Abstract
To assess the prognostic significance of blood pressure (BP) variability, we followed health outcomes in a family-based random population sample representative of the general population (n=2944; mean age: 44.9 years; 50.7% women). At baseline, BP was measured 5 times consecutively at each of 2 home visits 2 to 4 weeks apart. We assessed within-subject overall (10 readings), within- and between-visit systolic BP variability from variability independent of the mean, the difference between maximum and minimum BP, and average real variability. Over a median follow-up of 12 years, 401 deaths occurred and 311 participants experienced a fatal or nonfatal cardiovascular event. Overall systolic BP variability averaged (SD) 5.45 (2.82) units, 15.87 (8.36) mm Hg, and 4.08 (2.05) mm Hg for variability independent of the mean, difference between maximum and minimum BP, and average real variability, respectively. Female sex, older age, higher-mean systolic BP, lower body mass index, a history of peripheral arterial disease, and use of β-blockers were the main correlates of systolic BP variability. In multivariable-adjusted analyses, overall and within- and between-visit BP variability did not predict total or cardiovascular mortality or the composite of any fatal plus nonfatal cardiovascular end point. For instance, the hazard ratios for all cardiovascular events combined in relation to overall variability independent of the mean, difference between maximum and minimum BP, and average real variability were 1.05 (0.96–1.15), 1.06 (0.96–1.16), and 1.08 (0.98–1.19), respectively. By contrast, mean systolic BP was a significant predictor of all end points under study, independent of BP variability. In conclusion, in an unbiased population sample, BP variability did not contribute to risk stratification over and beyond mean systolic BP.
Keywords: blood pressure variability, cardiovascular disease, population science, risk factors, epidemiology
The prognostic significance of blood pressure variability remains controversial. Some studies reported association of end-organ damage,1–4 cardiovascular events,5–7 or mortality8 with blood pressure variability, whereas others failed to do so or found variability to be inferior to mean systolic pressure.9–11 Whether naturally occurring blood pressure variability predicts risk over and beyond blood pressure level remains uncertain.
Recent publications12,13 suggested that clinicians might reduce stroke risk more effectively by targeting systolic blood pressure variability along with systolic blood pressure level using specific classes of antihypertensive drugs. These recommendations12,13 originated mainly from clinical trials, which included high-risk groups, such as elderly14,15 or hypertensive16 patients or participants with a previous ischemic stroke or transient ischemic attack14 or diabetes mellitus.15,17 Other methodological issues that might have introduced bias are nonrandomization, possible lack of power,5,17 short follow-up time,17 categorization of continuous variability measures for risk prediction,8,14 the use of variability measures that are dependent on the level of blood pressure,8,15 limited adjustment,14 or failure to account for reverse causality.18 These factors render the current evidence on the prognostic significance of blood pressure variability inconclusive, especially in the general population. To address this issue, we investigated the predictive value of blood pressure variability in a randomly selected representative sample from the general population with sufficient power, follow-up time, and a wide age range.
Methods
Study Population
The Ethics Committee of the University of Leuven approved the Flemish Study on Environment, Genes and Health Outcomes.19,20 From August 1985 until November 1990, a random sample of the households living in a geographically defined area of Northern Belgium was investigated with the goal to recruit an equal number of participants in each of 6 subgroups by sex and age (20–39, 40–59, and ≥60 years). All household members with a minimum age of 20 years were invited to take part, provided that the quota of their sex-age group had not yet been fulfilled. From June 1996 until January 2004, recruitment of families continued using the former participants (1985–1990) as index persons, including teenagers. The participants, and in case of underaged offspring, their parents or custodians, gave informed consent.
The study population included 3318 participants. We excluded 394 subjects because they were younger than 18 years at enrollment (n=296) or because blood pressure had not been measured at home (n=48) or only at a single home visit (n=30). Thus, the number of participants statistically analyzed totaled 2944.
Measurements at Baseline
At baseline, trained nurses measured each participant’s blood pressure at 2 home visits at an interval of 2 to 4 weeks. At each home visit, after the participants had rested for 5 minutes in the sitting position, the nurses obtained 5 consecutive blood pressure readings to the nearest 2 mm Hg using mercury sphygmomanometers. Standard cuffs had a 12 × 24 cm inflatable portion, but if upper arm girth exceeded 31 cm, larger cuffs with 15 × 35 cm bladders were used. As described in detail elsewhere,21,22 we implemented a stringent program for quality assurance and quality control. Every 3 months, the observers had to pass a test requiring them to read blood pressures from a videotape featuring a falling mercury column with Korotkoff sounds (Blood Pressure Measurement, British Medical Association, London, United Kingdom). Their readings had to comply within 5 mm Hg of those of senior medical staff. Digit preference was checked at 6-month intervals. Hypertension was a blood pressure (average of 10 readings) ≥140 mm Hg systolic or ≥90 mm Hg diastolic or use of antihypertensive drugs.
At the enrollment visit, the nurses measured body height to the nearest 0.5 cm with a pliable measurer and the participant standing against the wall. For body weight measurement, participants wore light indoor clothing without shoes. Body mass index was weight in kilograms divided by the square of height in meters. Overweight and obesity were a body mass index ≥25 kg/m2 or ≥30 kg/m2, respectively.23 The nurses administered a questionnaire to collect information on each participant’s medical history, smoking and drinking habits, lifestyle, and intake of medications. Socioeconomic status was coded according to British coding rules24 and condensed into a scale with scores ranging from 1 (low) to 3 (high). Using published tables,25 we computed the energy spent in physical activity from body weight, time devoted to work and sports, and type of physical activity.
Venous blood samples, obtained at the second home visit, were analyzed by automated enzymatic methods for serum creatinine, total and high-density lipoprotein cholesterol, and plasma glucose. Hypercholesterolemia was a serum level of ≥5.16 mmol/L (200 mg/dL) or treatment with lipid-lowering drugs. Diabetes mellitus was a self-reported diagnosis, a fasting or random blood glucose level of ≥7.0 mmol/L (126 mg/dL) or ≥11.1 mmol/L (200 mg/dL), respectively, or use of antidiabetic drugs.26
Assessment of Outcome
Via the National Population Registry (Rijksregister) in Brussels, Belgium, we ascertained the vital status of all participants until December 31, 2009. We obtained the International Classification of Disease codes for the immediate and underlying causes of death from the Flemish Registry of Death Certificates. We collected information on the incidence of nonfatal events via follow-up visits with repeat administration of the same standardized questionnaire as that used at baseline. Physicians ascertained the diseases reported on the death certificates or via the questionnaires against the medical records of general practitioners or hospitals.
Fatal and nonfatal stroke did not include transient ischemic attacks. Coronary events included fatal and nonfatal myocardial infarction and coronary revascularization. Fatal and nonfatal cardiovascular events were composed of coronary end points, stroke, fatal and nonfatal left ventricular heart failure, aortic aneurysm, cor pulmonale, and pulmonary or arterial embolism. Hospitalizations for unstable angina were coded as ischemic heart disease. Heart failure was either a clinical diagnosis or the diagnosis on the death certificate but was always verified against hospital files or the records held by family doctors. For all end points, we censored participants from analysis after the occurrence of a first event.
Statistical Analysis
For database management and statistical analysis, we used SAS software, version 9.3 (SAS Institute Inc, Cary, NC). Departure from normality was evaluated by Shapiro-Wilk statistic and skewness by the computation of the coefficient of skewness, the third moment about the mean divided by the cube of the SD. We compared means and proportions by the standard normal z test and the χ2 statistic, respectively, and survival curves by Kaplan-Meier survival function estimates and the log-rank test. Statistical significance was a 2-sided significance level of 0.05 on 2-sided tests.
