Abstract
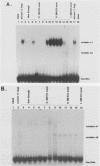
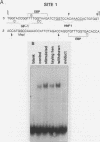
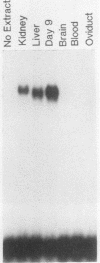
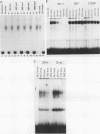
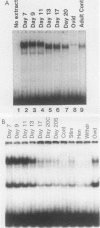
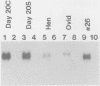
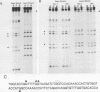
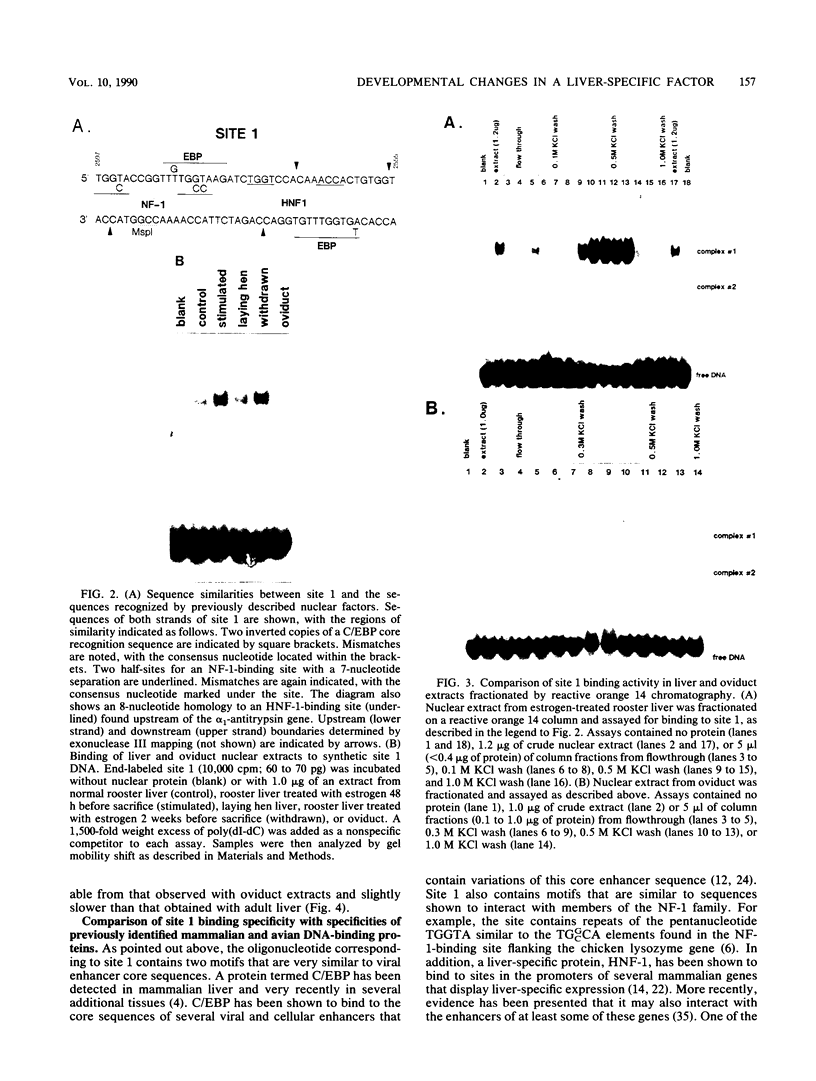
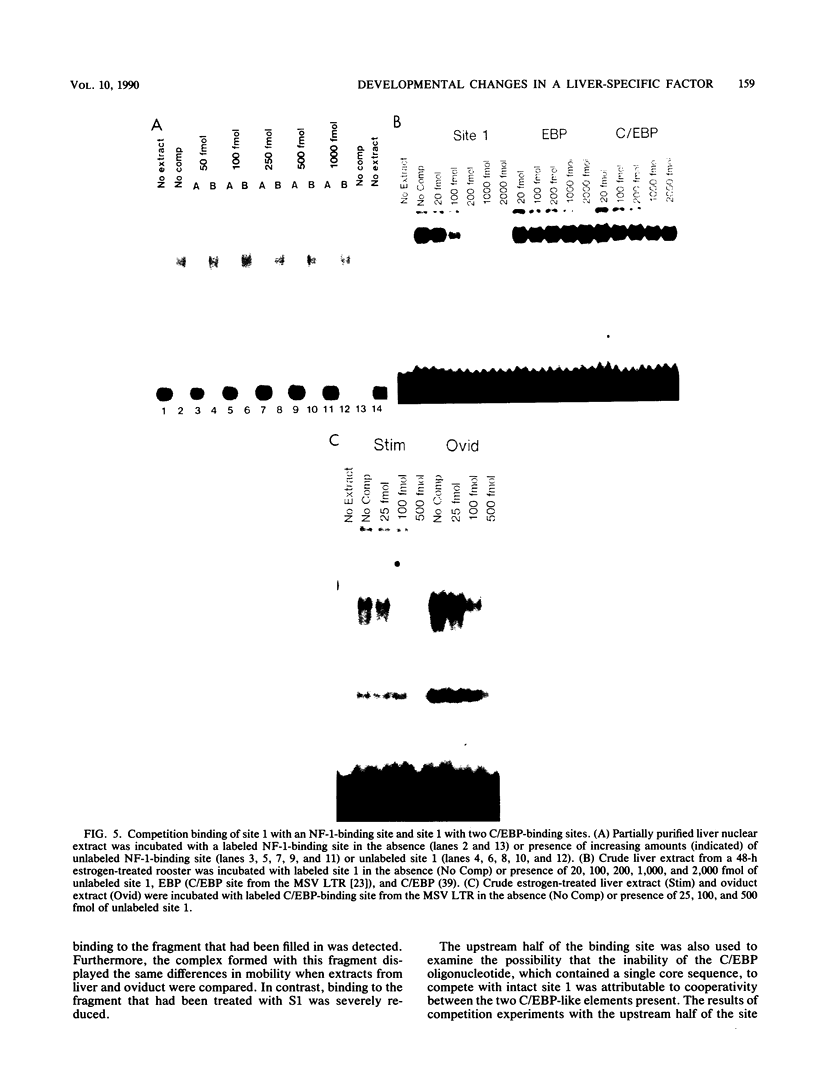
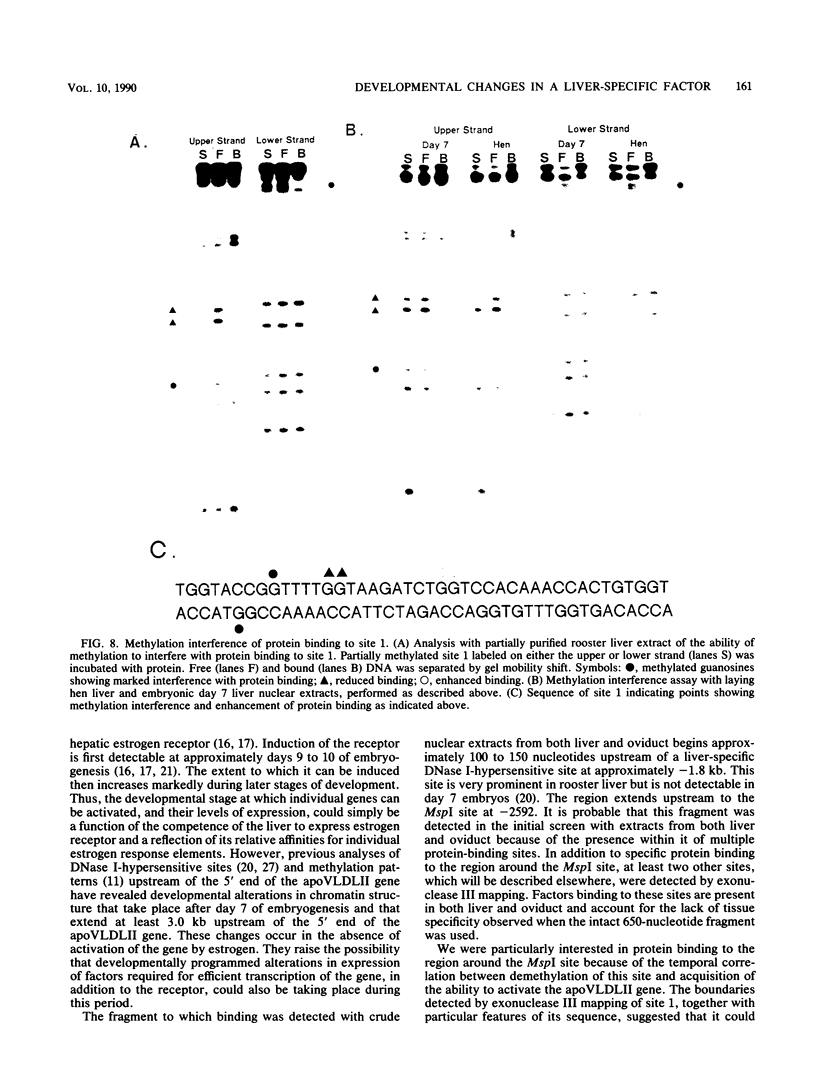
Expression of the avian very-low-density apolipoprotein II (apoVLDLII) gene is completely dependent on estrogen and restricted to the liver. We have identified binding sites for nonhistone nuclear proteins located between -1.96 and -2.61 kilobases. One of these sites, located at -2.6 kilobases (designated site 1), was found to span an MspI site that becomes demethylated between days 7 and 9 of embryogenesis, the stage of development at which competence to express the apoVLDLII gene begins to be acquired. Levels of the factor(s) involved were high at day 7 of embryogenesis, decreased two- to threefold by days 9 to 11, and continued to decline more slowly until hatching. Furthermore, the mobility of the complex formed underwent a well-defined shift between days 11 to 13 embryogenesis. Methylation interference studies showed that modification of the outer guanosines of the MspI site resulted in marked inhibition of the formation of the protein-DNA complex. Competition studies, fractionation of nuclear extracts, and tissue distribution indicated that the factor was not the avian homolog of hepatocyte nuclear factor 1, nuclear factor 1, or CCAAT/enhancer-binding protein (C/EBP). However, site 1 could complete for binding to an oligonucleotide, previously shown to be recognized by C/EBP, in a nonreciprocal fashion. These studies demonstrate that the sequence recognized by the protein includes a C/EBP consensus sequence but that elements in addition to the core enhancer motif are essential for binding.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer T. K., Tam S. P., Deugau K. V., Deeley R. G. Apolipoprotein C-II mRNA levels in primate liver. Induction by estrogen in the human hepatocarcinoma cell line, HepG2. J Biol Chem. 1985 Feb 10;260(3):1676–1681. [PubMed] [Google Scholar]
