Abstract
Background
To understand better the mechanism of left ventricular (LV) remodeling related to hypertension, it is important to evaluate LV function in relation to the changes in loading conditions. The aim of this study was to investigate changes in conventional ventricular-arterial coupling indexes, LV strain, and a new index reflecting regional myocardial work assessed noninvasively at rest and during isometric exercise in a random sample including participants with normal blood pressure and those with hypertension.
Methods
A total of 148 participants (53.4% women; mean age, 52.0 years; 39.2% with hypertension) underwent simultaneous echocardiographic and arterial data acquisition at rest and during increased afterload (handgrip exercise). End-systolic pressure was determined from the carotid pulse wave. Arterial elastance (Ea) and LV elastance (Ees) were calculated as end-systolic pressure/stroke volume and end-systolic pressure/end-systolic volume. Doppler tissue imaging and two-dimensional speckle tracking were used to derive LV longitudinal strain. Regional myocardial work (ejection work density [EWD]) was the area of the pressure-strain loop during ejection.
Results
At rest, with adjustments applied, Ees (3.06 vs 3.71 mmHg/mL,P = .0003), Ea/Ees (0.54 vs 0.47,P=.002) and EWD (670 vs 802 Pa/m2, P = .0001) differed significantly between participants with normal blood pressure and those with hypertension. During handgrip exercise, Ea and Ea/Ees significantly increased (P < .0001) in both groups. Doppler tissue imaging and two-dimensional LV strain decreased in participants with hypertension (P ≤ .008). Only in subjects with normal blood pressure EWD significantly increased (+14.7%, P = .0009).
Conclusions
Although patients with hypertension compared with those with normal blood pressure have increased LV systolic stiffness and regional myocardial work to match arterial load at rest, they might have diminished cardiac reserve to increase myocardial performance, as estimated by EWD during isometric exercise.
Keywords: Echocardiography, Hypertension, Ventricular-arterial coupling, Strain
The interaction of the heart with the systemic vasculature, ventricular-arterial coupling (VAC), is a key determinant of cardiovascular performance.1 The capacity of the body to augment cardiac output, regulate systemic blood pressure (BP) and respond appropriately to elevations in heart rate and preload and afterload depends on the properties of both the heart and the vasculature into which the left ventricle ejects blood.2 Hypertension is associated with the stiffening of the large arteries and left ventricle. Because a high arterial pressure opposes left ventricular (LV) ejection, it might lead in the short term to a reduction of LV stroke volume, which is compensated for by shifting the LV pump function to a higher energy level (the Frank-Starling mechanism) and by activating an autoregulation mechanism (the Anrep response). Thus, the heart responds to the greater afterload with an increase in LV stiffness.3 In the long term, this chronically increased cardiac loading leads to LV remodeling (concentrichypertrophy), increases LV oxygen requirements, and eventually causes LV failure. Moreover, to expand stiffened arteries, the heart needs to generate greater forces. Pulse-wave velocity also increases in stiffening arteries, leading to an early return of the reflected wave, which in turn augments late systolic LV load. Further damage to the left ventricle might be caused by increasing time required for systole and shortening of diastole.
Matching the properties of the left ventricle to those of the arteries might preserve cardiac mechanic efficiency at rest, but not necessarily during LV loading. Measurement of VAC generally requires invasive registration of LV pressures and LV volumes, recorded over a wide range of LV loads.3 Effective arterial elastance (Ea), commonly known as the ratio of LV end-systolic pressure (ESP) to stroke volume (SV), reflects the net arterial load imposed on the left ventricle.4 LV end-systolic elastance (Ees) provides an estimate of LV performance and is calculated by measuring the slope of the relations between ESP and end-systolic volume (ESV) registered over a range of LV loads.4 The Ea/Ees ratio is commonly used as an index of the interaction between the left ventricle and the arterial system. Some investigators have proven the feasibility of a noninvasive assessment of VAC in patients with hypertension.5-7 For instance, Osranek et al.6 measured LV volumes by echocardiography and estimated central aortic pressure from a tonometrically recorded pulse-wave signal at the radial artery at rest and during handgrip exercise.
Additional information about LV performance can also be derived from an assessment of myocardial deformation (strain). On the basis of Doppler tissue imaging (DTI) and two-dimensional (2D) speckle tracking, regional myocardial strain curves can be calculated.8,9 Moreover, strain in combination with simultaneously measured LV pressure can be used to estimate regional myocardial work.10 Thus, using the relation between regional LV deformation (strain) and loading, a measure of intrinsic myocardial performance can be obtained.
To understand better the mechanism of LV remodeling related to hypertension, it is important to evaluate LV function in relation to the changes in the loading conditions in subjects with normal BP and those with hypertension. Few studies6,7 have examined noninvasively the changes in VAC components during exercise in patients with hypertension. To our knowledge, no population study thus far has described changes in LV myocardial deformation (strain) and/or regional myocardial work derived from simultaneously obtained estimates of LV ESP and strain under different loading conditions. In the present study, we investigated changes in conventional VAC indexes, LV longitudinal strain, and a new index reflecting regional myocardial work assessed noninvasively at rest and during isometric exercise in a random sample including participants with normal BP and those with hypertension.
METHODS
Study Participants
Study participants were from the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO), consisting of a random population sample stratified by sex and age from a geographically defined area in northern Belgium.11 Seven municipalities gave listings of all inhabitants sorted by address. Households, defined as subjects living at the same address, were the sampling unit. We numbered households consecutively and generated a random-number list using SAS (SAS Institute Inc., Cary, NC). Households with numbers matching the list were invited. The ethics committee of the University of Leuven approved the study, and participants provided informed consent. FLEMENGHO participants were repeatedly visited at home and examined at a local examination center. At each contact, standardized and validated questionnaires were administered to collect detailed information about each participant’s personal and familial medical history, use of medications, and lifestyle. In 2009 and 2010, we reinvited 215 former FLEMENGHO participants for follow-up examinations at our field center, including echocardiography and the isometric exercise test (participation rate, 81.3%).