Because in middle-aged and older subjects systolic blood pressure is a stronger risk factor than diastolic blood pressure, we limited our analyses to systolic blood pressure.27 Within the context of this article, mean refers to the average of 10 blood pressure readings, that is, 5 readings at each of 2 home visits. For each individual, we computed overall, within-visit, and between-visit variability of systolic blood pressure. Overall variability was based on the 10 blood pressure readings, that is, 5 at each of 2 home visits. Within-visit variability was computed for both sets of 5 blood pressure readings at a single visit and the so-obtained parameters expressing variability were averaged over the 2 home visits. The between-visit blood pressure variability considered the variability (difference) between the mean blood pressure values at the 2 home visits.
We assessed blood pressure variability from the variability independent of the mean (VIM),12,14 the maximum minus minimum blood pressure difference (MMD), and average real variability (ARV).11,28 VIM is calculated as the SD divided by the mean to the power x and multiplied by the population mean to the power x.12,14 The power x is obtained by fitting a curve through a plot of SD against mean using the model SD=a × meanx, where x was derived by nonlinear regression analysis as implemented in the PROC NLIN procedure of the SAS package. The values of x for overall, within-visit 1, within-visit 2, and between visit variability were 1.58, 1.39, 1.34, and 1.70, respectively. ARV is the average of the absolute differences between consecutive blood pressure measurements.11,28 For between-visit variability, ARV reduces to MMD and only MMD is therefore reported.
We searched for covariables associated with blood pressure variability in stepwise multiple regression analyses with P values for explanatory variables to enter and stay in models set at 0.05. We considered as covariables sex, age, body mass index, systolic blood pressure, heart rate, total:high-density lipoprotein serum cholesterol ratio, plasma glucose, serum creatinine, energy expenditure in physical activity, triglycerides, history of cardiovascular disease, history of peripheral arterial disease, current smoking and alcohol intake, diabetes mellitus, and treatment with β-blockers, diuretics, or any antihypertensive drug. After identification of covariables, we applied a generalization of the standard linear model, as implemented in the PROC MIXED procedure of the SAS package to account for family clusters. We analyzed the prognostic significance of blood pressure variability using both categorical and continuous analyses. In categorical analyses, we plotted incidence rates by quartiles of the blood pressure variability distribution, while standardizing rates for sex and age (<40, 40-59, ≥60 years) by the direct method. For the continuous analyses, we used Cox proportional hazard regression as implemented in the PROC SURVIVAL procedure of the SUDAAN software (Research Triangle Institute, Chapel Hill, NC) version 10.01 to calculate standardized relative hazard ratios, while allowing for covariables, confounders, and family clusters. We adjusted Cox models for sex, age, body mass index, heart rate, current smoking and alcohol intake, total:high-density lipoprotein serum cholesterol ratio, plasma glucose, history of previous cardiovascular disease, and use of β-blockers. We checked the proportional hazards assumption by the Kolmogorov supremum test, as implemented in the PROC PHREG procedure of the SAS package. We tested heterogeneity in the hazard ratios across subgroups by introducing the appropriate interaction term in the Cox model. Finally, we applied the generalized R2 statistic to assess the risks explained in Cox regression29 by adding the indices of systolic blood pressure variability to models already including the mean systolic blood pressure level and covariables.
Results
Baseline Characteristics
Table 1 provides the baseline characteristics of the participants by quartiles of overall systolic blood pressure VIM. The 2944 participants included 1494 women (50.7%); 1500 and 427 subjects with overweight or obesity (51.0% and 14.5%, respectively); 1747 patients with hypercholesterolemia (59.3%), of whom 97 (5.6%) were on treatment with lipid-lowering drugs; and 717 hypertensive patients (24.4%), of whom 366 (51.0%) were on antihypertensive drug treatment. Among treated patients, 173 (47.3%) were on diuretics (including aldosterone antagonists), 201 (54.9%) on β-blockers, 55 (15.0%) on calcium channel blockers, 46 (12.6%) on angiotensin-converting enzyme inhibitors or blockers of the angiotensin II type-1 receptor, and 8 (2.2%) on other drug classes, including α-blockers; 350 (95.6%) patients were taking >1 drug. Of 1494 women and 1450 men, 395 (26.4%) and 502 (34.6%) were smokers; 222 women (14.9%) and 586 men (40.4%) reported intake of alcohol. In smokers, median tobacco use was 15 cigarettes per day (interquartile range, 10-20). In drinkers, the median alcohol consumption was 15 g per day (interquartile range, 8-28). Among women, 511 (34.2%) reported natural or surgical menopause.
Table 1.
Baseline Characteristics of Participants by Quartiles of Overall Systolic Blood Pressure Variability Independent of the Mean
| Characteristic | Categories of Systolic Blood Pressure Variability | P Value | |||
|---|---|---|---|---|---|
| Limits, units | |||||
| Women | (0.00–3.56) | (3.56–5.14) | (5.14–6.95) | (6.95–19.42) | |
| Men | (0.68–3.36) | (3.36–4.80) | (4.80–6.52) | (6.52–19.96) | |
| Number of subjects, % | |||||
| All participants in category | 735 | 737 | 736 | 736 | |
| Antihypertensive treatment | 85 (11.6) | 80 (10.9) | 98 (13.3) | 103 (14.0) | 0.075 |
| Smokers | 223 (30.3) | 221 (30.0) | 243 (33.0) | 210 (28.7) | 0.75 |
| Drinking alcohol | 196 (26.7) | 207 (27.3) | 218 (29.6) | 133 (26.2) | 0.89 |
| Diabetes mellitus | 19 (2.6) | 14 (1.9) | 16 (2.2) | 20 (2.7) | 0.79 |
| Cardiovascular disease | 48 (6.5) | 43 (5.8) | 63 (8.6)* | 51 (6.9) | 0.35 |
| Peripheral arterial disease | 4 (0.5) | 8 (1.2) | 9 (1.2) | 11 (1.5) | 0.089 |
| Mean (SD) of characteristic | |||||
| Age, y | 44.4 (15.8) | 43.6 (15.1) | 44.7 (15.7) | 46.8 (16.3)* | 0.002 |
| Body mass index, kg/m2 | 25.9 (4.5) | 25.8 (4.3) | 25.4 (4.2) | 25.5 (4.5) | 0.011 |
| Systolic blood pressure, mm Hg | 125.7 (15.1) | 125.2 (15.8) | 125.4 (15.7) | 125.7 (15.8) | 0.93 |
| Diastolic blood pressure, mm Hg | 75.6 (8.7) | 76.0 (9.4) | 75.7 (8.9) | 75.4 (9.2) | 0.67 |
| Heart rate, bpm | 69.4 (9.0) | 70.5 (8.7)* | 70.4 (9.1) | 70.7 (9.3) | 0.011 |
| Serum total cholesterol, mmol/L | 5.60 (1.23) | 5.45 (1.21)* | 5.57 (1.19)* | 5.61 (1.26) | 0.46 |
| Serum HDL cholesterol, mmol/L | 1.35 (0.40) | 1.37 (0.41) | 1.36 (0.42) | 1.37 (0.38) | 0.31 |
| Total:HDL cholesterol ratio | 4.55 (1.81) | 4.34 (1.66)* | 4.54 (2.11)* | 4.43 (1.72) | 0.58 |
| Plasma glucose, mmol/L | 5.07 (1.30) | 5.18 (1.48) | 5.21 (1.43) | 5.13 (1.11) | 0.31 |
| Serum creatinine, μmol/L | 92.9 (20.8) | 92.3 (17.3) | 92.4 (18.5) | 92.9 (17.1) | 0.98 |
| Median (interquartile range) | |||||
| Physical activity, kcal/d | 1444 (330–3047) | 1467 (418–3257) | 1460 (337–3257) | 1328 (237–3133)* | 0.041 |
HDL indicates high-density lipoprotein. Values are number of subjects (%), arithmetic mean±SD, or geometric mean (5th to 95th percentile interval). Systolic blood pressure variability is based on 10 blood pressure readings, that is, 5 consecutive blood pressure readings at each of 2 home visits at an interval of 2 to 4 weeks.
