Abstract
Background
Laboratory large animal models are important for establishing the efficacy of stem cell therapies that may be translated into clinical use. The similarity of ovine and human cardiovascular systems provides an opportunity to use the sheep as a large animal model in which to optimize cell-based treatments for the heart. Recent clinical trials in humans using endogenous cardiovascular progenitor cells report significant improvement in cardiac function following stem cell-based therapy. To date, however, endogenous cardiovascular progenitor cells have not been isolated from the sheep heart.
Methods
Cardiovascular cells expressing SSEA-4, CD105 and c-kit were isolated by flow cytometry and cloned from the right atrium of neonatal sheep. The expression of GATA-4, c-kit, and Isl1 was identified by PCR in the cloned cells. Immunohistochemical staining was used to compare the number of SSEA-4 positive cells in the right auricle, right atrium, left ventricle and the apex of the heart of fetal, neonatal and adult sheep. The number of SSEA4+cells was also compared in fetal, pregnant and non-pregnant adult sheep.
Results
Four distinct cardiac progenitor cell sub-populations were identified in sheep, including CD105+SSEA-4+c-kit+Isl1+GATA-4+cells, CD105+SSEA-4+c-kit+Isl1+GATA-4−cells, CD105+SSEA-4−c-kit-Isl1+GATA-4−cells, and CD105+SSEA-4−c-kit+Isl1+GATA-4−cells. Immunohistochemical staining for SSEA-4 showed that labeled cells were most abundant in the right atrium of fetal hearts where niches of progenitor cells could be identified.
Conclusion
We determined the phenotype and distribution of cardiac progenitor cells in the sheep heart. The availability of cloned endogenous cardiac progenitor cells from sheep will provide a valuable resource for optimizing the conditions for cardiac repair in the ovine model.
Keywords: Stem cells, Cardiovascular, Ovine
Introduction
Small animal models have been used widely to explore the impact of stem cell transplantation on cardiac function [1–4]. Stem cell-derived cardiovascular progenitors are capable of both neovascularization and cardiomyogenesis [5–6]. Consequently, administration of these cells for cardiovascular repair frequently provides a functional benefit in both mice and rats [7,8]. The extension of these findings into clinical trials, however, has been somewhat disappointing in that the marginal benefit obtained from cell-based treatments has not been substantially different from benefits obtained using conventional drug-based therapy [9].
The divergence between the results in rodents and those reported in humans may, in part, be due to differences in the heart rate of humans and small animals. Large animals, e.g. sheep, dogs or pigs are anatomically closer to humans in terms of structure, size, and heart rate, and therefore, represent models that may be more suitable for preclinical stem cell studies [10–12]. An additional challenge in optimizing stem cell-based treatments is the fact that the best candidate cell for expansion and administration into patients still remains to be determined. Cardiac progenitor cells (CPC) isolated from the heart itself have gained an increasing amount of attention due to the advantages of using an autologous cell type that can be isolated from patients. Data from several laboratories document the ability to expand these cells readily, and the ability of these progenitors to be differentiated in vitro into cardiovascular cells [13–15]. In clinical trials, these cells have been introduced into patients with ischemic cardiomyopathy (SCIPIO study) with promising results [16]. It is believed that CPC residing within the heart may have advantages over other stem cell types [17].
In order to optimize conditions under which CPC can be utilized to maximize their benefit for stem cell transplantation, cardiac progenitor cells need to be tested in large animals [18]. Several studies have been reported in pigs [19] and dogs [10], however, no studies have reported the isolation of cardiac progenitor cells from sheep or their effect on heart repair. The sheep is a commonly used animal model in the stem cell research field. Mesenchymal stem cells, embryonic stem cells, and skeletal myoblasts for heart repair have been studied in the sheep, but none of those reports used endogenous cardiac stem cells [11,20,21].
The successful clinical application of cardiac progenitor cells in patients could be expedited if the appropriate models were available for optimizing the experimental conditions necessary to maximize functional benefit. In the present study, we sought to isolate, characterize, and clone endogenous cardiovascular progenitors from the sheep heart. Our hypothesis was that the gene expression profile, surface phenotype and spatial distribution of endogenous cardiovascular progenitors in the sheep will be similar to that of humans. Hopefully, the availability of these cells will benefit the advancement of stem cell transplantation for cardiac repair.