We excluded 26 subjects from statistical analysis because of myocardial infarction or coronary revascularization (n = 6), moderate to severe valvular abnormalities (n = 9), atrial fibrillation (n = 5), or symptomatic heart failure (n = 6). We excluded a further 41 subject, because DTI studies or 2D echocardiograms (n = 15) or carotid artery pressure waves (n = 21) were of insufficient quality, as well as participants who did not comply with the study protocol (n = 5). Thus, the number of participants statistically analyzed totaled 148.
Echocardiography
Participants refrained from smoking, heavy exercise, and drinking alcohol or caffeine-containing beverages for ≥3 hours before echocardiography.
Data Acquisition
One experienced physician (T.K.) performed the ultrasound examinations according to a standardized protocol as published elsewhere,11 using a Vivid 7 Pro (GE Vingmed Ultrasound AS, Horten, Norway) interfaced with a 2.5-MHz phased-array probe. With the subject in the partial left decubitus position and breathing normally, the observer obtained images from the parasternal long and short axes and from the apical four-chamber and two-chamber and long-axis views. All recordings included at least five cardiac cycles and were digitally stored for offline analysis.
Using DTI, the observer recorded low-velocity, high-intensity myocardial signals at a high frame rate (>190 frames/sec), while adjusting the imaging angle to ensure parallel alignment of the ultrasound beam with the myocardial segment of interest. The Nyquist limit was set as low as possible avoiding aliasing.
Offline Analysis
The same observer (T.K.) analyzed recorded images, averaging three heart cycles for statistical analysis, using a workstation running EchoPAC version 4.0.4 (GE Vingmed Ultrasound AS). LV ESVand end-diastolic volume were measured offline from the apical four-chamber and two-chamber views, using the standard biplane Simpson’s method.12
On the basis of color Doppler myocardial motion data, one-dimensional longitudinal regional strain rate and strain curves were calculated by comparing local myocardial velocity profiles, using dedicated software.11 The SPEQLE package (version 4.6.2) allows M-mode tracking of the myocardium to ensure that the sample volume is maintained in the same anatomic position within myocardial image throughout the cardiac cycle. We positioned the sampling volume in the septal, lateral, inferior, and posterior walls at the level of the posterior chordae tendineae. To compute end-systolic strain, hereafter referred to as strain, we averaged three consecutive measurements and used their absolute values. We calculated the longitudinal strain rate of each interrogated LV wall by measuring the spatial velocity gradient over a computation area of 15 mm. Strain curves were obtained by integrating the mean strain rate profile over time. The beginning and ending of the ejection phase were determined from the simultaneously recorded electrocardiogram and the continuous-wave Doppler velocity trace at the level of the aortic valve. We used lateral averaging of 5 beams/pixel.
For measurement of 2D strain, the endocardial borders were manually traced at the end-systolic frame of the 2D images from the three long-axis views. The 2D strain software (Q-analysis; GE Vingmed Ultrasound AS) automatically tracks myocardial speckle motion, creating basal, mid, and apical regions of interest.
Carotid Pressure Waveform Analysis
During the echocardiographic examination, an experienced technician registered the carotid arterial waveform signal at the right side by applanation tonometry (TY-306; Fukuda Denshi, Tokyo, Japan). Brachial systolic and diastolic BP measured during the echocardiographic examination at rest and during the handgrip test with a validated Omron 705IT device (Omron Corporation, Tokyo, Japan) were used to calibrate the carotid pressure wave.13 ESP was determined from the carotid pressure wave at the incisura reflecting aortic valve closure.
Study Protocol
All participants underwent simultaneous echocardiographic and arterial data acquisition at rest and during an increase of LV afterload induced by isometric handgrip exercise. Maximal grip-force capacity was determined before the echocardiographic examination. Each participant performed three maximal voluntary left forearm contractions with a Stoelting handgrip dynamometer (Stoelting, Wood Dale, IL). The force of these three contractions was averaged. During the isometric exercise, each subject squeezed the dynamometer with the left arm at a submaximal target of 40% until fatigue. The last set of continuously recorded 2D and DTI echocardiographic data was used to derive exercise values.
Data Analysis
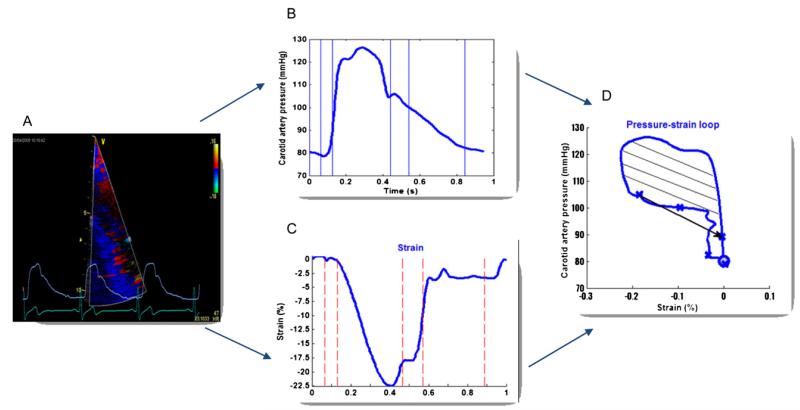
Ea and Ees were calculated as ESP/SV and ESP/ESV, respectively.14 The Ea/Ees ratio represents the VAC index.14 DTI strain and the carotid pressure curves were exported to SPEQLE software for further analysis. From the DTI strain curve and the simultaneously recorded pressure waveforms at the carotid artery, the regional myocardial work density was calculated as a quantitative measure of regional LV performance as previously described.10 Hereto, the instantaneous pressures were plotted against the instantaneous strain values (Figure 1) with indications of different mechanical phases of the cardiac cycle (i.e., onset of the cycle, isovolumic contraction, ejection, isovolumic relaxation, and early and late filling phases) as derived from pulse-wave Doppler tracings through the aortic and mitral valves. Because pressure recordings obtained in the carotid artery are only representative of LV pressures while the aortic valve is opened, ejection work density (EWD) was calculated as the area of the pressure-strain loop during the ejection phase because this area represents the cumulative work done by the muscle to instantaneously shorten a given amount (i.e., change in strain) at a given instantaneous resistance (i.e., pressure). Postprocessing was achieved within a MATLAB environment (The MathWorks Inc., Natick, MA).