P denotes the significance of the linear trend across categories of systolic blood pressure variability independent of the mean; significance of the difference with the adjacent lower quartile: P≤0.05.
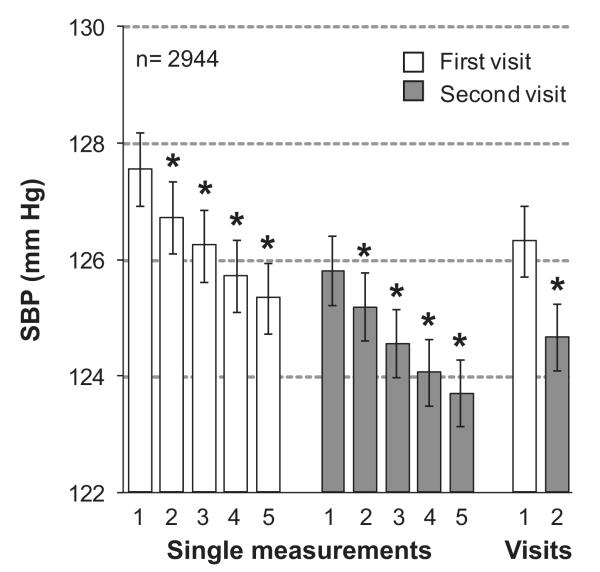
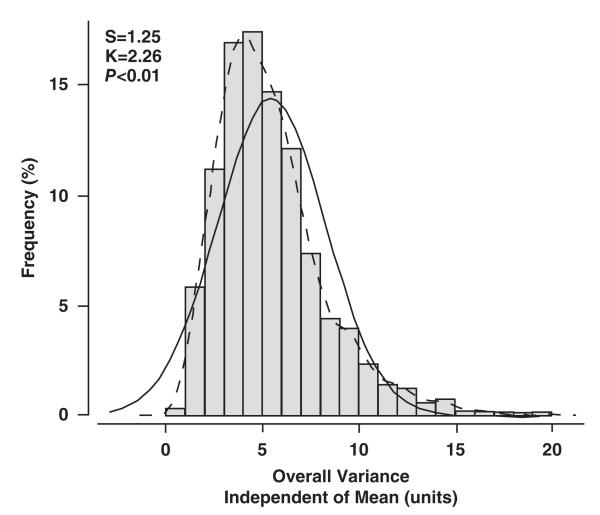
In the whole study population, systolic blood pressure averaged 126.3 mm Hg (SD, 16.8 mm Hg) at the first home visit (5 readings), 124.7 mm Hg (15.8 mm Hg) at the second home visit (5 readings; P<0.0001 versus first home visit; Figure 1), and 125.5 mm Hg (15.6 mm Hg) at the 2 visits combined (10 readings). Frequency histograms of the overall, within-visit and between-visit VIM appear in Figure 2 and Figures S1 and S2 in the online-only Data Supplement, respectively. Within-visit VIM decreased with higher systolic blood pressure with an opposite trend for between-visit VIM and no association with systolic blood pressure for overall variability (Table S1 in the online-only Data Supplement). Within-subject overall, within-visit, and overall variability as estimated from MMD and ARV showed a graded increase across quartiles of the distribution of systolic blood pressure (Table S1).
Figure 1.
Systolic blood pressure (SBP) on repeated measurement. Nurses measured blood pressure 5 times consecutively at each of 2 home visits 2 to 4 weeks apart. Values are means (±SE) of single readings or of all (n=5) readings obtained in 2944 participants at the first (open bars) or second (shaded bars) home visit. Numbers along the horizontal axis denote the order of the measurements or visits. An asterisk indicates a significant difference (P<0.0001) with the adjacent mean systolic blood pressure.
Figure 2.
Frequency distribution of overall variability of systolic blood pressure independent of the mean. Overall variability is based on 10 blood pressure readings in 2944 participants, that is, 5 at each of 2 home visits. The P value is for departure of the actually observed distribution (full line) from normality (dotted line). S indicates the coefficient of skewness; K, the coefficient of kurtosis.
Correlates of Variability
As captured by VIM, MMD, and ARV, overall variability of systolic blood pressure (10 readings) independently increased with age and decreased with body mass index (Table 2). Overall variability, as assessed by VIM, was lower in women than in men and was higher in patients with a history of peripheral arterial disease and those on treatment with β-blockers. VIM decreased and MMD and ARV increased with higher mean systolic blood pressure. Both MMD and ARV reflecting overall variability decreased with higher total:high-density lipoprotein serum cholesterol ratio. ARV increased with heart rate and plasma glucose. MMD was higher in participants with a history of peripheral arterial disease and on treatment with β-blockers and decreased with serum creatinine. Shared familial factors represented from 11.7% to 22.6% of the explained variance of the overall variability in systolic blood pressure. The aforementioned covariables explained 15.7% and 17.8% of the variance in MMD and AVR, respectively, but only 2.1% of VIM (Table 2). Variables not entering any model describing total variability included energy expenditure in physical activity, triglycerides, history of cardiovascular disease, current smoking and alcohol intake, diabetes mellitus, and classes of antihypertensive drugs other than β-blockers.
Table 2.
Correlates of Overall Systolic Variability in 2944 Participants
| Label | Variability Independent of the Mean (units) |
Difference Between Maximum and Minimum Blood Pressure (mm Hg) |
Average Real Variability (mm Hg) |
|---|---|---|---|
| Variance explained | |||
| Total | 0.138 | 0.306 | 0.403 |
| Pedigree | 0.117 | 0.150 | 0.226 |
| Fixed effects | 0.021 | 0.157 | 0.178 |
| Parameter estimates (SE) | |||
| Female sex | −0.245 (0.102)* | — | — |
| Age, +10 y | 0.205 (0.038)§ | 0.658 (0.105)§ | 0.138 (0.025)§ |
| Body mass index, +5 kg/m2 | −0.208 (0.064)† | −0.630 (0.178)‡ | −0.116 (0.043)† |
| Systolic blood pressure, +10 mm Hg | −0.077 (0.039)* | 1.787 (0.105)§ | 0.347 (0.025)§ |
| Heart rate, +5 bpm | — | — | 0.054 (0.019)† |
| Total/high-density lipoprotein cholesterol, +1 unit | — | −0.164 (0.086)* | −0.104 (0.021)§ |
| Plasma glucose, +1 mmol/L | — | — | 0.066 (0.026)* |
| Serum creatinine, +10 μmol/L | — | −0.246 (0.083)† | — |
| Peripheral arterial disease | 1.254 (0.492)* | 3.538 (1.338)† | — |
| Treated with β-blockers | 0.494 (0.209)* | 1.425 (0.567)* | — |
Systolic blood pressure was based on 10 blood pressure readings, that is, 5 consecutive blood pressure readings at each of 2 home visits at an interval of 2 to 4 weeks. The following variables were not related to any index of variability: energy expenditure in physical activity, triglycerides, history of cardiovascular disease, smoking and alcohol intake, diabetes mellitus, and classes of antihypertensive drugs other than β-blockers.
Significance of the parameter estimates:
P≤0.05;
P≤0.01;
P≤0.001; and
P≤0.0001.
The covariables associated with the within-visit and between-visit variability of systolic blood pressure were similar to those contributing to overall variability (Table S2). For the within-visit variability, VIM and MMD were also lower in patients on treatment with diuretics.
