shiny colony with distinct zone was picked and cultured in Brain Heart Infusion (BHI) broth at 37°C. Thus, grown S. aureus ATCC12600 culture was used to characterize α-amylase enzyme and extraction of chromosomal DNA [10].
α-amylase enzyme assay:
The α-amylase activity was measured with the Dinitro salycylic acid (DNS) according to the method described protocol by [11] using 10mg/ml starch dissolved in a 50 mM phosphate buffer pH 6.9 and 42° C. One unit of amylase activity was defined as the amount of enzyme that released 1mg of reducing end groups per minute at 42°C. Maltose was used as standard of reducing end sugar.
α-amylase gene was amplified from S. aureus chromosomal DNA using the primers α-amylase -F: 5'- CATGAATAAGCAATGG-3' and α-amylase -R: 5'- TTAATTTAGTTCGAT 3' which were designed from the α- amylase gene sequence of S. aureus Mu50 strain [12]. The reaction mixture contained in a final volume of 50µl which consisted of 100ρmoles of each primers, 100µmol of dNTPS mix, 10 mM Tris- HCl (pH 8.8), 1.5 mM MgCl2, 1U of hot start Taq DNA Polymerase (Bangalore Genei pvt ltd) and 0.25µg of chromosomal DNA. Amplification parameters included an initial denaturation step for 10 min at 94°C; 35 cycles of 94°C for 60 seconds of denaturation, 60 seconds of annealing at 33°C and 100 seconds of amplification at 72°C which was followed by a final extension step at 72°C for 5 min in a Mastercycler gradient Thermocycler (Eppendorf). Amplified products were purified with NP-PCR Purification kit, Taurus Scientific, USA and were sequenced by dye terminating method at MWG Biotech India Ltd. Thus, obtained α-amylase gene sequence was deposited at Gen Bank(http://www.ncbi.nlm.nih.gov/nuccore/HM067708).
Cloning, expression and purification of α-amylase gene:
α-amylase gene was cloned in the Sma I site of pQE 30 and transformed into E.coli DH5α. The resultant clone was named as PLAM1. The insert in the clone was sequenced and after ascertaining the sequence, the α-amylase was over expressed with 1mM IPTG. The r α-amylase was purified from the cytosolic fraction of PLAM 1 clone by passing through nickel metal chelate agarose column (by following QIA express expression system protocol) and protein was eluted using 300mM immidazole hydrochloride the product was analysed on 10% SDS-PAGE [10, 13]. The enzyme kinetics of purified α- amylase was performed as described earlier in section.
In silico structure prediction:
As the α-amylase structure of S. aureus and Streptococcus bovis were not available so far in the PDB we have constructed 3D structure using homology modelling method. The three dimensional models of S. aureus and Streptococcus bovis α- amylase was constructed by using Modeller 9v8 tool. The S. aureus α-amylase protein sequence was subjected to BLASTp [14] against PDB and the crystal structure of a putative α- amylase from Geobacillus sp (PDB ID: 2ZE0A) showing the maximum identity of 52% was chosen as template. Similarly the α-amylase of Streptococcus bovis was subjected to BLASTp against PDB and the crystal structure of putative α-amylase from (PDB ID:1WP6) showed 48% identity with maltohexose producing α-amylase from alkalophilic Bacillus sp. Alignment files were generated in PIR format for Query and template sequences using ClustalX tool [15]. Python scripts were written and 20 best models for each organism were generated. The model with the lowest discrete optimized protein energy (DOPE) score was selected for further analysis.
Validation of S. aureus and Streptococcus bovis α-amylases Model:
The stereo chemical quality of the predicted model was validated by PROCHECK and ProSA web servers. Both can read the atomic co-ordinates of the 3D model and judge the quality of the structure. Ramachandran plot generated from PROCHECK [16] validation server was used to access the quality of the model by looking into the allowed and disallowed regions of the plot. A Z-score value was generated from ProSA web server that can determine the overall quality of the model and its identity nearest to crystal structure.
Superimposition of S. aureus α-amylase:
The comparative structural prediction studies to ensure the identity and variability of S. aureus α-amylase structure with other α-amylase structures were carried out using MATRAS (MArkovian TRAnsition of Structure) program. This program has unique features where it can define the structural similarity score as the log-odds of two probabilities using a scheme similar to Dayhoff's amino acid substitution score. In the program we have assigned structures by inputting the PDB code and by uploading the PDB format files in the user's computer. An alignment, superimposed structures and various kinds of structural similarities, such as raw score, RMSD values were predicted for the following structures. The validated 3D model of S. aureus α-amylase was super imposed with available and built structures of α-amylases from other bacteria such as Bacillus subtilis (1BAG), Streptococcus bovis (built structure).