Figure 1.
From color Doppler myocardial imaging of the LV wall and simultaneously recorded carotid pressure waveforms (A), we derived carotid artery pressure (B) and DTI strain (C) curves to construct the pressure-strain loop (D). Regional myocardial work density during LV ejection phase was calculated (D) (dashed area).
Reproducibility
To determine the interobserver variability of LV DTI and 2D strain and EWD, two observers analyzed twice the LV deformation curves. Absolute and relative differences between two observers were calculated according to Bland and Altman’s method as (x1 – x2) versus averaged and (100 × [x1 – x2]/averaged) versus averaged, respectively. For DTI longitudinal strain, the absolute and relative differences were −0.29% (95% limits of agreement [LA], −3.73% to 3.14%) and −1.38% (95% LA, −16.8% to 14.1%), respectively. The absolute and relative differences for 2D strain across 37 pairwise readings were −0.30% (95% LA, −1.72% to 2.32%) and −1.42% (95% LA, −11.7% to 8.87%), respectively. The absolute and relative differences for EWD measurement across 34 pairwise readings were −14.5 Pa/m2 (95% LA, −82.3 to 53.4 Pa/m2) and −2.01% (95% LA, −11.6% to 7.54%), respectively. We also calculated intraclass correlation coefficients with 95% confidence intervals (CIs) while taking into account the bias across pairwise readings. The interobserver intraclass correlation coefficients for longitudinal DTI strain, 2D strain, and EWD were 0.87 (95% CI, 0.82 to 0.91), 0.89 (95% CI, 0.80 to 0.94), and 0.98 (95% CI, 0.97 to 0.99), respectively.
Other Measurements
At the examination center, trained study nurses administered a questionnaire to collect detailed information on each subject’s medical history, smoking and drinking habits, and intake of medications. The conventional BP was the average of five consecutive auscultatory readings obtained at the examination center with the subject in the seated position. Hypertension was defined as systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg or the use of antihypertensive drugs. Body mass index was calculated as weight in kilograms divided by the square of height in meters.
Statistical Methods
For database management and statistical analysis, we used SAS version 9.1. The central tendency and the spread of the data are reported as mean ± SD. We compared means and proportions using t tests and χ2 tests, respectively. Significance was set at P < .05 in two-sided tests. We applied a generalization of the standard linear model, as implemented in the PROC MIXED procedure of SAS, to investigate the associations between changes in LV volumes, VAC components, and LV strain and changes in ESP during handgrip exercise, while accounting for covariates such as sex, baseline values at rest, and age. We expressed multivariate-adjusted effect sizes for 10 mm Hg changes in LV ESP during handgrip exercise.
RESULTS
Characteristics of Participants
The 148 participants included 79 women (53.4%) and 58 patients with hypertension (39.2%), of whom 34 (23.0%) were on one or more antihypertensive drugs (β-blockers, n = 15; diuretics, n = 11; calcium channel blockers, n = 11; angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, n = 11). Table 1 shows the clinical characteristics of the study participants by hypertension status. Patients with hypertension were older and had higher body mass indexes compared with those with normal BP (Table 1). Mean handgrip force was similar in both groups, averaging 13.1 ± 4.3 kg over 286 ± 57 sec. As expected, LV mass index and relative wall thickness (Table 1) were significantly greater in patients with hypertension than those with normal BP. Participants with hypertension also had lower transmitral E/A ratios, mitral annular e′ and e′/a′ ratios, but higher E/e′ ratios (P < .0001). With adjustments applied for sex, age, and body mass index, only E/e′ ratio remained significantly different (P = .0075) according to hypertensive status, indicative of worse diastolic function (higher LV diastolic filling pressures) in patients with hypertension.
Table 1.
Characteristics of participants
| Characteristic | Normal BP (n = 90) |
Hypertension (n = 58) |
P |
|---|---|---|---|
| Anthropometrics | |||
| Age (y) | 44.6 ± 15.2 | 57.7 ± 10.6 | <.0001 |
| Women | 50 (55.6%) | 29 (50.0%) | .17 |
| Height (cm) | 171.± 6 9.7 | 169.1 ± 9.7 | .13 |
| Weight (kg) | 76.4 ± 15.4 | 79.1 ± 12.7 | .27 |
| Body mass index (kg/m2) | 25.8 ± 3.7 | 27.6 ± 3.0 | .003 |
| Body surface area (m2) | 1.89 ± 0.22 | 1.90 ± 0.19 | .77 |
| Questionnaire data | |||
| Treated for hypertension | – | 34 (58.6%) | – |
| Handgrip test performance | |||
| Handgrip force (kg) | 13.1 ± 4.3 | 13.0 ± 4.2 | .93 |
| Test duration (sec) | 288 ± 63 | 281 ± 47 | .45 |
| Echocardiographic data | |||
| LV mass index (g/m2) | 88.0 ± 19.2 | 99.5 ± 21.4 | .0006 |
| Relative wall thickness | 0.36 ± 0.047 | 0.40 ± 0.052 | <.0001 |
| Transmitral E/A ratio | 1.45 ± 0.54 | 1.05 ± 0.27 | <.0001 |
| Mitral annular e′ (cm/sec) | 12.2 ± 3.20 | 9.36 ± 2.26 | <.0001 |
| DTI e′/a′ ratio | 1.57 ± 0.76 | 0.96 ± 0.35 | <.0001 |
| E/e′ ratio | 6.38 ± 1.36 | 7.83 ± 1.86 | <.0001 |
Data are expressed as mean ± SD or as number (percentage). P values are for the differences between participants with normal BP and those with hypertension.