Incidence of Mortality and Morbidity
Median follow-up was 12.3 years (5th to 95th percentile interval, 2.7 to 23.7 years) for fatal end points and 12.0 years (5th to 95th percentile interval, 2.4 to 23.3 years) for all of the fatal and nonfatal cardiovascular events combined. Mortality included 164 cardiovascular (40.9%) and 200 non-cardiovascular (49.9%) deaths, 6 deaths (1.5%) attributed to renal failure, and 31 deaths (7.7%) from unknown causes. Considering cause-specific first cardiovascular events, the incidence of fatal and nonfatal stroke amounted to 35 and 14, respectively. Cardiac events consisted of 89 fatal and 135 nonfatal events, including 36 fatal and 30 nonfatal cases of acute myocardial infarction, 6 fatal and 8 nonfatal cases of other acute or subacute forms of ischemic heart disease, 6 sudden deaths, 36 fatal and 47 nonfatal cases of heart failure, 5 fatal cases of arrhythmia, 9 pacemaker implantations, and 41 cases of surgical or percutaneous coronary revascularization.
Risk Prediction by Mean and Variability of Systolic Blood Pressure
Mortality
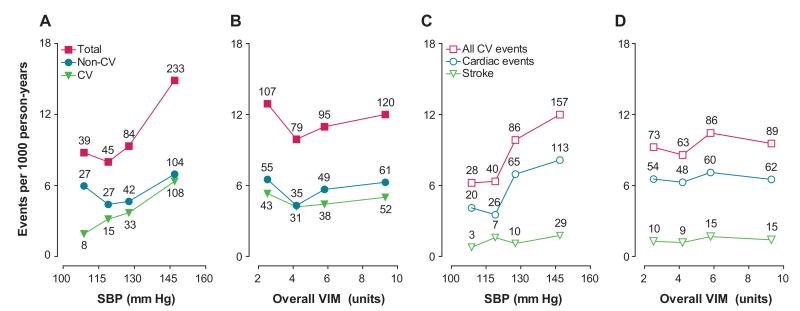
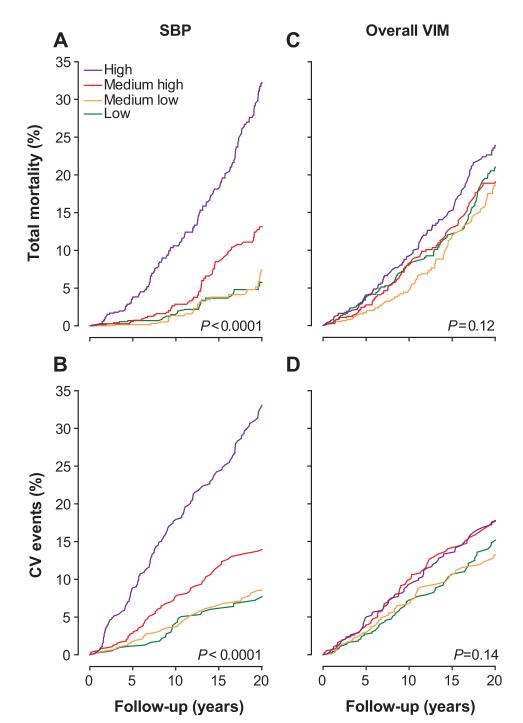
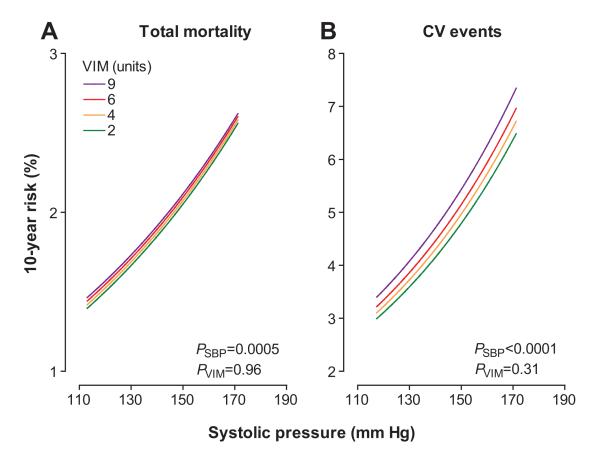
The sex- and age-standardized rates for total and cardiovascular mortality (Figure 3) increased across quartiles of mean systolic blood pressure (P<0.0001) but not across quartiles of overall VIM (P≥0.58). In analyses of Kaplan-Meier estimates, the log-rank test was significant for total mortality (Figure 4) across quartiles of mean systolic blood pressure (P<0.0001) but not across quartiles of overall VIM (P=0.12). Both mean systolic blood pressure and all measures of variability for overall, within visit, and between visits fulfilled the proportional hazard assumption (P≥0.055), with the exception of within-visit VIM in relation to total mortality (P=0.047). The multivariable-adjusted standardized hazard ratios for mortality in relation to mean and overall, within-visit, and between-visit variability of systolic blood pressure are presented in Table 3 and Tables S3 and S4, respectively. By including both mean systolic blood pressure and the indices of variability in multivariable-adjusted Cox models, mean systolic blood pressure, but not any measure of variability, predicted total and cardiovascular mortality. Figure 5 demonstrates that the 10-year multivariable-adjusted risk of death increased with mean systolic blood pressure (P=0.0005) but not with overall VIM (P=0.96).
Figure 3.
Mortality (A and B) and cardiovascular (CV) events (C and D) by quartiles of the distribution of mean systolic blood pressure (SBP) and overall variability independent of the mean (VIM) in 2944 participants. Incidence rates were standardized for sex and age by the direct method. The number of end points contributing to the rates is presented. The trend across quartiles of systolic pressure (A and C) was significant for total mortality (P<0.0001), non-CV mortality (P=0.044), CV mortality (P<0.0001), all CV events (P<0.0001), cardiac events (P<0.0001), and stroke (P=0.0001). The trends across quartiles of overall VIM (B and D) were all nonsignificant (P≥0.58).
Figure 4.
Kaplan-Meier survival function estimates for total mortality (A and C) and cardiovascular (CV) events (B and D) by sex-specific quartiles of mean systolic blood pressure (SBP) and overall systolic variability independent of the mean (VIM). CV events include all of the fatal and nonfatal CV end points. P values refer to the significance of the log-rank test across 4 quartiles.
Table 3.
