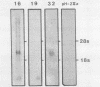
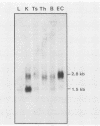
Abstract
The H-2 complex of mice contains many genes in addition to the gene families involved in immune reactions. Some of them are believed to function in mouse development, as suggested by the findings that several embryonic lethal mutations map within or near the H-2 complex. We have analyzed the H-2K/tw5 region in an attempt to study non-H-2 genes encoded in this region. Overlapping cosmid clones spanning about 170 kilobase pairs of DNA, including the H-2K/tw5 region of the mouse, have been screened for genes expressed in embryonic carcinoma cells. A transcript of 2.8 kilobase pairs (K. Abe. J.-F. Wei, F.-S. Wei, Y.-C. Hsu, H. Uehara, K. Artzt, and D. Bennett, EMBO J. 7:3441-3449, 1988) encoded by the KE 4 gene flanking H-2K distally was identified. The transcript was abundantly expressed in embryonic carcinoma cells but was present at low levels in other tissues in adults. A cDNA for this transcript was isolated from the F9 embryonic carcinoma cell line and sequenced. It potentially encodes a protein of 436 amino acids with several interesting features. First, it contains two regions made of well-conserved repeats unusually rich in histidine residues. In the repeats, histidine alternates with other amino acids, notably glycine or serine. Second, the two histidine-rich regions are separated by three putative membrane-spanning domains. Third, the N-terminal part of the sequence shows characteristics of a signal peptide. The results indicate that the protein coded by the gene may be a transmembrane protein with histidine-rich charge clusters. A similar sequence motif found in other known genes allows speculation on the possible functional of this gene.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Wei J. F., Wei F. S., Hsu Y. C., Uehara H., Artzt K., Bennett D. Searching for coding sequences in the mammalian genome: the H-2K region of the mouse MHC is replete with genes expressed in embryos. EMBO J. 1988 Nov;7(11):3441–3449. doi: 10.1002/j.1460-2075.1988.tb03218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor M., Tosi M., Duponchel C., Steinmetz M., Meo T. Liver mRNA probes disclose two cytochrome P-450 genes duplicated in tandem with the complement C4 loci of the mouse H-2S region. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4453–4457. doi: 10.1073/pnas.82.13.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artzt K., Abe K., Uehara H., Bennett D. Intra-H-2 recombination in t haplotypes shows a hot spot and close linkage of 1tw5 to H-2K. Immunogenetics. 1988;28(1):30–37. doi: 10.1007/BF00372526. [DOI] [PubMed] [Google Scholar]
- Artzt K., Shin H. S., Bennett D. Gene mapping within the T/t complex of the mouse. II. Anomalous position of the H-2 complex in t haplotypes. Cell. 1982 Mar;28(3):471–476. doi: 10.1016/0092-8674(82)90201-x. [DOI] [PubMed] [Google Scholar]
- Bennett D. T/t locus, its role in embryogenesis and its relation to classical histocompatibility systems. Prog Allergy. 1981;29:35–53. [PubMed] [Google Scholar]
- Berleth T., Burri M., Thoma G., Bopp D., Richstein S., Frigerio G., Noll M., Nüsslein-Volhard C. The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. EMBO J. 1988 Jun;7(6):1749–1756. doi: 10.1002/j.1460-2075.1988.tb03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewerton D. A., Hart F. D., Nicholls A., Caffrey M., James D. C., Sturrock R. D. Ankylosing spondylitis and HL-A 27. Lancet. 1973 Apr 28;1(7809):904–907. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- Brickell P. M., Latchman D. S., Murphy D., Willison K., Rigby P. W. Activation of a Qa/Tla class I major histocompatibility antigen gene is a general feature of oncogenesis in the mouse. Nature. 1983 Dec 22;306(5945):756–760. doi: 10.1038/306756a0. [DOI] [PubMed] [Google Scholar]
- Burch M. K., Blackburn M. N., Morgan W. T. Further characterization of the interaction of histidine-rich glycoprotein with heparin: evidence for the binding of two molecules of histidine-rich glycoprotein by high molecular weight heparin and for the involvement of histidine residues in heparin binding. Biochemistry. 1987 Nov 17;26(23):7477–7482. doi: 10.1021/bi00397a042. [DOI] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frigerio G., Burri M., Bopp D., Baumgartner S., Noll M. Structure of the segmentation gene paired and the Drosophila PRD gene set as part of a gene network. Cell. 1986 Dec 5;47(5):735–746. doi: 10.1016/0092-8674(86)90516-7. [DOI] [PubMed] [Google Scholar]
- Furie B., Furie B. C. The molecular basis of blood coagulation. Cell. 1988 May 20;53(4):505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Goldbard S. B., Verbanac K. M., Warner C. M. Genetic analysis of H-2 linked gene(s) affecting early mouse embryo development. J Immunogenet. 1982 Apr;9(2):77–82. doi: 10.1111/j.1744-313x.1982.tb00966.x. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Han Y. N., Kato H., Iwanaga S., Suzuki T. Primary structure of bovine plasma high-molecular-weight kininogen. The amino acid sequence of a glycopeptide portion (fragment 1) following the C-terminus ot the bradykinin moiety. J Biochem. 1976 Jun;79(6):1201–1222. doi: 10.1093/oxfordjournals.jbchem.a131175. [DOI] [PubMed] [Google Scholar]