VAC Components and LV Longitudinal Strain at Rest in Participants with Hypertension and Those with Normal BP
Table 2 shows the hemodynamic and VAC characteristics and LV longitudinal strain by hypertension status at rest and during handgrip exercise. As expected, at rest, systolic and diastolic BP and ESP were significantly higher in patients with hypertension than in subjects with normal BP (P < .0001). ESV (P = .04) but not SV (P = .10) was lower in patients with hypertension (Table 2). The Ea/Ees ratio was 16.4% lower (P < .0001) in patients with hypertension. Examining the components of this ratio, both Ea (P = .02) and Ees (P < .0001) were higher in patients with hypertension compared with subjects with normal BP. With adjustments applied for sex, age, and body mass index, only Ees (3.06 vs 3.71 mm Hg/mL, P = .0003) and Ea/Ees ratio (0.54 vs 0.47, P = .002) differed significantly according to hypertension status.
Table 2.
Hemodynamic measurements, VAC components, and LV strain by hypertension status
| Normal BP (n = 90) |
Hypertension (n = 58) |
P (normal BP vs hypertension) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Rest | Handgrip exercise |
Δ | P | Rest | Handgrip exercise |
Δ | P | Rest | Handgrip exercise |
| Hemodynamic | ||||||||||
| Heart rate (beats/min) | 58.0 ± 8.5 | 65.3 ± 10.0 | 7.3 ± 5.3 | <.0001 | 55.9 ± 7.3 | 62.6 ± 11.1 | 6.7 ± 6.6 | <.0001 | .13 | .13 |
| Brachial systolic BP (mm Hg) | 109.9 ± 12.3 | 127.7 ± 15.4 | 17.8 ± 13.3 | <.0001 | 127.5 ± 17.2 | 144.8 ± 17.5 | 17.3 ± 15.0 | <.0001 | <.0001 | <.0001 |
| Brachial diastolic BP (mm Hg) | 63.6 ± 8.7 | 76.0 ± 10.8 | 12.4 ± 10.4 | <.0001 | 72.3 ± 12.2 | 81.8 ± 11.7 | 9.6 ± 11.9 | <.0001 | <.0001 | .002 |
| End-systolic BP (mm Hg) | 94.7 ± 12.1 | 108.4 ± 14.6 | 13.7 ± 11.7 | <.0001 | 112.0 ± 15.5 | 122.4 ± 13.5 | 10.4 ± 13.5 | <.0001 | <.0001 | <.0001 |
| VAC components | ||||||||||
| End-diastolic volume (mL) | 99.3 ± 22.6 | 102.5 ± 24.0 | 3.2 ± 7.4 | .0001 | 100.8 ± 21.8 | 101.6 ± 21.8 | 0.83 ± 8.1 | .45 | .88 | .83 |
| ESV (mL) | 34.9 ± 10.0 | 38.7 ± 11.0 | 3.7 ± 4.7 | <.0001 | 31.8 ± 10.1 | 35.1 ± 11.3 | 3.3 ± 4.5 | <.0001 | .04 | .07 |
| SV (mL) | 64.4 ± 14.5 | 63.9 ± 15.0 | −0.48 ± 7.0 | .51 | 69.0 ± 14.2 | 66.4 ± 13.0 | −2.6 ± 8.7 | .03 | .10 | .31 |
| Ea (mm Hg/mL) | 1.55 ± 0.40 | 1.80 ± 0.49 | 0.25 ± 0.29 | <.0001 | 1.69 ± 0.40 | 1.90 ± 0.38 | 0.21 ± 0.26 | <.0001 | .02 | .16 |
| Ees (mm Hg/mL) | 2.99 ± 1.11 | 3.06 ± 1.06 | 0.07 ± 0.44 | .14 | 3.87 ± 1.33 | 3.83 ± 1.24 | 60.04 ± 0.73 | .70 | <.0001 | .0002 |
| Ea/Ees ratio | 0.55 ± 0.12 | 0.61 ± 0.13 | 0.06 ± 0.10 | <.0001 | 0.47 ± 0.12 | 0.53 ± 0.13 | 0.06 ± 0.10 | <.0001 | <.0001 | .0004 |
| LV strain | ||||||||||
| TDI strain | ||||||||||
| Basal-mid strain (%) | 20.8 ± 2.54 | 21.1 ± 2.95 | 0.31 ± 2.64 | .27 | 21.2 ± 2.67 | 19.6 ± 3.02 | −1.6 ± 3.0 | .0002 | .39 | .004 |
| EWD (Pa/m2) | 652 ± 174 | 748 ± 203 | 96 ± 190 | <.0001 | 859 ± 231 | 839 ± 246 | −20 ± 217 | .48 | <.0001 | .02 |
| 2D speckle-tracking strain | ||||||||||
| Basal-mid strain (%) | 20.0 ± 2.35 | 19.6 ± 2.63 | 60.39 ± 2.0 | .07 | 20.7 ± 1.98 | 19.8 ± 2.40 | −0.87 ± 2.3 | .009 | .07 | .59 |
| Apical strain (%) | 23.2 ± 2.61 | 22.3 ± 3.01 | 60.94 ± 3.2 | .007 | 24.3 ± 3.41 | 23.0 ± 3.59 | 61.36 ± 4.0 | .01 | .03 | .22 |
| Averaged strain (%) | 21.2 ± 1.77 | 20.6 ± 2.39 | −0.61 ± 2.1 | .007 | 21.7 ± 1.79 | 21.0 ± 2.02 | −0.67 ± 1.9 | .01 | .13 | .30 |
Data are expressed as mean ± SD.
Participants with hypertension and those with normal BP had similar longitudinal DTI strain and 2D strain measured at the basal-mid LV wall segments (P ≥ .07; Table 2). However, in both unadjusted and adjusted analyses, EWD at rest was about 24% higher in patients with hypertension than in subjects with normal BP (P < .0001).
VAC Components and LV Longitudinal Strain Changes during Handgrip Exercise in Participants with Hypertension and Those with Normal BP
In participants with hypertension and those with normal BP (P < .0001 for all; Table 2), handgrip exercise increased heart rate by +7.3 beats/min (relative change with exercise [Δ] = +12.4%) and +6.7 beats/min (Δ = +12.0%), respectively, and ESP by +13.7 mm Hg (Δ = +14.5%) and +10.4 mm Hg (Δ = +9.3%), respectively, with no between-group differences (P ≥ .09; Table 3). Because a short duration of the isometric test (mean time, 4.8 ± 0.93 min), a modest increase in BP during the test (mean ΔESP, 12.3 mm Hg) and the exclusion of patients with clinically overt heart failure or established coronary artery disease, no symptoms were reported during the handgrip test.