Result & Discussion
In S. aureus cell wall biosynthesis is a crucial factor for its survival in the host and in the formation of small colony variants, which is one of the key factors observed in the host with increased relapsed episodes of S. aureus infections [13, 17] this is probably augmented in S. aureus due to the high activity of α-amylase which is secreted in the culture filtrate. This increased production of monosaccharides elevates the glucose levels which is phosphorylated by glk A [18] in this pathogen and is primarily utilized in the synthesis of PIA, exopolysaccharides and in cell wall biosynthesis [2, 19, 20]. This in turn suppresses the TCA cycle resulting in reductive conditions [20, 21] and in this situation rate of biofilm formation is very high. This type of scenario is seen in all multi drug resistant strains (MDR) and Vancomycin resistant strains (VRSA) S. aureus [19, 20, 21]. Therefore in the present study α- amylase gene of S. aureus ATCC 12600 was cloned, sequenced and expressed in E.coli DH5α, the resultant clone was named as PLAM 1.
The α-amylase gene expression in PLAM 1 clone was obtained by adding extra “ C ” residue in the 5' end of the forward primer such that the gene is cloned in −1 frame in the Sma I site of pQE 30, and thereby, correct expression of enzyme could be achieved [10, 13]. The insert in the PLAM 1 clone was confirmed by sequencing and ascertaining the sequence which is the same as the one deposited in the GenBank (accession number HM067708). The α-amylase gene expression was induced with 1mM IPTG. The product of α-amylase was purified with nickel metal chelate chromatography column and the pure recombinant enzyme showed similar properties with that of native α-amylase [10, 13]. The α-amylase identified in the extracellular fraction of S. aureus ATCC 12600 showed an enzyme activity of 0.0193±0.0015 mg/ml/min, Vmax 0.032±0.0036 (mg/ml)/mg/min and Km 11.57±0.409 mg/ml. The pure recombinant α-amylase showed single band in SDS–PAGE (10%) with a molecular weight of 58kDa, which is corresponding to the insert cloned and monomeric size of the protein (Figure 1). The kinetics of pure recombinant α-amylase was close to the secreted native α-amylase of S. aureus ATCC12600 Table 1 (see supplementary material) which are in accordance with the results reported earlier [9].
Figure 1.
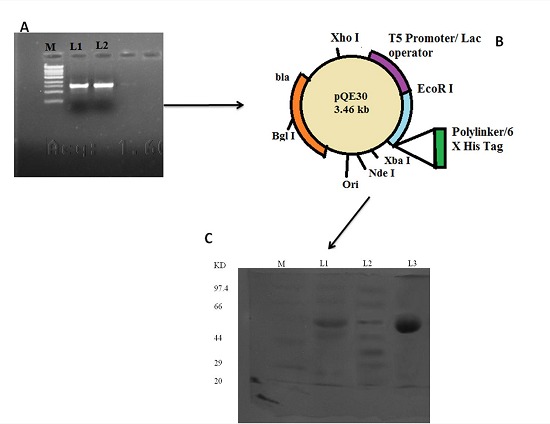
Cloning of α-amylase gene from S.aureus ATCC 12600. A. PCR amplification of α-amylase gene using α-amylase primers from the S. aureus ATCC12600 following the method described. Lane M molecular size marker obtained from Bangalore genei pvt ltd, lane L1 and L2 PCR amplified products. B. Schematic representation of pQE 30 plasmid vector, C. Electrophoretogram showing the expression of recombinant α-amylase from PLAM1clone in SDS-PAGE (10%). Lane M: Molecular weight markers obtained from Bangalore Genei Pvt ltd. Lane 1: induced cell lysate of PLAM1 clone Lanes 2 : uninduced cell lysate of PLAM1 clone. Lane 3: pure recombinant α-amylase eluted from nickel metal chelate chromatographic column.
The sequence of S. aureus α- amylase (GenBank accession number for amylase gene is HM067708) showed complete homology with amylase gene of all the S. aureus strains reported in the database. The multiple sequence alignment of α- amylase protein sequence of S. aureus ATCC12600 showed highly conserved regions with Bacillus subtilis and Streptococcus bovis while very low identity in the sequence was observed with both human salivary α- amylase (Figure 2). As the crystal structure of S. aureus α- amylase was not available hence in the present study we have built the 3D structure using homology modeling method using X-ray crystal structure of Geobacillus sp as template which showed 52% identity. Similarly, the 3D α- amylase for Streptococcus bovis was also constructed using the Xray crystal structure of Alkalophilic Bacillus Sp as template however, the comparative structural analysis showed extensive variations as indicated from the RMSD values which also correlated with the enzyme kinetic results of α-amylase Table 2 (see supplementary material) & Figure 3). These results also suggest that in various MDR and VRSA strains of S. aureus α- amylase expression may be critical in the colonization and rate of biofilm formation.
Figure 2.