Materials and Methods
Animal preparation
A total of 13 female Suffolk sheep were used in this study, including 2 neonates (9 days and 10 days after birth, respectively), 5 fetuses (130 to 150 days gestation), 3 pregnant ewes (2 years of age) and 3 age-matched non-pregnant ewes. All experimental procedures were performed within the regulations of the Animal Welfare Act, the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Institutional Animal Care and Use Committee of Loma Linda University.
Cardiac progenitor cell isolation and clonogenic culture
Enzymatic digestion was used to dissociate CPCs from the right atrium of neonatal sheep. The tissue was cut into 1 mm3 pieces in ice cold M-buffer, washed, and digested with collagenase A (Roche Diagnostics Corp, Indianapolis, IN) at 1 mg/ml for two hours at 37 degrees. The digested cells were then resuspended in supplemented M199 medium and cloned at a density of 0.8 cells/well on 0.1% gelatin-coated 96 well plates. CPCs were also isolated from surgical biopsies of age-matched human neonates (8 days after birth) under Institutional Review Board approval. The availability of human tissue for the isolation of endogenous cardiovascular cells allowed us to compare CPC in sheep and in humans.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from cultured cells at passage 5, and RNA was reverse transcribed into cDNA. Sheep primer sequences were designed by using Primer-BLAST (www.ncbi.nlm.nih.gov/tools/primer-blast/). Sheep specific primers for GATA-4, c-kit, Isl1 transcription factor (Isl1) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) used for this study are shown in table 1. Real-Time PCR was performed using Fast SYBR Green Master Mix (Promega, Madison, WI) and an IQ™5 Multicolor Real-Time PCR Detection System (BioRad, Hercules, CA). The PCR conditions were: 94°C for 10 minutes, 94°C for 15 seconds, 52°C for 60 seconds, 72°C for 30 seconds for a total of 40 cycles. The presence of a PCR product of the correct size was verified by gel electrophoresis.
Table 1.
Sheep-Specific Primers.
| Genes | Forward | Reverse |
|---|---|---|
| GATA-4 | CTTCCAGCAGCTCCAGCAGTGTG | TTGCGGGGAGAGCTTCAGAGCG |
| Isl1 | GTGCAGCATCGGCTTCAGCAAAAA | CCTCCCGCAGCGCGAACTCAT |
| c-kit | GGCATATCCCAAACCTGAACACCGA | CCCTGCGGCCACGCACTGTA |
| GAPDH | CCAGCCGCATCCCTGAGA CAAG | GACCCTCTTGGCGCCACCCT |
Flow cytometry
Flow cytometry experiments were performed to examine surface marker expression on cardiovascular cell clones at passage 5 following isolation from the sheep heart. Cells were tested using monoclonal phycoerythrin (PE) conjugated mouse anti-human CD105 antibody (Biolegend, San Diego, CA), fluorescein isothiocyanate (FITC) conjugated mouse anti-human stage-specific embryonic antigen 4 (SSEA-4) (Biolegend) and mouse anti-human c-kit (Millipore, Billerica, MA). All antibodies were used at concentrations recommended by the manufacturer for flow cytometry and the appropriate isotype controls were included for each assay. The data was acquired using a MACSQuant Analyzer (MiltenyiBiotec, Auburn, CA). Labeling experiments were repeated three times for this study.
Proliferation assay
The Proliferation assay was performed using the Quick Cell Proliferation Assay Kit II (BioVision, Milpitas, CA). Briefly, cultured cells (5×103/well) at passage 5 were distributed in a 96-well plate in a final volume of 100 μl/well culture medium and were cultured overnight. WST-1 solution was added to each well and the cells were incubated for 4 additional hours in standard culture conditions. The absorbance of the samples was then measured using a micro titer plate reader at 450 nm. Cardiac progenitor cells from age-matched human heart biopsies (patients at 8 days after birth) at passage 5 were used to compare the proliferation rates in sheep and human CPC. All clones were analyzed in triplicate.
SSEA-4 immunofluorescence staining
Frozen sections from the right auricle, right atrium, left ventricle and apex of the heart were obtained from fetal sheep (n=5), pregnant adult ewes (n=3) and non-pregnant adult ewes (n=3) for SSEA-4 immunofluorescence staining. Samples from neonatal sheep were divided to allow a portion of the tissue to be used for cell culture and a portion to be used for immunostaining. Tissues were cut into sections at 5 μm thickness for immunofluorescence staining and 10 μm for confocal microscope analyses. The FITC conjugated antibody for SSEA-4 was added (1:100 dilution) overnight at 4 degrees. Staining was observed under fluorescent microscopy (Olympus BH-2, Japan) and confocal microscopy (Zeiss Microscopy LLC, Thornwood, NY). The SSEA-4 labeled cells were quantified using Image-Pro plus 5.0 (Media Cybernetics, Rockville, MD). SSEA-4 positive labeling was calculated as the total SSEA-4 positive area (in pixels)/total image area (192000 pixels)×100%. Five representative labeled areas were selected for quantification and the average labeling was reported for each sample. For confocal microscope analyses, the Z stack mode was applied to localize the FITC and DAPI labeling areas.