Table 3.
Adjusted changes of hemodynamic measurements, VAC components, and LV strain during the handgrip test by hypertension status
| Normal BP |
Hypertension |
||||||
|---|---|---|---|---|---|---|---|
| Variable | Δ adjusted mean ± SE |
95% CI | P | Δ adjusted mean ± SE |
95% CI | P |
P (normal BP vs hypertension) |
| Hemodynamic | |||||||
| Heart rate (beats/min) | 6.87 ± 0.61 | 5.67 to 8.09 | <.0001 | 7.50 ± 0.77 | 5.97 to 9.02 | <.0001 | .55 |
| Brachial systolic BP (mm Hg) | 16.1 ± 1.45 | 13.3 to 19.0 | <.0001 | 20.5 ± 1.87 | 16.8 to 24.2 | <.0001 | .09 |
| Brachial diastolic BP (mm Hg) | 10.7 ± 1.07 | 8.6 to 12.9 | <.0001 | 12.5 ± 1.37 | 9.8 to 15.2 | <.0001 | .33 |
| End-systolic BP (mm Hg) | 11.5 ± 1.26 | 9.03 to 14.0 | <.0001 | 14.2 ± 1.63 | 11.0 to 17.4 | <.0001 | .24 |
| VAC components | |||||||
| End-diastolic volume (mL) | 3.18 ± 0.85 | 1.50 to 4.86 | .0003 | 0.86 ± 1.11 | −1.33 to 3.05 | .44 | .12 |
| ESV (mL) | 3.82 ± 0.51 | 2.81 to 4.83 | <.0001 | 3.17 ± 0.66 | 1.87 to 4.46 | <.0001 | .46 |
| SV (mL) | −0.90 ± 0.80 | −2.47 to 0.68 | .26 | −1.61 ± 1.04 | −3.67 to 0.45 | .12 | .61 |
| Ea (mm Hg/mL) | 0.24 ± 0.03 | 0.18 to 0.30 | <.0001 | 0.21 ± 0.04 | 0.14 to 0.29 | <.0001 | .49 |
| Ees (mm Hg/mL) | −0.005 ± 0.06 | −0.12 to 0.11 | .94 | 0.08 ± 0.08 | −0.076 to 0.23 | .32 | .43 |
| Ea/Ees ratio | 0.072 ± 0.011 | 0.051 to 0.093 | <.0001 | 0.051 ± 0.014 | 0.023 to 0.078 | .0004 | .25 |
| DTI strain | |||||||
| Basal-mid (%) | 0.0003 ± 0.28 | −0.55 to 0.55 | .99 | −1.13 ± 0.35 | −1.82 to −0.43 | .008 | .018 |
| EWD (Pa/m2) | 72.3 ± 21.3 | 30.2 to 114.4 | .0009 | 16.4 ± 27.8 | −38.6 to 71.4 | .56 | .14 |
| 2D strain | |||||||
| Basal-mid (%) | −0.33 ± 0.22 | −0.77 to 0.20 | .15 | −1.05 ± 0.29 | −1.63 to −0.48 | .0004 | .06 |
| Apical (%) | −1.44 ± 0.34 | −2.12 to −0.76 | <.0001 | −1.17 ± 0.44 | −2.06 to −0.29 | .01 | .65 |
| Averaged (%) | −0.78 ± 0.20 | −1.18 to −0.38 | .0002 | −0.86 ± 0.26 | −1.37 to −0.34 | .0014 | .83 |
Changes were adjusted for sex, values at rest, and age.
The responses of the VAC components and LV longitudinal strain to handgrip exercise with adjustment applied for values at rest, sex, and age are shown in Table 3 by hypertension status. During handgrip exercise ESV, Ea and Ea/Ees ratio significantly increased (P < .001 for all) in both participants with hypertension and those with normal BP, with no between-group differences (P ≥ .25; Table 3). DTI and 2D longitudinal strain of the basal-mid LV wall segments decreased significantly only in participants with hypertension (P ≤ .008; Table 3). EWD increased by 14.7% (P = .0009) in subjects with normal BP and did not change (Δ = +2.5%, P = .56) in those with hypertension during handgrip. Apical and averaged 2D strain decreased similarly in both group (P ≤ .01; Table 3).
Association between Changes in LV Volumes and Longitudinal Strain with Increase in ESP during Handgrip Exercise
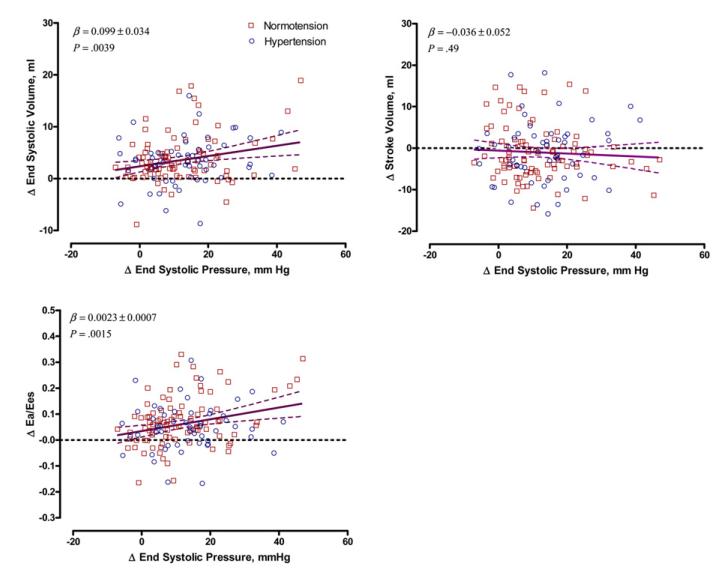
We analyzed the association between changes in LV volumes, Ea/Ees ratio, and LV longitudinal strain with increase in ESP during handgrip exercise in analyses adjusted for baseline values at rest, sex, and age. Exercise-induced changes in ESV and Ea/Ees ratio, but not SV (P = .49), were significantly associated with changes in ESP (Figure 2). For each 10 mm Hg increase in ESP during handgrip exercise, ESV and Ea/Ees ratio rose by 0.99 mL (P = .0039) and 0.023 (P = .0015), respectively. There was a significant interaction (P ≤ .02) between ΔESP and sex, indicating that the exercise-induced changes in ESV and Ea/Ees ratio were more pronounced in women than in men. These associations did not reach statistical significance (P ≥ .32) in men, whereas in women, they were +1.37 mL (P = .0018) for ΔESV and +0.029 (P = .0012) for ΔEa/Ees.