- Baumhueter S., Courtois G., Crabtree G. R. A variant nuclear protein in dedifferentiated hepatoma cells binds to the same functional sequences in the beta fibrinogen gene promoter as HNF-1. EMBO J. 1988 Aug;7(8):2485–2493. doi: 10.1002/j.1460-2075.1988.tb03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., Gwynn B., Howard S., Jerry J., Gordon J. I., Landschulz W. H., McKnight S. L. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989 Aug;3(8):1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- Bond J. P., Notides A. C. Estrogen receptor purification by affinity chromatography using an orange triazine dye. Anal Biochem. 1987 Jun;163(2):385–390. doi: 10.1016/0003-2697(87)90239-9. [DOI] [PubMed] [Google Scholar]
- Borgmeyer U., Nowock J., Sippel A. E. The TGGCA-binding protein: a eukaryotic nuclear protein recognizing a symmetrical sequence on double-stranded linear DNA. Nucleic Acids Res. 1984 May 25;12(10):4295–4311. doi: 10.1093/nar/12.10.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw M. S., Tsai M. J., O'Malley B. W. A far upstream ovalbumin enhancer binds nuclear factor-1-like factor. J Biol Chem. 1988 Jun 15;263(17):8485–8490. [PubMed] [Google Scholar]
- Burch J. B., Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983 May;33(1):65–76. doi: 10.1016/0092-8674(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Cereghini S., Blumenfeld M., Yaniv M. A liver-specific factor essential for albumin transcription differs between differentiated and dedifferentiated rat hepatoma cells. Genes Dev. 1988 Aug;2(8):957–974. doi: 10.1101/gad.2.8.957. [DOI] [PubMed] [Google Scholar]
- Codina-Salada J., Moore J. P., Chan L. Kinetics of primary and secondary stimulation of the mRNA for APOVLDL-II, a major yolk protein, in the cockerel liver by estrogen. Endocrinology. 1983 Sep;113(3):1158–1160. doi: 10.1210/endo-113-3-1158. [DOI] [PubMed] [Google Scholar]
- Colgan V., Elbrecht A., Goldman P., Lazier C. B., Deeley R. The avian apoprotein II very low density lipoprotein gene. Methylation patterns of 5' and 3' flanking regions during development and following induction by estrogen. J Biol Chem. 1982 Dec 10;257(23):14453–14460. [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Xanthopoulos K. G., Darnell J. E., Jr A liver-specific DNA-binding protein recognizes multiple nucleotide sites in regulatory regions of transthyretin, alpha 1-antitrypsin, albumin, and simian virus 40 genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3840–3844. doi: 10.1073/pnas.85.11.