- Han Y. N., Komiya M., Iwanaga S., Suzuki T. Studies on the primary structure of bovine high-molecular-weight kininogen. Amino acid sequence of a fragment ("histidine-rich peptide") released by plasma kallikrein. J Biochem. 1975 Jan 1;77(1?):55–68. [PubMed] [Google Scholar]
- Hood L., Steinmetz M., Malissen B. Genes of the major histocompatibility complex of the mouse. Annu Rev Immunol. 1983;1:529–568. doi: 10.1146/annurev.iy.01.040183.002525. [DOI] [PubMed] [Google Scholar]
- Karlin S., Blaisdell B. E., Mocarski E. S., Brendel V. A method to identify distinctive charge configurations in protein sequences, with application to human herpesvirus polypeptides. J Mol Biol. 1989 Jan 5;205(1):165–177. doi: 10.1016/0022-2836(89)90373-2. [DOI] [PubMed] [Google Scholar]
- Karlin S., Brendel V. Charge configurations in viral proteins. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9396–9400. doi: 10.1073/pnas.85.24.9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbiriou D. M., Griffin J. H. Human high molecular weight kininogen. Studies of structure-function relationships and of proteolysis of the molecule occurring during contact activation of plasma. J Biol Chem. 1979 Dec 10;254(23):12020–12027. [PubMed] [Google Scholar]
- Koide T., Foster D., Yoshitake S., Davie E. W. Amino acid sequence of human histidine-rich glycoprotein derived from the nucleotide sequence of its cDNA. Biochemistry. 1986 Apr 22;25(8):2220–2225. doi: 10.1021/bi00356a055. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lévi-Strauss M., Carroll M. C., Steinmetz M., Meo T. A previously undetected MHC gene with an unusual periodic structure. Science. 1988 Apr 8;240(4849):201–204. doi: 10.1126/science.3353717. [DOI] [PubMed] [Google Scholar]
- Murphy D., Brickell P. M., Latchman D. S., Willison K., Rigby P. W. Transcripts regulated during normal embryonic development and oncogenic transformation share a repetitive element. Cell. 1983 Dec;35(3 Pt 2):865–871. doi: 10.1016/0092-8674(83)90119-8. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Unity in function in the absence of consensus in sequence: role of leader peptides in export. Science. 1989 Mar 3;243(4895):1156–1159. doi: 10.1126/science.2646712. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosstein L., Terasaki P. I., Bluestone R., Pearson C. M. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973 Apr 5;288(14):704–706. doi: 10.1056/NEJM197304052881403. [DOI] [PubMed] [Google Scholar]
- Shin H. S., Bargiello T. A., Clark B. T., Jackson F. R., Young M. W. An unusual coding sequence from a Drosophila clock gene is conserved in vertebrates. Nature. 1985 Oct 3;317(6036):445–448. doi: 10.1038/317445a0. [DOI] [PubMed] [Google Scholar]
- Shin H. S., Flaherty L., Artzt K., Bennett D., Ravetch J. Inversion in the H-2 complex of t-haplotypes in mice. Nature. 1983 Nov 24;306(5941):380–383. doi: 10.1038/306380a0. [DOI] [PubMed] [Google Scholar]
- Shin H. S. The T/t complex and the genetic control of mouse development. Immunol Ser. 1989;43:443–471. [PubMed] [Google Scholar]
- Spies T., Blanck G., Bresnahan M., Sands J., Strominger J. L. A new cluster of genes within the human major histocompatibility complex. Science. 1989 Jan 13;243(4888):214–217. doi: 10.1126/science.2911734. [DOI] [PubMed] [Google Scholar]
- Spies T., Morton C. C., Nedospasov S. A., Fiers W., Pious D., Strominger J. L. Genes for the tumor necrosis factors alpha and beta are linked to the human major histocompatibility complex. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8699–8702. doi: 10.1073/pnas.83.22.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. E., Mandle R., Jr, Kaplan A. P. Studies of binding of prekallikrein and Factor XI to high molecular weight kininogen and its light chain. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4862–4866. doi: 10.1073/pnas.76.10.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara H., Abe K., Park C. H., Shin H. S., Bennett D., Artzt K. The molecular organization of the H-2K region of two t-haplotypes: implications for the evolution of genetic diversity. EMBO J. 1987 Jan;6(1):83–90. doi: 10.1002/j.1460-2075.1987.tb04722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., Chaplin D. D., Weis J. H., Dupont B., New M. I., Seidman J. G. Two steroid 21-hydroxylase genes are located in the murine S region. 1984 Nov 29-Dec 5Nature. 312(5993):465–467. doi: 10.1038/312465a0. [DOI] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- Wiggins R. C., Bouma B. N., Cochrane C. G., Griffin J. H. Role of high-molecular-weight kininogen in surface-binding and activation of coagulation Factor XI and prekallikrein. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4636–4640. doi: 10.1073/pnas.74.10.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]