Statistics
The percentage of SSEA-4 labeling and the proliferation rates were expressed as mean ± standard error. The SSEA-4 labeled area was compared in the fetal, neonatal, pregnant and non-pregnant sheep using ANOVA analyses. The proliferation rate between human and sheep cells was compared by student test. P<0.05 was considered statistically significant. All analyses were performed using SPSS software (Statistical Package for the Social Sciences, version 16.0, SPSS Inc, Chicago, IL).
Results
Cell surface markers expressed on neonatal sheep cardiovascular cell clones
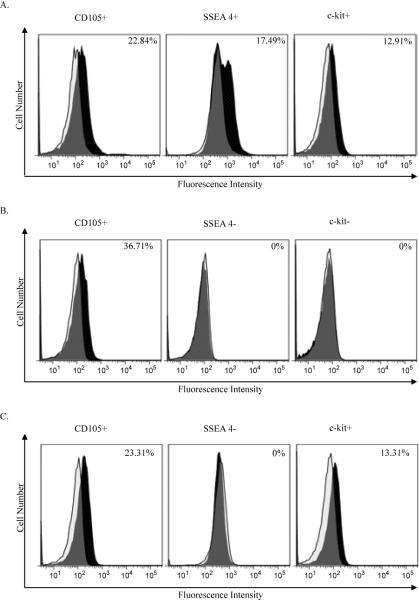
A total of 36 clones were derived from neonatal sheep hearts and were tested for SSEA-4, CD105 and c-kit expression levels by flow cytometry (Figure 1). Our data showed several distinct sub-populations of isolated cells defined by the presence of CD105 co-expressing either SSEA-4 or c-kit (Figure 1,2). Of all clones tested, 26 (72%) were CD105 positive (mean percentage: 25.8 ± 3.7%); 9 clones (25%) were SSEA-4 positive (mean percentage: 9.9 ± 2.0%) and 10 clones (28%) were c-kit positive (mean percentage: 9.5 ± 2.9%). Five cell clones (14%) co-expressed SSEA-4, CD105 and c-kit. PCR was used to verify that transcripts for c-kit were present in the cells where surface labeling for c-kit was low (Figure 2). These results suggested that the cardiac progenitor cells that were isolated from the neonatal sheep heart were heterogeneous.
Figure 1. Cell surface marker expression patterns in sheep cardiovascular cell clones.
Sheep cardiovascular progenitor cell clones were examined for expression of early cardiovascular progenitor cell markers CD105, SSEA-4 and c-kit using flow cytometry. Cell surface marker expression on three representative cardiovascular progenitor cell clones is shown here. The CD105+SSEA4+c-kit+ cell clone shown is FC1, the CD105+SSEA4-c-kit-clone is FC2 and the CD105+SSEA4-c-kit+ clone is HB1. A total of 36 clones were examined by flow cytometry. Positively labeled cells are represented in black, isotype controls are shown as a clear tracing.
Figure 2. c-kit expression on cardiovascular progenitor cells.
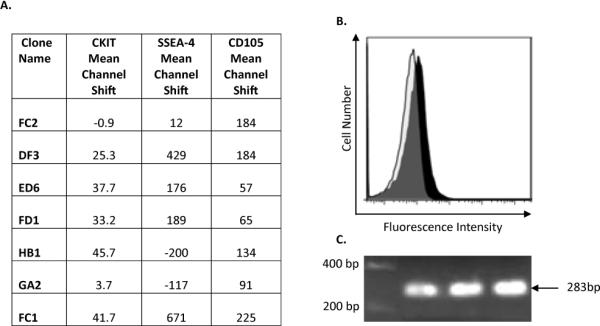
The levels of c-kit were low, as shown by the mean channel shift (A) for the seven representative clones. The expression levels of CD105 and SSEA4 are provided for comparison. The expression of c-kit identified by flow cytometry (B) was confirmed by PCR (C, c-kit size=283bp).