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G., Baumhueter S., Crabtree G. R. Purified hepatocyte nuclear factor 1 interacts with a family of hepatocyte-specific promoters. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7937–7941. doi: 10.1073/pnas.85.21.7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G., Morgan J. G., Campbell L. A., Fourel G., Crabtree G. R. Interaction of a liver-specific nuclear factor with the fibrinogen and alpha 1-antitrypsin promoters. Science. 1987 Oct 30;238(4827):688–692. doi: 10.1126/science.3499668. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Udell D. S., Burns A. T., Gordon J. I., Goldberger R. F. Kinetics of avian vitellogenin messenger RNA induction. Comparison between primary and secondary response to estrogen. J Biol Chem. 1977 Nov 25;252(22):7913–7915. [PubMed] [Google Scholar]
- Elbrecht A., Lazier C. B., Protter A. A., Williams D. L. Independent developmental programs for two estrogen-regulated genes. Science. 1984 Aug 10;225(4662):639–641. doi: 10.1126/science.6740331. [DOI] [PubMed] [Google Scholar]
- Evans M. I., O'Malley P. J., Krust A., Burch J. B. Developmental regulation of the estrogen receptor and the estrogen responsiveness of five yolk protein genes in the avian liver. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8493–8497. doi: 10.1073/pnas.84.23.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. I., Silva R., Burch J. B. Isolation of chicken vitellogenin I and III cDNAs and the developmental regulation of five estrogen-responsive genes in the embryonic liver. Genes Dev. 1988 Jan;2(1):116–124. doi: 10.1101/gad.2.1.116. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haché R. J., Deeley R. G. Organization, sequence and nuclease hypersensitivity of repetitive elements flanking the chicken apoVLDLII gene: extended sequence similarity to elements flanking the chicken vitellogenin gene. Nucleic Acids Res. 1988 Jan 11;16(1):97–113. doi: 10.1093/nar/16.