Figure 2.
Scatterplots of the changes in LV ESV, SV, and Ea/Ees ratio versus changes in ESP during handgrip exercise in multivariate-adjusted analyses of 148 participants, including 90 subjects with normal BP (squares) and 58 patients with hypertension (circles). The solid and dotted lines represent the regression line and the 95% CI, respectively. The regression slopes were standardized to the means of the distributions of sex, values at rest, and age.
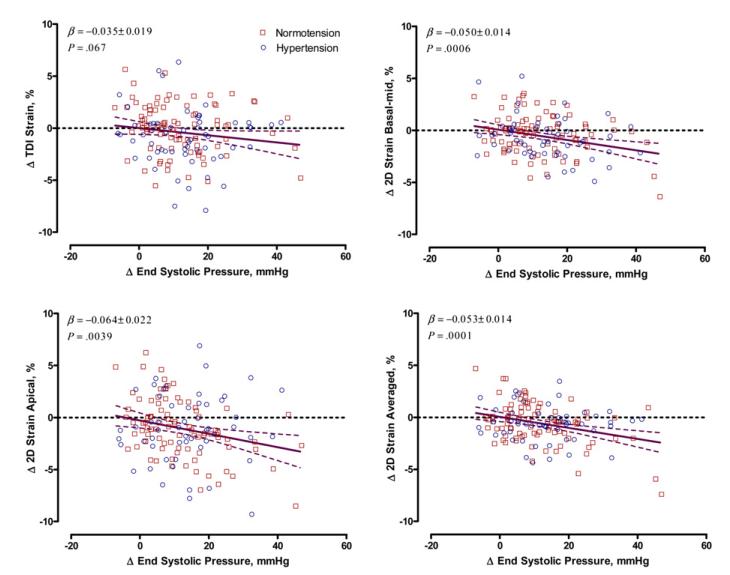
In multivariate-adjusted analyses, LV longitudinal strain measured during exercise by speckle tracking and DTI decreased with increased ESP (Figure 3). For a 10 mm Hg increment in ESP during handgrip, basal-mid, apical, and averaged 2D LV strain decreased by 0.50% (P = .0006), 0.64% (P = .0039), and 0.53% (P = .0001), respectively. The multivariate-adjusted association between the exercise-induced responses of basal-mid DTI strain and ESP were slightly attenuated compared with the associations seen with 2D strain, but trends were similar (−0.35%, P = .067). We did not observe significant interaction between ΔESP and sex in relation to exercise-induced changes in LV strain (P ≥ .70). These findings remained consistent when we additionally adjusted our models for antihypertensive drug treatment (data not shown).
Figure 3.
Scatterplots of the changes in DTI and 2D strain versus changes in ESP during handgrip exercise in multivariate-adjusted analyses of 148 participants, including 90 subjects with normal BP (squares) and 58 patients with hypertension (circles). The solid and dotted lines represent the regression line and the 95% CI, respectively. The regression slopes were standardized to the means of the distributions of sex, values at rest, and age.
DISCUSSION
Using a noninvasive technique involving simultaneous recordings of LV and arterial function, we demonstrated differences in LV performance at rest and in cardiac adaptation to increased afterload between patients with hypertension and subjects with normal BP. To our knowledge, our report is the first study documenting the responses of LV longitudinal strain and regional myocardial work density to isometric exercise. The present study also proved the feasibility of noninvasive simultaneous assessment of LV strain and ESP.
Even before the development of clinically overt heart failure or LV hypertrophy, hypertension accelerates the age-related stiffening of the left ventricle and large arteries. In agreement with previous studies,3,5,7,15 our data show that effective arterial and LV elastance on the basis of determination of LV volumes and ESP are increased in hypertension. Cohen-Solal et al.15 showed that Ea and Ees as measured by angiography were significantly higher in 19 men with hypertension compared with 25 men with normal BP. These findings were later confirmed by Saba et al.,5 who used echocardiography to estimate LV volumes and carotid pressure waveforms to estimate ESP in 81 subjects with normal BP and 174 patients with hypertension. Chantler et al.7 reported that the VAC ratio was about 25% lower in women with hypertension compared with those with normal BP, with no difference seen in men. Along similar lines, we found that in patients with hypertension, the VAC ratio at rest was 16.4% lower compared with subjects with normal BP. The lower VAC ratio in patients with hypertension was due to a disproportionate increase in Ees compared with Ea (32% vs 10%). In line with our findings, Borlaug et al.16 reported that 719 patients with hypertension had higher Ees and circumferential midwall fractional shortening compared with 617 controls with normal BP. Ees is an index of myocardial contractility reflecting the ability of the left ventricle to eject blood opposed to a given pressure. An increase in Ees is generally associated with enhanced myocardial contractility. On the other hand, chronic changes in Ees also reflect chamber geometry and passive myocardial stiffening, and both have been observed to be altered in patients with hypertension in previous studies, including ours. Therefore, in patients with hypertension, higher Ees at rest might not only mean greater myocardial contractility but also reflect geometric and passive structural changes in hypertensive hearts.
Our study is the first to demonstrate a difference between participants with hypertension and those with normal BP in regional myocardial work density during ejection (EWD), which we assessed noninvasively using DTI longitudinal strain–LV ESP loops. EWD at rest was about 24% higher in patients with hypertension than in subjects with normal BP. A previous experimental study17 showed that the peak rate of changes of LV pressure (dP/dt), as an invasive index of myocardial contractility, was greater by 51% in the hypertensive compared with normotensive animals.