Stem cell marker transcripts expressed in neonatal sheep cardiovascular cell clones
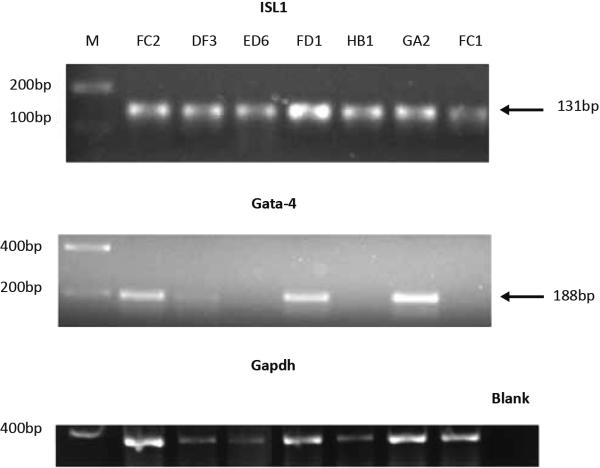
RT-PCR was performed to determine whether Isl1 and GATA-4 were expressed in the proliferating cells that were isolated and cloned from the neonatal sheep heart (Figure 3). A total of 12 cell clones at passage 5 were tested by PCR. Isl1 was expressed in all cell clones. GATA-4 was detected in a proportion of the clones (Figure 3). Combined with the data from flow cytometry mentioned above, four distinct cardiac progenitor cell sub-populations were identified that expressed the following cardiovascular progenitor cell markers: CD105+SSEA-4+c-kit+Isl1+GATA-4+, CD105+SSEA-4+c-kit+Isl1+GATA-4−, CD105+SSEA-4−c-kit-Isl1+GATA-4−, and CD105+SSEA-4−c-kit+Isl1+GATA-4−.
Figure 3. Cardiovascular transcription factor expression in isolated neonatal sheep cell clones.
Neonatal sheep cardiovascular cell clones shown were tested by PCR for expression of Isl 1 and GATA-4. All seven clones expressed transcripts for Isl1 (A, Isl1 size=131bp) and a proportion of these clones expressed transcripts for GATA-4 (B, GATA-4 size=188bp).
The proliferation rate of sheep vs. human cardiovascular progenitor cell clones
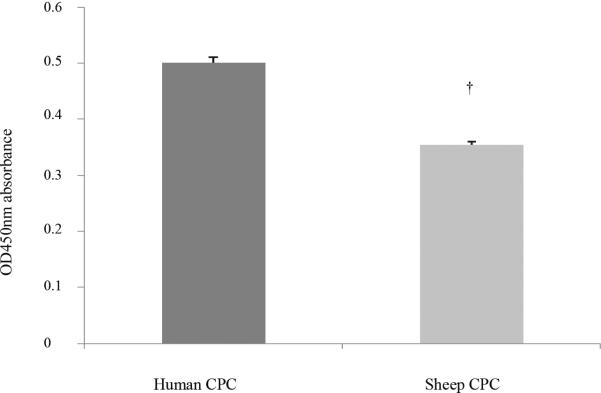
We compared the proliferation rate of sheep CPC with that of comparable clones isolated from human neonatal patients. This information was intended to determine whether or not the time needed to expand sheep CPC to numbers sufficient for transplantation would be the same or different than the time necessary to expand human CPC to numbers sufficient for human cardiac transplantation. Eight cardiovascular progenitor clones at passage 5 were compared (n=4 in each group). The proliferation rate was measured using a readout of optical absorbance at OD 450. The optical absorbance at OD 450 of the 4 human CPC clones was: 0.48 ± 0.01, 0.51 ± 0.02, 0.34 ± 0.01, 0.72 ± 0.01; the absorbance in the wells containing the sheep cell clones was: 0.26 ± 0.01, 0.40 ± 0.01, 0.46 ± 0.01 and 0.30 ± 0.01, respectively. The mean optical absorbance for the human cardiac progenitor cells was 0.51 ± 0.01 vs. 0.35 ± 0.01 for the sheep. Compared to cardiac progenitor cells isolated from humans, the cardiac progenitor cells isolated from sheep had a lower proliferation rate (Figure 4, P=0.017).
Figure 4.
Proliferation rate of sheep cardiac progenitor cells when compared with similar clones isolated from humans. The proliferation rate of neonatal sheep and neonatal human cardiovascular progenitor clones was indicated by the OD 450 absorbance using a proliferation assay kit. Sheep CPC had a lower proliferation rate when compared with human CPC (0.35 ± 0.01 vs. 0.51 ± 0.01, P=0.017), suggesting that CPC from sheep proliferate more slowly than comparable clones isolated from age-matched humans.