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haché R. J., Tam S. P., Cochrane A., Nesheim M., Deeley R. G. Long-term effects of estrogen on avian liver: estrogen-inducible switch in expression of nuclear, hormone-binding proteins. Mol Cell Biol. 1987 Oct;7(10):3538–3547. doi: 10.1128/mcb.7.10.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardon E. M., Frain M., Paonessa G., Cortese R. Two distinct factors interact with the promoter regions of several liver-specific genes. EMBO J. 1988 Jun;7(6):1711–1719. doi: 10.1002/j.1460-2075.1988.tb03000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R. S., Friedman N., Darnell J. E., Jr, Babiss L. E. Positive and negative regulatory elements in the mouse albumin enhancer. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1553–1557. doi: 10.1073/pnas.86.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Keller R., Dierks-Ventling C. Deoxyribonucleic acid and ribonucleic acid synthesis during phosvitin induction by 17beta-estradiol in immature chicks. J Biol Chem. 1973 Aug 10;248(15):5262–5266. [PubMed] [Google Scholar]
- Jost J. P., Ohno T., Panyim S., Schuerch A. R. Appearance of vitellogenin mRNA sequences and rate of vitellogenin synthesis in chicken liver following primary and secondary stimulation by 17 beta-estradiol. Eur J Biochem. 1978 Mar 15;84(2):355–361. doi: 10.1111/j.1432-1033.1978.tb12175.x. [DOI] [PubMed] [Google Scholar]
- Kok K., Snippe L., Ab G., Gruber M. Nuclease-hypersensitive sites in chromatin of the estrogen-inducible apoVLDL II gene of chicken. Nucleic Acids Res. 1985 Jul 25;13(14):5189–5202. doi: 10.1093/nar/13.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtsteiner S., Wuarin J., Schibler U. The interplay of DNA-binding proteins on the promoter of the mouse albumin gene. Cell. 1987 Dec 24;51(6):963–973. doi: 10.1016/0092-8674(87)90583-6. [DOI] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noteborn M. H., Bakker O., de Jonge M. A., Gruber M., Ab G. Differential estrogen responsiveness of the vitellogenin and apo very low density lipoprotein II genes in the rooster liver. J Steroid Biochem. 1986 Jan;24(1):281–285. doi: 10.1016/0022-4731(86)90065-8. [DOI] [PubMed] [Google Scholar]
