Abstract
The term “ultima ratio” has multiple, though related, meanings. The motto “ultima ratio regum,” cast on the cannons of the French army of King Louis XIV, meant that war is the last argument of kings, that is, the one to be used after all diplomatic arguments have failed. Along similar lines, we propose that, given the current evidence, renal denervation should be used as a last resort, after state-of-the-art drug treatment optimized at expert centers failed to control blood pressure.
Hypertension affects an estimated 20% to 30% of the world’s adult population.1 Despite the availability of numerous safe and effective pharmacological therapies, including single-pill combinations of 2 to 3 drugs, the percentage of patients achieving adequate blood pressure control meeting guideline targets remains low.1,2 Resistant hypertension is a blood pressure that remains above goal in spite of the concomitant use of antihypertensive medications from ≥3 drug classes.3 Patients who require >4 drug classes to have their blood pressure controlled are also considered to have resistant hypertension. Preferably, the regimen should include a diuretic, and all of the doses should be optimal.3,4
Treatment-Resistant Hypertension
The online-only Data Supplement provides an overview of the epidemiology of treatment-resistant hypertension and the role of the sympathetic nervous system in maintaining uncontrolled hypertension.
Results of the SYMPLICITY Studies
SYMPLICITY Hypertension-1
In 2009, Krum et al5 reported a nonrandomized proof-of-concept study (NCT 00483808 and NCT 00664638) showing that percutaneous radiofrequency catheter-based renal sympathetic denervation was feasible, effective, and safe. Among 45 analyzed patients enrolled in this first-in-human open study, on treatment with 4.5 antihypertensive drugs, blood pressure at entry was 177/101 mmHg and decreased by 27/17 mmHg 12 months after renal denervation.5
SYMPLICITY Hypertension-2
After the proof-of-concept study,5 the SYMPLICITY Hypertension-2 (SIMPLICITY HTN-2) investigators published a randomized clinical trial.6 Patients were eligible if they had a baseline systolic blood pressure of ≥160 mmHg (150 mmHg for patients with type 2 diabetes mellitus) while taking ≥3 antihypertensive drugs. Of 190 patients screened at 24 centers, 106 (55.8%) were randomly allocated to undergo renal denervation plus previous treatment (n=52) or to maintain previous treatment alone (control group; n=54); 49 (94.2%) who underwent renal denervation and 51 (94.4%) of controls had their systolic blood pressure measured at the office at 6 months, the primary end point. In the renal denervation group, office blood pressure decreased by 32/12 mmHg (P<0.0001) from the baseline value of 178/96 mmHg, whereas the corresponding 1/0-mmHg change from 178/97 to 179/97 mmHg in the control group was not significant (P≥0.77). At 6 months, the between-group difference in the office blood pressure averaged 33/11 mmHg (P<0.0001).6 Of the patients who completed the trial, 41 (83.7%) who underwent renal denervation had a reduction in systolic blood pressure of ≥10 mmHg compared with 18 controls (35.3%; P<0.0001).6
Among the patients with a 6-month follow-up, more had drug reductions in the renal denervation group than in the control group (20.4% versus 5.9%; P=0.04), with no between-group differences in the proportion of patients who had their drug treatment intensified (8.2% versus 11.8%; P=0.74).6 There were no serious procedure-related or device-related complications, and occurrence of adverse events did not differ between groups. In particular, renal function and the albumin:creatinine ratio at 6 months were not significantly different from baseline.6
SYMPLICITY HTN-1 Registry
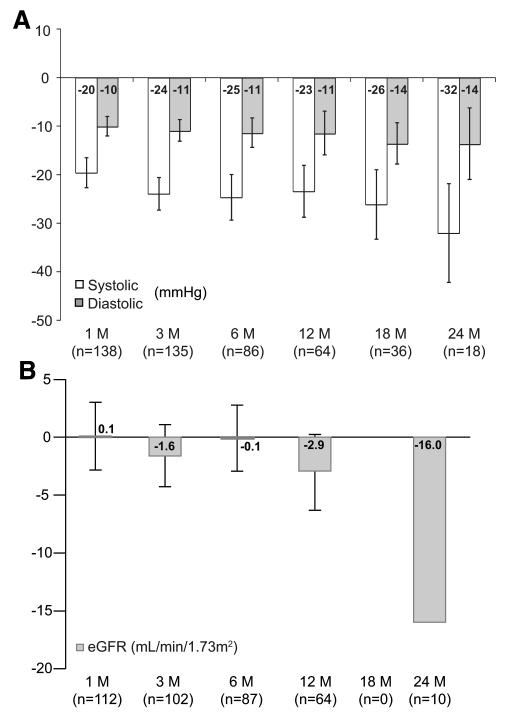
Between June 6, 2007, and May 1, 2010, the SYMPLICITY HTN-1 investigators applied renal sympathetic denervation in 153 patients,7 including the 45 patients from the SYMPLICITY HTN-1 Study.5 They published follow-up information in May 2011.7 Mean age was 57 years, 39% were women, 31% were diabetic, and 22% had coronary artery disease. Before renal denervation, office blood pressure measured on a mean of 5 antihypertensive medications averaged 176/98 mmHg. At 1, 3, 6, 12, 18, and 24 months, the percentage of patients followed up for blood pressure amounted to 90.2%, 88.2%, 56.2%, 41.8%, 23.5%, and 11.8%, respectively.7 At these time points, the blood pressure reductions averaged 20/10, 24/11, 25/11, 23/11, 26/14, and 32/14 mmHg (Figure, A). These findings were consistent after censoring for increases in antihypertensive medication and in a cohort of 18 patients (11.8%) with a 2-year follow-up.