The distribution of SSEA-4 positive cells in the neonatal sheep heart
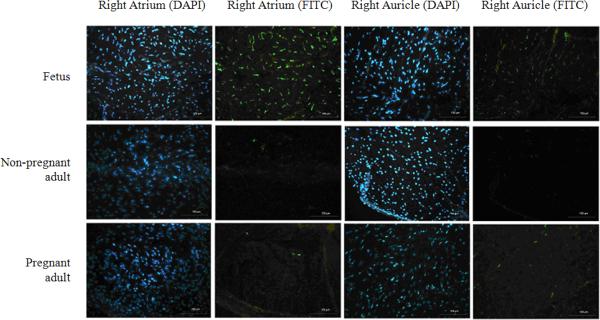
SSEA-4 has been used as a stem cell marker to identify resident progenitor cells in the human heart [22]. In order to determine the relative frequency and distribution of SSEA-4 labeled cells in sheep, we used immunostaining to identify labeled areas in the atrium, auricle, left ventricle and apex of the heart. Age-matched adult pregnant and non-pregnant sheep, fetal sheep at 130 to 150 days of gestation and neonatal sheep at 8 days after birth were used to obtain cardiac tissue samples for this analysis. In general, a markedly higher level of staining was identified in the right auricle and right atrium when compared to staining identified in the left ventricle and apex. We observed clusters of SSEA-4 labeled cells distributed in the right auricle and right atrium of fetal sheep samples, but not in the left ventricle and apex tissues. These clusters of SSEA-4 labeled areas were not observed in samples derived from adult pregnant or non-pregnant sheep.
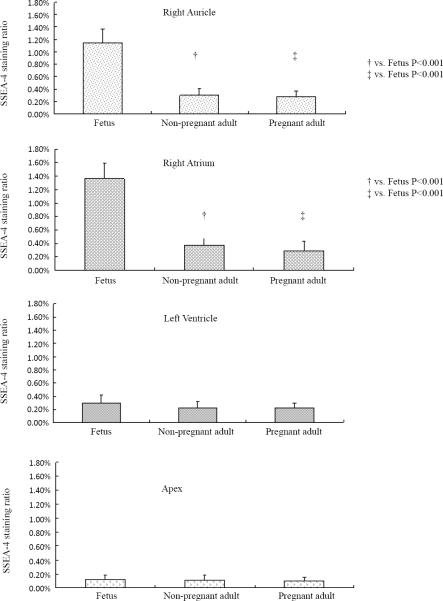
Typical images representing the SSEA-4 labeled areas in the auricle, right atrium, left ventricle and apex of fetal, pregnant and non-pregnant sheep are shown in figure 5. A representative confocal microscope image of a CPC isolated from the right atrium of a fetal sheep is shown in figure 6. Quantitative analysis showed that SSEA-4 labeling in the auricle was 1.15 ± 0.22% in fetal sheep; 0.30 ± 0.11% in pregnant and 0.28 ± 0.08% in the non-pregnant adult sheep (1.15 ± 0.22% vs. 0.30 ± 0.11% and 0.28 ± 0.08%, both P<0.001) (Figure 7). SSEA-4 labeling was also significantly higher in the right atrium of the fetus when compared to pregnant and non-pregnant adults (1.36% ± 0.23% vs. 0.37% ± 0.16% and 0.29% ± 0.14%, both P<0.001). There were no differences in SSEA-4 staining area ratio in the left ventricle and apex. SSEA-4 labeling area ratio in the right atrium of neonates (0.96 ± 0.17%) was lower than that in fetuses, but was significantly higher than that in pregnant or non-pregnant adults (P=0.0042 and P=0.0022, respectively).
Figure 5. SSEA-4 labeling distribution in the right atrium and right auricle of fetal, pregnant and adult sheep.
Representative images of the right auricle and right atrium from fetal, pregnant and adult sheep are shown (magnitude 200×). Niche-like SSEA-4 positive areas labeled with FITC were observed in right auricle and right atrium tissues from fetal sheep samples, but were not identified in the left ventricle and apex. The SSEA-4 positive areas in the right auricle, right atrium and left ventricle from pregnant adults and non-pregnant adults were sparse.