Adjusted Standardized Hazard Ratios for End Points in Relation to the Mean and Overall Variability of Systolic Blood Pressure
| Basic Model |
Full Model |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean Systolic Blood Pressure |
Variability Independent of the Mean |
Difference Between Maximum and Minimum Blood Pressure |
Average Real Variability |
|||||
| End Point (N) | HR (95% CI) | R2 (%) | HR (95% CI) | R2 (%) | HR (95% CI) | R2 (%) | HR (95% CI) | R2 (%) |
| Mortality | ||||||||
| Total (401) | 1.18 (1.08–1.30)‡ | 28.6 | 1.00 (0.91–1.10) | <0.01 | 0.99 (0.91–1.09) | <0.01 | 1.03 (0.93–1.13) | <0.10 |
| Cardiovascular (164) | 1.37 (1.18–1.59)§ | 18.4 | 1.11 (0.97–1.27) | 0.10 | 1.08 (0.95–1.22) | <0.01 | 1.08 (0.94–1.25) | <0.01 |
| Cardiac (112) | 1.44 (1.22–1.70)§ | 13.5 | 1.05 (0.89–1.25) | <0.01 | 1.02 (0.87–1.19) | <0.01 | 1.07 (0.90–1.27) | <0.01 |
| Cardiovascular events | ||||||||
| All (311) | 1.25 (1.12–1.40)‡ | 16.8 | 1.05 (0.96–1.15) | <0.01 | 1.06 (0.96–1.16) | <0.01 | 1.08 (0.98–1.19) | <0.01 |
| Cardiac (164) | 1.29 (1.13–1.46)‡ | 12.4 | 1.03 (0.92–1.15) | <0.01 | 1.02 (0.92–1.14) | <0.01 | 1.08 (0.97–1.21) | 0.10 |
| Coronary (133) | 1.21 (1.03–1.41)* | 8.5 | 0.93 (0.80–1.09) | 0.10 | 0.96 (0.82–1.11) | 0.10 | 0.99 (0.85–1.15) | <0.01 |
| Stroke (49) | 1.44 (1.04–1.98)* | 4.9 | 1.13 (0.88–1.46) | <0.01 | 1.17 (0.92–1.48) | <0.01 | 1.10 (0.81–1.49) | <0.01 |
HR indicates hazard ratio. Systolic blood pressure (SBP) level and variability were based on 10 blood pressure readings, that is, 5 consecutive blood pressure readings at each of 2 home visits, 2 to 4 weeks apart. The basic model accounts for relatedness and includes in addition to mean SBP, sex, age, body mass index, heart rate, smoking and drinking, total:high-density lipoprotein serum cholesterol ratio, plasma glucose, history of cardiovascular disease, and use of β-blockers as covariables. Full models include the aforementioned covariables and both mean SBP and an index of SBP variability. HRs given with 95% CIs express the risk associated with a 1-SD increase in the explanatory variables: 15.6 mm Hg for level of blood pressure and 2.82 units, 8.36 mm Hg, and 2.05 mm Hg for variability independent of the mean, difference between maximum and minimum blood pressure, and average real variability, respectively. The R2 statistic is a measure for the risk prediction provided by the basic model, including mean SBP and the additive contribution of the indices of variability. The cause of death was renal in 6 cases and unknown in 31 cases.
Significance of the hazard ratios:
P≤0.05;
P≤0.01;
P≤0.001; and
P≤0.0001.
Figure 5.
Absolute 10-year risk of death (A) or a cardiovascular (CV) event (B) in relation to mean systolic blood pressure (SBP) at different levels of overall systolic variability independent of the mean (VIM). Mean systolic blood pressure along the x axis covers the 5th to 95th percentile interval. The variability independent of the mean is presented by 4 risk functions corresponding with 2, 4, 6, and 9 units (approximate quartile midpoints). The risk functions were standardized to the distributions (mean or ratio) of sex, age, body mass index, heart rate, smoking and drinking, total:high-density lipoprotein serum cholesterol ratio, plasma glucose, history of CV disease, and use of β-blockers. Among 2944 participants, 401 deaths and 311 composite CV end points occurred. PSBP and PVIM indicate the significance of mean systolic blood pressure and the overall variability independent of the mean.
Fatal and Nonfatal Cardiovascular Events
The sex- and age-standardized rates of all of the fatal and nonfatal cardiovascular events, cardiac events, and stroke (Figure 3) increased across quartiles of mean systolic blood pressure (P≤0.0001) but not across quartiles of overall VIM (P≥0.63). As illustrated by Figure 4, the log-rank test was significant for the composite cardiovascular end point across quartiles of mean systolic blood pressure (P<0.0001) but not across quartiles of overall VIM (P=0.14). Both mean systolic blood pressure and all measures of variability for overall, within visit, and between visits fulfilled the proportional hazard assumption (P≥0.11). In fully adjusted models (Table 3), including both mean systolic blood pressure and a measure of variability, mean systolic blood pressure predicted fatal and nonfatal cardiovascular outcomes. By contrast, none of the overall variability indices predicted any of the end points. The 10-year multivariable-adjusted risk of a composite cardiovascular end point increased with mean systolic blood pressure (P<0.0001) but not with overall VIM (P=0.31).
Sensitivity Analyses
Analyses of the indices of variability within visits (Table S3) and between visits (Table S4) confirmed the above findings on overall variability. Furthermore, we stratified our analyses of VIM as predictor of total mortality and the composite cardiovascular end point (Table S5) for sex, age (60 years), history of cardiovascular disease, the use of antihypertensive drugs (0, 1), and hypertensive status (0, 1). In models adjusted as in Table 3, all hazard ratios for VIM in relation to total mortality were nonsignificant (P≥0.27). VIM contributed to the prediction of the composite cardiovascular end point in women and in treated patients but not in any other group (Table S5). Among 1494 women, the hazard ratios were 1.21 (95% CI, 1.06–1.39; P=0.005) for VIM, 1.20 (1.05–1.36; P=0.006) for MMD, and 1.27 (1.10–1.46; P=0.001) for ARV. Among 366 treated patients, the hazard ratios were 1.21 (1.06–1.38; P=0.005) for VIM, 1.25 (1.10–1.41; P=0.0005) for MMD, and 1.31 (1.14–1.51; P=0.0002) for ARV. None of the interaction terms between the indices of variability and treatment status were significant (P>0.10), with the exception of ARV (P<0.038). All interaction terms of sex with the indices of variability were significant (P≤0.036).
Discussion
We investigated in an unbiased population sample whether blood pressure variability adds to risk prediction over and beyond mean systolic blood pressure. We showed that within-subject blood pressure variability, assessed overall, within, and between visits, did not have any independent prognostic value, except in women and patients on antihypertensive drug treatment. Mean systolic pressure consistently predicted total mortality and fatal and nonfatal cardiovascular events.
The attempts to anchor blood pressure variability as an established cardiovascular risk factor span >30 years.9–11,18,30 In the late 1970s, Clement et al31 assessed variability from the SD and coefficient of variation of blood pressure measurements obtained every 5 minutes for 3 hours in 70 untreated hypertensive patients. The study demonstrated a positive relation between sympathetic activity and blood pressure variability as captured by the SD. However, an important finding was that blood pressure level and SD were correlated, so that the correlations of variability with the indexes of sympathetic activity disappeared when variability was expressed as coefficient of variation. In the early 1980s, Mancia and coworkers32,33 showed that SD and coefficient of variation of mean arterial pressure derived from 24-h continuous intra-arterial recordings in small studies involving normotensive and hypertensive patients (n<100) increased with age and decreased during sleep. SD correlated positively with blood pressure level and fell with antihypertensive drug treatment, whereas coefficient of variation was independent of level before and after drug intervention.33 Notwithstanding these initial findings,32,33 the same group only used SD to provide some of the first evidence relating target organ damage to blood pressure variability,34 which was confirmed after a mean follow-up of 7.4 years.35 Prospective studies using noninvasive 24-h ambulatory blood pressure followed, providing multivariable-adjusted results relating especially daytime systolic SD to early carotid atherosclerosis progression in 286 hospitalized subjects (mean age, 68 years) followed up for 3.3 years.36 In hypertensive patients, Verdecchia et al37 failed to provide any outcome-based evidence after adjusting for confounders and covariables for both daytime and nighttime systolic blood pressure variability as reflected by the SD. On the other hand, Kikuya et al5 provided prognostic evidence for daytime systolic SD from the Ohasama population study involving 1542 participants followed up for a median 8.5 years. In a large randomly selected population sample of 8938 participants followed up for a median of 11.3 years, Hansen et al11 demonstrated that the prognostic significance of the level of 24-h blood pressure outperformed reading-to-reading blood pressure variability. More recently, prospective studies supporting the prognostic significance of blood pressure variability emerged in patients with diabetes mellitus15 and in aged patients at high-stroke risk in the context of clinical trials, as reported by Rothwell et al.14