- Nowock J., Sippel A. E. Specific protein-DNA interaction at four sites flanking the chicken lysozyme gene. Cell. 1982 Sep;30(2):607–615. doi: 10.1016/0092-8674(82)90257-4. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Rupp R. A., Sippel A. E. Chicken liver TGGCA protein purified by preparative mobility shift electrophoresis (PMSE) shows a 36.8 to 29.8 kd microheterogeneity. Nucleic Acids Res. 1987 Dec 10;15(23):9707–9726. doi: 10.1093/nar/15.23.9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryden T. A., Beemon K. Avian retroviral long terminal repeats bind CCAAT/enhancer-binding protein. Mol Cell Biol. 1989 Mar;9(3):1155–1164. doi: 10.1128/mcb.9.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadaishi K., Morinaga T., Tamaoki T. Interaction of a hepatoma-specific nuclear factor with transcription-regulatory sequences of the human alpha-fetoprotein and albumin genes. Mol Cell Biol. 1988 Dec;8(12):5179–5187. doi: 10.1128/mcb.8.12.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüle R., Muller M., Kaltschmidt C., Renkawitz R. Many transcription factors interact synergistically with steroid receptors. Science. 1988 Dec 9;242(4884):1418–1420. doi: 10.1126/science.3201230. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Shen R. F., Li Y., Sifers R. N., Wang H., Hardick C., Tsai S. Y., Woo S. L. Tissue-specific expression of the human alpha 1-antitrypsin gene is controlled by multiple cis-regulatory elements. Nucleic Acids Res. 1987 Oct 26;15(20):8399–8415. doi: 10.1093/nar/15.20.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C. R., LaMarco K. L., Johnson P. F., Landschulz W. H., McKnight S. L. In situ detection of sequence-specific DNA binding activity specified by a recombinant bacteriophage. Genes Dev. 1988 Jul;2(7):801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]
- Wiskocil R., Bensky P., Dower W., Goldberger R. F., Gordon J. I., Deeley R. G. Coordinate regulation of two estrogen-dependent genes in avian liver. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4474–4478. doi: 10.1073/pnas.77.8.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J. H., Davidson I., Macchi M., Rosales R., Vigneron M., Staub A., Chambon P. In vitro binding of several cell-specific and ubiquitous nuclear proteins to the GT-I motif of the SV40 enhancer. Genes Dev. 1987 Oct;1(8):794–807. doi: 10.1101/gad.1.8.794. [DOI] [PubMed] [Google Scholar]