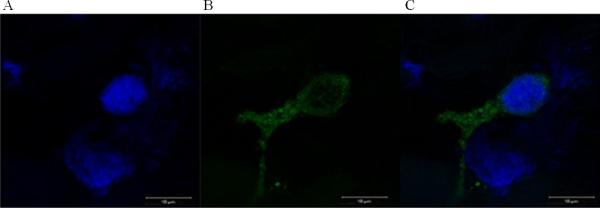
Figure 6. SSEA-4-labeling on a representative cardiac progenitor cell.
Confocal laser scanning microscope images (magnitude 630×) were taken after immunohistochemical staining of cardiac progenitor cells in the right atrium of fetal sheep using a FITC-labeled anti-SSEA-4 antibody: A) DAPI stained cell nuclei B) FITC labeled cell surface and cytoplasm identifies SSEA4 expression on these cells C) merged image of DAPI and FITC staining.
Figure 7. Quantitation of SSEA-4 labeled cells in the sheep heart.
SSEA-4 positive labeling in the right auricle, right atrium, left ventricle and apex of fetal, pregnant and non-pregnant female adult sheep hearts were compared. By quantitative analysis, SSEA-4 labeling was significantly higher in the right atrium of the fetus when compared with pregnant and non-pregnant adults (1.36% ± 0.23% vs. 0.37% ± 0.16% and 0.29% ± 0.14%, both P<0.001). SSEA-4+ labeling in the auricle was 1.15 ± 0.22% in fetal sheep; 0.30 ± 0.11% in pregnant and 0.28 ± 0.08% in the non-pregnant adult sheep (1.15 ± 0.22% vs. 0.30 ± 0.11% and 0.28 ± 0.08%, both P<0.001). There were no differences in SSEA-4 staining in the left ventricle or apex of fetal, pregnant or adult sheep.
Discussion
In the present study, we provide the first report of cloned, endogenous cardiac progenitor cells isolated from neonatal sheep. Sheep and human endogenous cardiovascular cells are similar, expressing CD105, SSEA-4, c-kit and Isl1, a transcription factor identifying cardiac-lineage progenitor cells. These cells, in neonatal sheep and fetal humans are clustered in vivo in the right atrium where they are most abundant. The number of SSEA4+cells and Isl1 positive cells declines significantly during the neonatal period in humans [22]. The availability of sheep CPC will provide new opportunities to optimize the use of endogenous cardiac progenitor cells for the repair of the heart in humans using the sheep as a preclinical large animal model.
Isl1+precursors have the potential of self-renewal and differentiation into endothelial, cardiomyocyte, and smooth muscle lineages [23]. Cardiovascular progenitor cells that express Isl1 were shown to cluster within the right atrial wall in fetal and newborn humans [22], but occurred at lower frequency when compared with our findings in sheep of comparable age [24]. All CPC clones isolated from neonatal sheep expressed Isl1, possibly due to the method of progenitor cell isolation used in our study which allowed us to examine transcription factor expression in proliferating cardiovascular cell clones. Isl1 expression defines these cells as cardiac progenitors [25,26].
Although Isl1 expression is limited, a higher percentage of cardiovascular progenitors expressing c-kit can be isolated from human patients (approximately 24%) [24]. A similar percentage of c-kit expressing cells were identified in sheep. The ability to isolate higher numbers of c-kit+cells from some older individuals may be a consequence of the medical condition of the patient [16]. The expression of c-kit has been identified on cardiovascular progenitors, cardiospheres and cardiosphere-derived cells (CDCs) [27,28]. In rats, transplantation of progenitor cells expressing c-kit improves cardiac function [28] and the c-kit+cardiac stem cells are beneficial when administered in human clinical trials [16]. In the present study, we also observed the co-expression of c-kit and CD105 on sheep CPC, which is consistent with a prior report in humans [27]. The sheep clones can be used to optimize administration of c-kit expressing cells for cell–based treatment of the heart.
The ovine model will also provide an opportunity to address the relevance of the heterogeneity in the CPC population. Sheep CPC that are c-kit+Isl1+ and c-kit-isl1+ are present and clonable in normal sheep. Interestingly, sheep CPC can co-express c-kit and Isl1 whereas, a CPC population expressing c-kit and Isl1 has not been identified in humans [24,29]. As some c-kit+ cells also may be mast cells [29], the frequent co-expression of Isl1 and c-kit in our study indicates that c-kit positive cells in the sheep heart are cardiovascular progenitors. Pouly et al. identified c-kit+CD45+ cells from human endomyocardial biopsies that do not express the stem cell markers MDR-1, Isl1 or CD105 [29], whereas, Koninckx et al. identified c-kit+CD105+ cells that were CD45 negative [30]. Isl1 was not examined in these cells. In cardiac stem cells isolated from human atrial appendages, GATA4+ or Nkx2.5+ cells were always c-kit positive, but c-kit+ cells were not always GATA4+/Nkx2.5+ positive [31].