Rothwell38 questioned the usual blood pressure hypothesis by providing evidence that visit-to-visit systolic blood pressure variability is a strong predictor of subsequent stroke. These researchers14 investigated blood pressure variability in high-risk and aged subjects receiving aspirin in the United Kingdom Transient Ischaemic Attack aspirin trial39 and aspirin or antihypertensive drugs in 3 validation cohorts (European Stroke Prevention Study-1,40 Dutch Transient Ischaemic Attack trial,41 and the Anglo-Scandinavian Cardiac Outcomes Trial - Blood Pressure Lowering Arm [ASCOT-BPLA]).42,43 The United Kingdom Transient Ischaemic Attack aspirin trial39 was also studied in detail,14 because systolic blood pressure was a strong predictor of stroke in this cohort, and therefore blood pressure readings were deemed to be reliable.44 As the office blood pressure was measured only once at 4-month intervals, reproducible results are unlikely45 because of various confounding factors, such as lifestyle and changing behavioral and environmental conditions.8,46,47 The authors14 did also not specify the baseline covariables for which they accounted in the analysis. Most of the reported results were unadjusted or based on VIM derived from systolic blood pressure.14 Findings from the Transient Ischaemic Attack validation cohort41 revealed inconsistencies among the rather overwhelming amount of provariability results, with VIM failing to predict stroke before (hazard ratio, 1.76; CI, 0.73–4.23) and after (1.83; CI, 0.76–4.39) adjustment for mean systolic pressure. In addition, the ASCOT-BPLA trial43 was included to test the generalizability of the Transient Ischaemic Attack cohort findings. In this large trial,43 blood pressure was measured every 6 months in triplicate and the mean of the last 2 readings was used. Even though the results seemed confirmatory, the ASCOT-BPLA cohort included aged (mean age, 63 years) hypertensive patients with ≥3 other vascular risk factors, making the study population highly selective. In addition, analyses based on categorization of continuous variability estimates into deciles to predict risk were not confirmed in analyses of variability as a continuous variable. The categorical approach is generally not recommended for continuously distributed variables,48 because it enables the reporting of extreme-case hazard ratios by comparing top with bottom deciles. For instance, the top decile hazard ratio for SD over 7 visits in the United Kingdom Transient Ischaemic Attack trial was 6.22 (CI, 4.16–9.29).14
The issue of the clinical applicability of blood pressure variability raised renewed interest when a recent meta-analysis49 identified drug-class effects on interindividual variability and stroke risk reduction. Webb et al49 reported from 389 of 1372 eligible trials that variation in systolic blood pressure was reduced by calcium-channel blockers and non-loop diuretics, but increased on treatment with angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, and β-blockers. However, the results for this metaanalysis rested on the between-patient SD, which is dependent on the mean31–33 and only a surrogate for within-individual variability.49 Rothwell et al12 investigated the effects of β-blockers and calcium-channel blockers on within-individual variability in the ASCOT-BPLA42,43 and the Medical Research Council50 trials. In the ASCOT-BPLA trial42,43 they compared amlodipine-based and atenolol-based regimens in 19 257 patients, and in the Medical Research Council trial,50 atenolol-based and diuretic-based regimens versus placebo in 4396 patients (mean age, 70 years). Rothwell et al12 reported that the amlodipine- and atenolol-based treatments had opposite effects on within-individual variability with amlodipine reducing both variability and stroke risk. In the Medical Research Council trial,12 atenolol increased visit-to-visit variability compared with placebo, and temporal trends in variability in the atenolol group were associated with stroke risk. These randomized controlled trials allowed add-on medications and dose titration and differed in mean systolic blood pressure and variability.12,42,43 It remains therefore uncertain whether the effects on blood pressure variability were dose dependent and persistent in combination with other drugs. To address this issue, Webb and Rothwell13 performed a metaanalysis of trials with randomization to fixed combination or fixed doses. In this study, the authors also showed that higher doses of calcium channel blockers as monotherapy or in combination reduced interindividual blood pressure variability more than lower doses, and higher doses of β-blockers increased variability. However, again SD was used and findings were based on rather small, short duration (<26 weeks), nonoutcome-based studies.13 However convincing these findings may be, they should be interpreted with caution. As the authors correctly state,12 more work is needed to understand the nature and consequences of variability in blood pressure. Yet, recommendations based on post hoc interpretations are made prematurely for the clinical management of hypertension and the development and use of different classes of blood pressure-lowering drugs.12,13,49 This is alarming because no evidence to date exists on, for example, the new-generation β-blockers. Although in the present study we did find VIM to be predictive of a composite cardiovascular outcome in treated participants, the interaction with treatment was not significant.
Our findings support the usual blood pressure hypothesis51,52 and challenge the predictive value of blood pressure variability because of the following reasons: (1) our study sample was representative of the general population by applying random recruitment and long-term follow-up; (2) we had sufficient power with the inclusion of 2944 participants and with 401 deaths and 311 fatal and nonfatal cardiovascular events; (3) the study sample had a wide age range of 18.0 to 91.1 years; (4) results were consistent for overall, within-, and between-visit variability, and all known covariables were included; (5) as suggested by Rothwell and colleagues,12,14 we used VIM as measure of variability; and (6) blood pressure variability indices were analyzed as continuous variables along with mean systolic blood pressure in risk prediction. Nevertheless, our current study must also be interpreted within the context of its potential limitations. Although our analyses were adjusted at the level of individual participants, we cannot exclude residual confounding. Blood pressure was measured at 2 home visits by research nurses, and intra- and inter-observer variability could have influenced the results. However, the nurses were highly trained in blood pressure measurement and subjected to a stringent program for quality assurance and quality control.21,22 Lastly, nurses measured blood pressure 5 times at each of 2 visits, 2 to 4 weeks apart. More time points and longer time periods between visits could perhaps have yielded different results. However, we believe that reproducibility was enhanced with the short between-visit time and 5 blood pressure measurements at each visit.
Perspectives
Our current findings suggest that, in the general population, within-subject blood pressure variability does not have any prognostic significance over and beyond mean systolic pressure. Present guidelines53,54 for the management of blood pressure should be followed. Blood pressure level is a reversible risk factor, overriding all other modifiable risk factors. The rule of halves still applies across Europe and worldwide.55,56 Half of the hypertensive patients are on treatment and of those treated only half are controlled.56 Clinicians should focus on blood pressure level and controlling hypertension.56 The suggestion to consider blood pressure variability as an additional risk indicator and to reduce it by drugs detracts from what really matters in the management of hypertension, controlling the blood pressure level, and should be ignored to avoid confusion among clinicians.
Supplementary Material
Table S1: Variability by Quartiles of Mean Systolic Blood Pressure
Table S2: Correlates of the Within- and Between Visit Systolic Variability in 2944 Participants
Table S3: Adjusted Standardized Hazard Ratios for Endpoints in Relation to the Mean and Within Visit Variability of Systolic Pressure
Table S4: Adjusted Standardized Hazard Ratios for Endpoints in Relation to the Mean and Between Visit Variability of Systolic Pressure
Table S5: Adjusted Standardized Hazard Ratios for Total Mortality and All Cardiovascular Events in Relation to Mean and Overall Variability of Systolic Pressure in Different Strata
Figure S1: Frequency Distribution of Within Visit Variability of Systolic Blood Pressure Independent of the Mean
Figure S2: Frequency Distribution of Between Visit Variability of Systolic Blood Pressure Independent of the Mean
Novelty and Significance.
What Is New?
• Recent publications suggested that clinicians might reduce cardiovascular risk more effectively by targeting blood pressure variability along with blood pressure level using specific classes of antihypertensive drugs. Few population studies addressed whether blood pressure variability is a risk factor over and beyond blood pressure level.
What Is Relevant?
• We assessed within-subject blood pressure variability from 5 blood pressure readings at each of 2 home visits. We estimated blood pressure variability as variability independent of the mean, the difference between maximum and minimum blood pressure, and average real variability. None of these variability indices predicted total or cardiovascular mortality or fatal combined with nonfatal cardiovascular events, whereas mean blood pressure was a strong and consistent predictor.