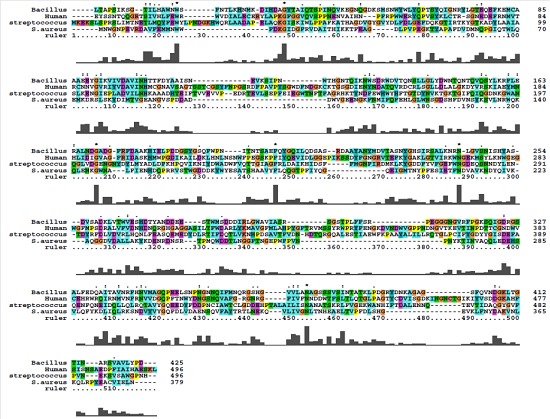
Multiple Sequence alignment of S.aureus α-amylase with Bacillus subtilis and Streptococcus bovis.
Figure 3.
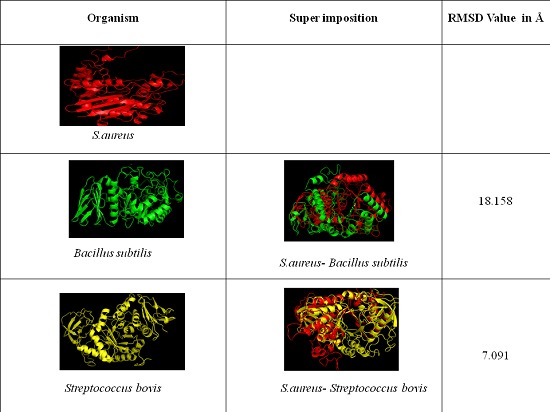
Structural comparison of S. aureus α-amylase with Bacillus subtilis and Streptococcus bovis.
Conclusion
S. aureus can colonize and adopt on any anatomical locales in the human host for which the organism requires large amount of monosaccharides which can be easily generated by α- amylase. In this context this enzyme may be playing pivotal role in the colonization and biofilm formation which are one of the key factors in the pathogenesis of S. aureus.
Supplementary material
Acknowledgments
This paper forms a part of Ph.D thesis work going to be submitted to JNT University, Hyderabad- 500085, A.P, India.
Footnotes
Citation:Lakshmi et al, Bioinformation 9(6): 281-285 (2013)
References
- 1.Lew DP, et al. Drugs. 1995;2:100. doi: 10.2165/00003495-199500492-00016. [DOI] [PubMed] [Google Scholar]
- 2.Gotz F. Mol Microbiol. 2002;43:1367. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 3.Fux CA, et al. Trends Microbiol. 2005;13:34. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Hall-Stoodley L, et al. Nat Rev Microbiol. 2004;2:95. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 5.Jespersen HM, et al. J Protein Chem. 1993;12:791. doi: 10.1007/BF01024938. [DOI] [PubMed] [Google Scholar]
- 6.Buisson G, et al. EMBO J. 1987;6:3909. doi: 10.1002/j.1460-2075.1987.tb02731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu M, et al. Protein J. 2010;29:591. doi: 10.1007/s10930-010-9290-0. [DOI] [PubMed] [Google Scholar]
- 8.Bano S, et al. AAPS PharmSciTech. 2011;12:255. doi: 10.1208/s12249-011-9586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thudt K, et al. Gene. 1985;37:163. doi: 10.1016/0378-1119(85)90269-0. [DOI] [PubMed] [Google Scholar]
- 10.Prasad UV, et al. Appl Biochem Biotechnol. 2013;169:862. doi: 10.1007/s12010-012-0027-8. [DOI] [PubMed] [Google Scholar]
- 11.Bernfeld P. Methods in Enzymology. 1955;1:149. [Google Scholar]
- 12.Ohta T. DNA Res. 2004;11:51. doi: 10.1093/dnares/11.1.51. [DOI] [PubMed] [Google Scholar]
- 13.Hari Prasad O, et al. Protein J. 2012;31:345. doi: 10.1007/s10930-012-9410-0. [DOI] [PubMed] [Google Scholar]
- 14.Altschul SF, et al. Nucleic Acids Res. 1997;25:3389. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson JD, et al. Nucleic Acids Res. 1997;25:4876. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laskowski RA, et al. J Appl Cryst. 1993;26:283. [Google Scholar]
- 17.Kahl BC, et al. Infect Immun. 2005;73:4119. doi: 10.1128/IAI.73.7.4119-4126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasanna Lakshmi H, et al. Bioinformation. 2013;9:169. doi: 10.6026/97320630009169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui L, et al. J Clin Microbiol. 2003;41:5. [Google Scholar]
- 20.Zhu Y, et al. Infect Immun. 2009;77:4256. doi: 10.1128/IAI.00195-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson JL, et al. Antimicrob Agents Chemother. 2007;51:616. doi: 10.1128/AAC.01057-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freer SN. Appl Environ Microbiol. 1993;59:1398. doi: 10.1128/aem.59.5.1398-1402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