Although CPCs identified in humans include separate populations of SSEA-4+ cells, c-kit+ cells, and Isl1+ cells, [22] substantial overlap appears to exist in the sheep. Whether or not these cells represent various stages of differentiation from a common progenitor in the sheep remains to be determined. Sheep CPC clones identified in this report represent a novel resource with which to clarify the functional potential of distinct subpopulations of progenitors when administered for cardiovascular repair. Of particular interest will be the differentiation and functional capacity of clones expressing both Isl1 and c-kit. The impact of these cells on cardiac function in vivo will provide new insight on the efficacy of endogenous cardiovascular stem cells utilized for the repair of the heart.
Acknowledgements
The confocal microscopy work was done at the Loma Linda University School of Medicine Advanced Imaging and Microscopy Core. This Core is supported by the National Science Foundation under Major Research Instrumentation, Division of Biological Infrastructure Grant No. 0923559 (Sean M Wilson) and Loma Linda University School of Medicine. This work was also supported in part by NIH grant HD031226 (LDL). We thank Monica Rubalcava for technical assistance with confocal microscopy, Peter Gifford and John Hough for their help with immunofluorescence staining, Nina Chu and Megan Russell for their help with obtaining cardiac tissue samples.
Abbreviations
- CPC
Cardiac Progenitor Cells
- SSEA-4
Stage-Specific Embryonic Antigen 4
- Isl1
Islet-1 Transcription Factor
- GATA-4
Transcription Factor GATA-4
- FITC
Fluorescein Isothiocyanate
- GAPDH
Glyceraldehyde-3-Phosphate Dehydrogenase
Footnotes
This an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Publisher's Disclaimer: This article was originally published in a journal by OMICS Publishing Group, and the attached copy is provided by OMICS Publishing Group for the author's benefit and for the benefit of the author's institution, for commercial/research/educational use including without limitation use in instruction at your institution, sending it to specific colleagues that you know, and providing a copy to your institution's administrator.
All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution's website or repository, are requested to cite properly.
References
- 1.Mauritz C, Martens A, Rojas SV, Schnick T, Rathert C, et al. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. Eur Heart J. 2011;32:2634–2641. doi: 10.1093/eurheartj/ehr166. [DOI] [PubMed] [Google Scholar]
- 2.Caspi O, Huber I, Kehat I, Habib M, Arbel G, et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 3.Kearns-Jonker M, Dai W, Gunthart M, Fuentes T, Yeh HY, et al. Genetically Engineered Mesenchymal Stem Cells Influence Gene Expression in Donor Cardiomyocytes and the Recipient Heart. J Stem Cell Res Ther. 2012;S1:005. doi: 10.4172/2157-7633.s1-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong F, Harvey J, Finan A, Weber K, Agarwal U, et al. Myocardial CXCR4 expression is required for mesenchymal stem cell mediated repair following acute myocardial infarction. Circulation. 2012;126:314–324. doi: 10.1161/CIRCULATIONAHA.111.082453. [DOI] [PubMed] [Google Scholar]
- 5.Ye J, Boyle A, Shih H, Sievers RE, Zhang Y, et al. Sca-1+ cardiosphere-derived cells are enriched for Isl1-expressing cardiac precursors and improve cardiac function after myocardial injury. PLoS One. 2012;7:e30329. doi: 10.1371/journal.pone.0030329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zakharova L, Mastroeni D, Mutlu N, Molina M, Goldman S, et al. Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function. Cardiovasc Res. 2010;87:40–49. doi: 10.1093/cvr/cvq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonios M, Chang CY, Pinheiro A, Dimaano VL, Higuchi T, et al. Cardiac resynchronization by cardiosphere-derived stem cell transplantation in an experimental model of myocardial infarction. J Am Soc Echocardiogr. 2011;24:808–814. doi: 10.1016/j.echo.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li TS, Cheng K, Malliaras K, Matsushita N, Sun B, et al. Expansion of human cardiac stem cells in physiological oxygen improves cell production efficiency and potency for myocardial repair. Cardiovasc Res. 2011;89:157–165. doi: 10.1093/cvr/cvq251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tongers J, Losordo DW, Landmesser U. Stem and progenitor cell-based therapy in ischaemic heart disease: promise, uncertainties, and challenges. Eur Heart J. 2011;32:1197–1206. doi: 10.1093/eurheartj/ehr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci USA. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grieve SM, Bhindi R, Seow J, Doyle A, Turner AJ, et al. Microvascular obstruction by intracoronary delivery of mesenchymal stem cells and quantification of resulting myocardial infarction by cardiac magnetic resonance. Circ Heart Fail. 2010;3:e5–6. doi: 10.1161/CIRCHEARTFAILURE.109.931360. [DOI] [PubMed] [Google Scholar]