Summary
• Clinicians should focus on blood pressure level and controlling hypertension. The suggestion to consider blood pressure variability as an additional risk indicator and to reduce it by drugs detracts from what really matters in the management of hypertension, that is lowering blood pressure, and creates confusion among clinicians.
Acknowledgments
Sources of Funding The European Union (grants IC15-CT98-0329-EPOGH, LSHM-CT-2006-037093-InGenious HyperCare, HEALTH-2007-2.1.1-2-HyperGenes, HEALTH-2011.2.4.2-2-EU-MASCARA, and the European Research Council Advanced Researcher Grant-2011-294713-EPLORE) and the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Brussels, Belgium (grant G.0734.09), gave support to the Studies Coordinating Centre.
Footnotes
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.112.202143/-/DC1.
Disclosures None.
References
- 1.Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens. 1987;5:93–98. doi: 10.1097/00004872-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Tatasciore A, Renda G, Zimarino M, Soccio M, Bilo G, Parati G, Schillaci G, De Caterina R. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension. 2007;50:325–332. doi: 10.1161/HYPERTENSIONAHA.107.090084. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Ohishi M, Kamide K, Onishi M, Takeya Y, Tatara Y, Oguro R, Yamamoto K, Sugimoto K, Rakugi H. The impact of visit-to-visit variability in blood pressure on renal function. Hypertens Res. 2012;35:239–243. doi: 10.1038/hr.2011.170. [DOI] [PubMed] [Google Scholar]
- 4.Matsui Y, Ishikawa J, Eguchi K, Shibasaki S, Shimada K, Kario K. Maximum value of home blood pressure: a novel indicator of target organ damage in hypertension. Hypertension. 2011;57:1087–1093. doi: 10.1161/HYPERTENSIONAHA.111.171645. [DOI] [PubMed] [Google Scholar]
- 5.Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, Ota M, Nagai K, Araki T, Satoh H, Ito S, Hisamichi S, Imai Y. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension. 2000;36:901–906. doi: 10.1161/01.hyp.36.5.901. [DOI] [PubMed] [Google Scholar]
- 6.Johansson JK, Niiranen TJ, Puukka PJ, Jula AM. Prognostic value of the variability in home-measured blood pressure and heart rate: the Finn-Home Study. Hypertension. 2012;59:212–218. doi: 10.1161/HYPERTENSIONAHA.111.178657. [DOI] [PubMed] [Google Scholar]
- 7.Shimbo D, Newman JD, Aragaki AK, Lamonte MJ, Bavry AA, Allison M, Manson JE, Wassertheil-Smoller S. Association between annual visit-to-visit blood pressure variability and stroke in postmenopausal women: data from the women’s health initiative. Hypertension. 2012;60:625–630. doi: 10.1161/HYPERTENSIONAHA.112.193094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: findings from NHANES III, 1988 to 1994. Hypertension. 2011;57:160–166. doi: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 9.Schutte AE, Schutte R, Huisman HW, van Rooyen JM, Fourie CM, Malan NT, Malan L. Blood pressure variability is significantly associated with ECG left ventricular mass in normotensive Africans: the SABPA Study. Hypertens Res. 2011;34:1127–1134. doi: 10.1038/hr.2011.104. [DOI] [PubMed] [Google Scholar]
- 10.Pierdomenico SD, Lapenna D, Di Tommaso R, Di Carlo S, Esposito AL, Di Mascio R, Ballone E, Cuccurullo F, Mezzetti A. Blood pressure variability and cardiovascular risk in treated hypertensive patients. Am J Hypertens. 2006;19:991–997. doi: 10.1016/j.amjhyper.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Imai Y, Wang J, Ibsen H, O’Brien E, Staessen JA, International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55:1049–1057. doi: 10.1161/HYPERTENSIONAHA.109.140798. [DOI] [PubMed] [Google Scholar]
- 12.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Poulter NR, Sever PS, ASCOT-BPLA and MRC Trial Investigators Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–480. doi: 10.1016/S1474-4422(10)70066-1. [DOI] [PubMed] [Google Scholar]
- 13.Webb AJ, Rothwell PM. Effect of dose and combination of antihypertensives on interindividual blood pressure variability: a systematic review. Stroke. 2011;42:2860–2865. doi: 10.1161/STROKEAHA.110.611566. [DOI] [PubMed] [Google Scholar]
- 14.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh YT, Tu ST, Cho TJ, Chang SJ, Chen JF, Hsieh MC. Visit-to-visit variability in blood pressure strongly predicts all-cause mortality in patients with type 2 diabetes: a 5·5-year prospective analysis. Eur J Clin Invest. 2012;42:245–253. doi: 10.1111/j.1365-2362.2011.02574.x. [DOI] [PubMed] [Google Scholar]
- 16.Verdecchia P, Angeli F, Gattobigio R, Rapicetta C, Reboldi G. Impact of blood pressure variability on cardiac and cerebrovascular complications in hypertension. Am J Hypertens. 2007;20:154–161. doi: 10.1016/j.amjhyper.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Eguchi K, Ishikawa J, Hoshide S, Pickering TG, Schwartz JE, Shimada K, Kario K. Night time blood pressure variability is a strong predictor for cardiovascular events in patients with type 2 diabetes. Am J Hypertens. 2009;22:46–51. doi: 10.1038/ajh.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen TW, Li Y, Staessen JA. Blood pressure variability remains an elusive predictor of cardiovascular outcome. Am J Hypertens. 2009;22:3–4. doi: 10.1038/ajh.2008.322. [DOI] [PubMed] [Google Scholar]
- 19.Staessen JA, Wang JG, Brand E, Barlassina C, Birkenhäger WH, Herrmann SM, Fagard R, Tizzoni L, Bianchi G. Effects of three candidate genes on prevalence and incidence of hypertension in a Caucasian population. J Hypertens. 2001;19:1349–1358. doi: 10.1097/00004872-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerová J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, Filipovský J, Kawecka-Jaszcz K, Nikitin Y, Staessen JA, European Project on Genes in Hypertension (EPOGH) Investigators Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305:1777–1785. doi: 10.1001/jama.2011.574. [DOI] [PubMed] [Google Scholar]
- 21.Staessen J, Bulpitt CJ, Fagard R, Joossens JV, Lijnen P, Amery A. Familial aggregation of blood pressure, anthropometric characteristics and urinary excretion of sodium and potassium–a population study in two Belgian towns. J Chronic Dis. 1985;38:397–407. doi: 10.1016/0021-9681(85)90135-3. [DOI] [PubMed] [Google Scholar]
- 22.Kuznetsova T, Staessen JA, Kawecka-Jaszcz K, Babeanu S, Casiglia E, Filipovsky J, Nachev C, Nikitin Y, Peleskã J, O’Brien E. Quality control of the blood pressure phenotype in the European Project on Genes in Hypertension. Blood Press Monit. 2002;7:215–224. doi: 10.1097/00126097-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization . Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. World Health Organization; Geneva, Switzerland: 2000. [PubMed] [Google Scholar]
- 24.Office of Population Censuses and Surveys . Classification of Occupations and Coding Index. Her Majesty’s Stationery Office; London, United Kingdom: 1980. [Google Scholar]
- 25.Åstrand PO, Rodahl K. Textbook of Work Physiology. Physiological Bases of Exercise. McGraw-Hill Book Company; New York, NY: 1986. pp. 486–522. [Google Scholar]
- 26.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 27.Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation. 2001;103:1245–1249. doi: 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- 28.Mena L, Pintos S, Queipo NV, Aizpúrua JA, Maestre G, Sulbarán T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. 2005;23:505–511. doi: 10.1097/01.hjh.0000160205.81652.5a. [DOI] [PubMed] [Google Scholar]
- 29.Gillespie BW. [Accessed August 15, 2012];Use of generalized R-squared in Cox regression. APHA Scientific Session and Event Listing 2006. http://apha.confex.com/apha/134am/techprogram/paper_135906.htm.