No previous population study has examined how hypertension affects VAC components and LV strain during an acute increase in afterload induced by isometric exercise. In a small group of patients with hypertension (n = 18) Osranek et al.6 found increased Ea/Ees ratio and its components during handgrip exercise before and after antihypertensive treatment. Chantler et al.7 reported that Ea/Ees ratio decreased to the same extent with aerobic bicycle exercise in subjects with normal BP and those with hypertension. The interaction between Ea and Ees can be used to evaluate global LV systolic performance in the context of its interaction with the systemic arterial system. In fact, Ea/Ees ratio is inversely related to ejection fraction (Ea/Ees = ESV/SV = [1/EF] – 1). On the other hand, by examining the components of the Ea/Ees ratio, we might separately evaluate the elastance of the arterial system and of the left ventricle. During handgrip exercise, Ea/Ees ratio increased to the same extent in the normotensive and hypertensive groups because of similar increases in Ea, whereas global myocardial contractility (Ees) did not change significantly in both groups (Table 3).
On the other hand, changes in LV deformation are determined by changes in active force development as well as changes in local wall stress during the ejection phase. Thus, acute increase in BP, as occurring during isometric exercise, might lead to increased wall stress and thus reduced deformation, particularly in the longitudinally oriented subendocardial fibers, because they have lower curvature (and thus experience higher fiber stress) than the midmyocardial (circumferential) fibers. This was recently confirmed in an experimental study involving an aortic banding model that showed disparate changes in longitudinal and radial myocardial strain in response to acute alternations to LV afterload.18 Indeed, in this experimental study, longitudinal systolic deformation dramatically fell as afterload increased, whereas LV fractional shortening and radial strain were still preserved after a mild banding.18 Because the subendocardium is more vulnerable to increased wall stress, ischemia, and interstitial fibrosis, longitudinal systolic dysfunction could already been seen at the early stages of progressive myocardial disease, including hypertrophy and myocardial ischemia.19
Along similar lines, in our study, for the first time we observed differences in exercise-induced changes in LV longitudinal strain and regional work density between participants with hypertension and those with normal BP. Thus, DTI and speckle tracking generate valuable information about regional LV performance not only at rest but also under different loading conditions. Indeed, during handgrip exercise, DTI and 2D longitudinal strain of basal-mid LV wall segments decreased significantly in subjects with hypertension, whereas apical strain decreased similarly in both groups. In our study, during handgrip exercise, regional myocardial work density increased in subjects with normal BP but did not significantly change in patients with hypertension. The exercised-induced increase in EWD in participants with normal BP might be explained by the Anrep effect, an autoregulation mechanism in which myocardial contractility increases with increased afterload. This allows the heart to compensate for changes in LV volume that occur when afterload increases. On the other hand, patients with hypertension might have diminished cardiac reserve to increase myocardial performance (estimated in our study by EWD) at least in the longitudinal direction to compensate for volume changes during the isometric test.
The present study must be interpreted within the context of its potential limitations and strengths. First, the DTI technique to estimate LV longitudinal strain offers superior temporal resolution but is prone to measurement error due to signal noise, acoustic artifacts, and angle dependency. This may limit the application of DTI in routine clinical practice. Two-dimensional speckle tracking might be less vulnerable to these artifacts. However, the algorithm to reconstruct pressure-strain curves on the basis of 2D speckle images has not yet been implemented in the commercially available software.
Second, we estimated regional myocardial work density based only on LV longitudinal strain. However, the full spectrum of myocardial deformation includes along with longitudinal also circumferential and radial components. Further research is required to evaluate LV regional myocardial work including all components of LV deformation.
Third, although we optimized 2D grayscale echocardiographic images as well as the angle of the ultrasound beam, the velocity range settings and noise level of color myocardial Doppler images, we could not always acquire images of optimal quality. Thus, we had to discard 15 from the analysis because of poor quality of LV images. Moreover, in 21 participants, the carotid pulse-wave curve was not of optimal quality.
Finally, we estimated LV volumes by 2D echocardiography using the biplane Simpson’s method. We might therefore have underestimated the absolute values of LV volumes.20 On the other hand, the Ea/Ees ratio should not be affected by the method of LV volumes assessment or anthropometric characteristics.
CONCLUSIONS
Our study demonstrated differences in LV performance at rest and during cardiac adaptation to increased afterload between patients with hypertension and subjects with normal BP. Although patients with hypertension compared with subjects with normal BP have increased LV systolic stiffness and regional myocardial work to match arterial load at rest, they might have diminished cardiac reserve to increase myocardial performance as estimated by EWD, at least in the longitudinal direction, to compensate for volume changes during the isometric test. Our study proved the feasibility of assessment of myocardial performance by simultaneous recording of LV strain and ESP. Although the reconstructed area of the pressure-strain loop during LV ejection is an index rather than a direct measure of regional myocardial performance, it has the advantage of being simple to obtain and noninvasive in nature.10 From the clinical point of view, the pressure-strain loop along with the pressure-volume loop obtained from noninvasively measured ESP, LV SV, and strain might give insight into how different antihypertensive drugs might modify and/or improve myocardial function.6 Moreover, these indexes of myocardial performance might be used to follow the evolution of LV regional function after a myocardial infarction in relation to the changes in local loading conditions.21 From the research point of view, future longitudinal population studies should clarify whether alterations in the VAC ratio and its components as well as in LV regional work density provide any prognostic information for adverse outcomes, such as heart failure. Clarifying these mechanisms might open new opportunities for the prevention and treatment of early LV dysfunction.
ACKNOWLEDGMENTS
We gratefully acknowledge the expert assistance of Sandra Covens, Linda Custers, Marie-Jeanne Jehoul, and Hanne Truyens (Leuven, Belgium).
The Studies Coordinating Centre was supported by grants HEALTH-2011-278249-EU-MASCARA and ERC Advanced Grant-2011-294713-EPLORE from the European Union and grants G.0575.06 and G.0734.09 from Fonds Voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community (Brussels, Belgium).