- 12.Mazo M, Hernández S, Gavira JJ, Abizanda G, Araña M, et al. Treatment of reperfused ischemia with adipose-derived stem cells in a preclinical swine model of myocardial infarction. Cell Transplant. 2012 doi: 10.3727/096368912X638847. [DOI] [PubMed] [Google Scholar]
- 13.Mishra R, Vijayan K, Colletti EJ, Harrington DA, Matthiesen TS, et al. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation. 2011;123:364–373. doi: 10.1161/CIRCULATIONAHA.110.971622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forte G, Pietronave S, Nardone G, Zamperone A, Magnani E, et al. Human cardiac progenitor cell grafts as unrestricted source of supernumerary cardiac cells in healthy murine hearts. Stem Cells. 2011;29:2051–2061. doi: 10.1002/stem.763. [DOI] [PubMed] [Google Scholar]
- 15.Gaetani R, Doevendans PA, Metz CH, Alblas J, Messina E, et al. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials. 2012;33:1782–1790. doi: 10.1016/j.biomaterials.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59:942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon JA, Spinale FG. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail. 2009;2:262–271. doi: 10.1161/CIRCHEARTFAILURE.108.814459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellison GM, Torella D, Dellegrottaglie S, Perez-Martinez C, Perez de Prado A, et al. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol. 2011;58:977–986. doi: 10.1016/j.jacc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Menard C, Hagege AA, Agbulut O, Barro M, Morichetti MC, et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: a preclinical study. Lancet. 2005;366:1005–1012. doi: 10.1016/S0140-6736(05)67380-1. [DOI] [PubMed] [Google Scholar]
- 21.Bourahla B, Shafy A, Meilhac O, Elmadbouh I, Michel JB, et al. Mesothelial cells vs. skeletal myoblasts for myocardial infarction. Asian Cardiovasc Thorac Ann. 2010;18:153–160. doi: 10.1177/0218492310361793. [DOI] [PubMed] [Google Scholar]
- 22.Amir G, Ma X, Reddy VM, Hanley FL, Reinhartz O, et al. Dynamics of human myocardial progenitor cell populations in the neonatal period. Ann Thorac Surg. 2008;86:1311–1319. doi: 10.1016/j.athoracsur.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 23.Kwon C, Qian L, Cheng P, Nigam V, Arnold J, et al. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol. 2009;11:951–957. doi: 10.1038/ncb1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itzhaki-Alfia A, Leor J, Raanani E, Sternik L, Spiegelstein D, et al. Patient characteristics and cell source determine the number of isolated human cardiac progenitor cells. Circulation. 2009;120:2559–2566. doi: 10.1161/CIRCULATIONAHA.109.849588. [DOI] [PubMed] [Google Scholar]
- 25.Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–117. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 26.Moretti A, Lam J, Evans SM, Laugwitz KL. Biology of Isl1+ cardiac progenitor cells in development and disease. Cell Mol Life Sci. 2007;64:674–682. doi: 10.1007/s00018-007-6520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 28.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125:100–112. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pouly J, Bruneval P, Mandet C, Proksch S, Peyrard S, et al. Cardiac stem cells in the real world. J Thorac Cardiovasc Surg. 2008;135:673–678. doi: 10.1016/j.jtcvs.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 30.Koninckx R, Hensen K, Rummens JL, Hendrikx M. Cardiac stem cells in the real world. J Thorac Cardiovasc Surg. 2008;136:797–798. doi: 10.1016/j.jtcvs.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 31.He JQ, Vu DM, Hunt G, Chugh A, Bhatnagar A, et al. Human cardiac stem cells isolated from atrial appendages stably express c-kit. PLoS One. 2011;6:e27719. doi: 10.1371/journal.pone.0027719. [DOI] [PMC free article] [PubMed] [Google Scholar]