- 30.Zanchetti A. Wars, war games, and dead bodies on the battlefield: variations on the theme of blood pressure variability. Stroke. 2011;42:2722–2724. doi: 10.1161/STROKEAHA.111.626747. [DOI] [PubMed] [Google Scholar]
- 31.Clement DL, Mussche MM, Vanhoutte G, Pannier R. Is blood pressure variability related to activity of the sympathetic system? Clin Sci. 1979;57(suppl 5):S217–S219. doi: 10.1042/cs057217s. [DOI] [PubMed] [Google Scholar]
- 32.Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, Grassi G, Zanchetti A. Blood pressure variability in man: its relation to high blood pressure, age and baroreflex sensitivity. Clin Sci. 1980;59(suppl 6):S401–S404. doi: 10.1042/cs059401s. [DOI] [PubMed] [Google Scholar]
- 33.Mancia G. Blood pressure variability at normal and high blood pressure. Chest. 1983;83(suppl 2):317–320. doi: 10.1378/chest.83.2.317. [DOI] [PubMed] [Google Scholar]
- 34.Parati G, Pomidossi G, Casadei R, Groppelli A, Trazzi S, Di Rienzo M, Mancia G. Role of heart rate variability in the production of blood pressure variability in man. J Hypertens. 1987;5:557–560. doi: 10.1097/00004872-198710000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Frattola A, Parati G, Cuspidi C, Albini F, Mancia G. Prognostic value of 24-hour blood pressure variability. J Hypertens. 1993;11:1133–1137. doi: 10.1097/00004872-199310000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Sander D, Kukla C, Klingelhöfer J, Winbeck K, Conrad B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: A 3-year follow-up study. Circulation. 2000;102:1536–1541. doi: 10.1161/01.cir.102.13.1536. [DOI] [PubMed] [Google Scholar]
- 37.Verdecchia P, Borgioni C, Ciucci A, Gattobigio R, Schillaci G, Sacchi N, Santucci A, Santucci C, Reboldi G, Porcellati C. Prognostic significance of blood pressure variability in essential hypertension. Blood Press Monit. 1996;1:3–11. [PubMed] [Google Scholar]
- 38.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–948. doi: 10.1016/S0140-6736(10)60309-1. [DOI] [PubMed] [Google Scholar]
- 39.Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom Transient Ischaemic Attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatr. 1991;54:1044–1054. doi: 10.1136/jnnp.54.12.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The ESPS Group The European Stroke Prevention Study (ESPS): principal end-points. Lancet. 1987;12:1351–1354. [PubMed] [Google Scholar]
- 41.The Dutch TIA Trial Study Group A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. N Engl J Med. 1991;325:1261–1266. doi: 10.1056/NEJM199110313251801. [DOI] [PubMed] [Google Scholar]
- 42.Poulter NR, Wedel H, Dahlöf B, Sever PS, Beevers DG, Caulfield M, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J, Pocock S, ASCOT Investigators Role of blood pressure and other variables in the differential cardiovascular event rates noted in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA) Lancet. 2005;366:907–913. doi: 10.1016/S0140-6736(05)67186-3. [DOI] [PubMed] [Google Scholar]
- 43.Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J, ASCOT Investigators Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 44.Rodgers A, MacMahon S, Gamble G, Slattery J, Sandercock P, Warlow C. Blood pressure and risk of stroke in patients with cerebrovascular disease: the United Kingdom Transient Ischaemic Attack Collaborative Group. BMJ. 1996;313:147. doi: 10.1136/bmj.313.7050.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerin W, Rosofsky M, Pieper C, Pickering TG. A test of reproducibility of blood pressure and heart rate variability using a controlled ambulatory procedure. J Hypertens. 1993;11:1127–1131. doi: 10.1097/00004872-199310000-00018. [DOI] [PubMed] [Google Scholar]
- 46.Weder AB, Julius S. Behavior, blood pressure variability, and hypertension. Psychosom Med. 1985;47:406–414. doi: 10.1097/00006842-198509000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Brook RD, Weder AB, Rajagopalan S. Environmental hypertensionology the effects of environmental factors on blood pressure in clinical practice and research. J Clin Hypertens (Greenwich) 2011;13:836–842. doi: 10.1111/j.1751-7176.2011.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naggara O, Raymond J, Guilbert F, Roy D, Weill A, Altman DG. Analysis by categorizing or dichotomizing continuous variables is inadvisable: an example from the natural history of unruptured aneurysms. AJNR Am J Neuroradiol. 2011;32:437–440. doi: 10.3174/ajnr.A2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375:906–915. doi: 10.1016/S0140-6736(10)60235-8. [DOI] [PubMed] [Google Scholar]
- 50.MRC Working Party Medical research council trial of treatment of hypertension in older adults: principal results. Br Med J. 1992;304:405–412. doi: 10.1136/bmj.304.6824.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 52.Gasowski J, Li Y, Kuznetsova T, Richart T, Thijs L, Grodzicki T, Clarke R, Staessen JA. Is “usual” blood pressure a proxy for 24-h ambulatory blood pressure in predicting cardiovascular outcomes? Am J Hypertens. 2008;21:994–1000. doi: 10.1038/ajh.2008.231. [DOI] [PubMed] [Google Scholar]
- 53.British Cardiac Society. British Hypertension Society. Diabetes UK. HEART UK. Primary Care Cardiovascular Society. The Stroke Association JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular diseases in clinical practice. Heart. 2006;91:1–52. doi: 10.1136/hrt.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Kjeldsen SE, Erdine S, Narkiewicz K, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Cifkova R, Dominiczak A, Fagard R, Heagerty AM, Laurent S, Lindholm LH, Mancia G, Manolis A, Nilsson PM, Redon J, Schmieder RE, Struijker-Boudier HA, Viigimaa M, Filippatos G, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Kiowski W, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O’Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Viigimaa M, Waeber B, Williams B, Zamorano JL, The task force for the management of arterial hypertension of the European Society of Hypertension. The task force for the management of arterial hypertension of the European Society of Cardiology 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 55.Staessen JA, Kuznetsova T, Stolarz K. Hypertension prevalence and stroke mortality across populations. JAMA. 2003;289:2420–2422. doi: 10.1001/jama.289.18.2420. [DOI] [PubMed] [Google Scholar]
- 56.Weinehall L, Ohgren B, Persson M, Stegmayr B, Boman K, Hallmans G, Lindholm LH. High remaining risk in poorly treated hypertension: the ‘rule of halves’ still exists. J Hypertens. 2002;20:2081–2088. doi: 10.1097/00004872-200210000-00029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Variability by Quartiles of Mean Systolic Blood Pressure
Table S2: Correlates of the Within- and Between Visit Systolic Variability in 2944 Participants
Table S3: Adjusted Standardized Hazard Ratios for Endpoints in Relation to the Mean and Within Visit Variability of Systolic Pressure
Table S4: Adjusted Standardized Hazard Ratios for Endpoints in Relation to the Mean and Between Visit Variability of Systolic Pressure
Table S5: Adjusted Standardized Hazard Ratios for Total Mortality and All Cardiovascular Events in Relation to Mean and Overall Variability of Systolic Pressure in Different Strata
Figure S1: Frequency Distribution of Within Visit Variability of Systolic Blood Pressure Independent of the Mean
Figure S2: Frequency Distribution of Between Visit Variability of Systolic Blood Pressure Independent of the Mean