Abbreviations
- BP
Blood pressure
- CI
Confidence interval
- DTI
Doppler tissue imaging
- Ea
Arterial elastance
- Ees
End-systolic elastance
- ESP
End-systolic pressure
- ESV
End-systolic volume
- EWD
Ejection work density
- FLEMENGHO
Flemish Study on Environment, Genes and Health Outcomes
- LA
Limits of agreement
- LV
Left ventricular
- SV
Stroke volume
- 2D
Two-dimensional
- VAC
Ventricular-arterial coupling
REFERENCES
- 1.Kass DA. Ventricular arterial stiffening: integrating the pathophysiology. Hypertension. 2005;46:185–93. doi: 10.1161/01.HYP.0000168053.34306.d4. [DOI] [PubMed] [Google Scholar]
- 2.de Tombe PP, Jones S, Burkhoff D, Hunter WC, Kass DA. Ventricular stroke and efficiency both remain nearly optimal despite altered vascular loading. Am J Physiol. 1993;264:H1817–24. doi: 10.1152/ajpheart.1993.264.6.H1817. [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi M, Hay I, Fetics BJ, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–20. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 4.Sunagawa K, Sagawa K, Maughan WL. Ventriculo-vascular coupling: clinical, physiologic, and engineering aspects. Springer Verlag; New York: 1987. Ventricular interaction with the vascular system in terms of pressure-volumes relationships; pp. 210–39. [Google Scholar]
- 5.Saba PS, Ganau A, Devereux RB, Pini R, Pickering TG, Roman MJ. Impact of arterial elastance as a measure of vascular load on left ventricular geometry in hypertension. J Hypertens. 1999;17:1007–15. doi: 10.1097/00004872-199917070-00018. [DOI] [PubMed] [Google Scholar]
- 6.Osranek M, Eisenach JH, Khandheria BK, Chandrasekaran K, Seward JB, Belohlavek M. Arterioventricular coupling and ventricular efficiency after antihypertensive therapy: a noninvasive prospective study. Hypertension. 2008;51:275–81. doi: 10.1161/HYPERTENSIONAHA.107.097964. [DOI] [PubMed] [Google Scholar]
- 7.Chantler PD, Melenovsky V, Schulman SP, Gerstenblith G, Becker LC, Ferrucci L, et al. The sex-specific impact of systolic hypertension and systolic blood pressure on arterial-ventricular coupling at rest and during exercise. Am J Physiol Heart Circ Physiol. 2008;295:H145–53. doi: 10.1152/ajpheart.01179.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Hooge J, Heimdal A, Jamal F, Kukulski T, Bijnens B, Rademakers FE, et al. Regional strain and strain rate measurements by cardiac ultrasound: principles, implementation and limitations. Eur J Echocardiography. 2000;1:154–70. doi: 10.1053/euje.2000.0031. [DOI] [PubMed] [Google Scholar]
- 9.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–69. doi: 10.1016/j.echo.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Urheim S, Rabben SI, Skulstad H, Lyseggen E, Ihlen H, Smiseth OA. Regional myocardial work by strain Doppler echocardiography and LV pressure: a new method for quantifying myocardial function. Am J Physiol Heart Circ Physiol. 2005;288:H2375–80. doi: 10.1152/ajpheart.00946.2004. [DOI] [PubMed] [Google Scholar]
- 11.Kuznetsova T, Herbots L, Richart T, D’hooge J, Thijs L, Fagard RH, et al. Left ventricular strain and strain rate in a general population. Eur Heart J. 2008;29:2014–23. doi: 10.1093/eurheartj/ehn280. [DOI] [PubMed] [Google Scholar]
- 12.Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, et al. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. A report from the American Society of Echocardiography’s Guidelines and Standard Committee and the Task Force on Echocardiography in Clinical Trials. J Am Soc Echocardiogr. 2004;17:1086–119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Mahieu D, Kips J, Rietzschel ER, De Buyzere ML, Verbeke F, Gillebert TC, et al. Asklepios Investigators Noninvasive assessment of central and peripheral arterial pressure (waveforms): implications of calibration methods. J Hypertens. 2010;28:300–5. doi: 10.1097/HJH.0b013e3283340a1a. [DOI] [PubMed] [Google Scholar]
- 14.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol. 2008;105:1342–51. doi: 10.1152/japplphysiol.90600.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen-Solal A, Caviezel B, Himbert D, Gourgon R. Left ventricular-arterial coupling in systemic hypertension: analysis by means of arterial effective and left ventricular elastances. J Hypertens. 1994;12:591–600. [PubMed] [Google Scholar]
- 16.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–8. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aylward PE, McRitchie RJ, Chalmers JP, West MJ. Baroreflex control of myocardial contractility in conscious normotensive and renal hypertensive rabbits. Hypertension. 1983;5:916–26. doi: 10.1161/01.hyp.5.6.916. [DOI] [PubMed] [Google Scholar]
- 18.Donal E, Bergerot C, Thibault H, Ernande L, Loufoua J, Augeul L, et al. Influence of afterload on left ventricular radial and longitudinal systolic functions: a two-dimensional strain imaging study. Eur J Echocardiogr. 2009;10:914–21. doi: 10.1093/ejechocard/jep095. [DOI] [PubMed] [Google Scholar]
- 19.Sengupta PP, Narula J. Reclassifying heart failure: predominantly subendocardial, subepicardial, and transmural. Heart Fail Clin. 2008;4:379–82. doi: 10.1016/j.hfc.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Gardner BI, Bingham SE, Allen MR, Blatter DD, Anderson JL. Cardiac magnetic resonance versus transthoracic echocardiography for the assessment of cardiac volumes and regional function after myocardial infarction: an intrasubject comparison using simultaneous intrasubject recordings. Cardiovasc Ultrasound. 2009;7:38. doi: 10.1186/1476-7120-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rademakers F, Van de Werf F, Mortelmans L, Marchal G, Bogaert J. Evolution of regional performance after an acute anterior myocardial infarction in humans using magnetic resonance tagging. J Physiol. 2003;546:777–87. doi: 10.1113/jphysiol.2002.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]