Figure.
Mean changes in systolic and diastolic blood pressures (A) and estimated glomerular filtration rate (B) after renal sympathetic denervation over 24 months of follow-up. Error bars represent 95% CIs. A was reprinted from Symplicity HTN-1 Investigators7 with permission of the publisher. Copyright © 2011, American Heart Association, Inc. B was drawn according to results reported in reference 7 (no eGFR data available at 18 months; the error term for the 24-month eGFR data was not reported).
At baseline, the estimated glomerular filtration rate (eGFR) was 83 mL/min per 1.73 m2. During the first year of follow-up, eGFR remained stable, with changes at 1, 3, 6, and 12 months of +0.1, −1.6, −0.1, and −2.9 mL/min per 1.73 m2, when the percentage of patients remaining in follow-up for renal function was 73.2%, 66.7%, 56.9%, and 41.8%, respectively.7 Only 10 patients (6.5%), had eGFR measured at 2 years. eGFR fell by 16.0 mL/min per 1.73 m2 in all of the patients (Figure B) and by 7.8 and 24.2 mL/min per 1.73 m2 in patients who did not have (n=5) or did have (n=5) a diuretic added to their treatment. No patient experienced a doubling of serum creatinine, developed class IV chronic kidney disease (15–29 mL/min per 1.73 m2), or progressed to dialysis.7 In 149 patients (97.4%), renal denervation was without complication. Acute procedural complications included 3 groin pseudoaneurysms and 1 renal artery dissection, all managed without further sequelae.7
Limitations of the Evidence Supporting Renal Denervation
Duration and Completeness of Follow-Up
The SYMPLICITY HTN-15 and SYMPLICITY HTN-26 studies covered only 6 months. The proportion of patients in the SYMPLICITY HTN-1 registry,7 with a follow-up of 1 and 2 years, was 41.8% and 11.8% for blood pressure and 41.8% and 6.5% for eGFR (Figure). To what extent incomplete follow-up beyond 6 months reflects dropout of patients is not documented in the report on the SYMPLICITY Registry.7
Definition and Management of Resistant Hypertension
The definition of treatment-resistant hypertension in the SYMPLICITY reports, although in line with the contemporary guidelines,8–10 was not stringent. In SYMPLICITY HTN-1,5 treatment resistance included intolerance to blood pressure–lowering drugs, which often occurs in nonadherent patients. At screening for SYMPLICITY HTN-2,6 patients recorded the intake of medications during 2 weeks, but the number of patients excluded because of nonadherence was not reported. No attempt was made to ascertain in a verifiable manner adherence to or persistence of antihypertensive drug treatment.
Furthermore, the management of hypertension was not optimal in all of the patients. The SYMPLICITY researchers did not report how lifestyle measures were reinforced before enrollment and followed up by serial measurements of body mass index or 24-hour urinary sodium.11 At inclusion, 11% and 5% of the patients enrolled in SYMPLICITY HTN-26 and the SYMPLICITY HTN-1 registries7 were not taking diuretics, and only 17% and 22% were taking aldosterone antagonists, a drug class strongly recommended in treatment-resistant patients,12 particularly if plasma renin activity is low.13 Risk of hyperkalemia or degradation of renal function cannot explain the underuse of aldosterone antagonists, because patients with an eGFR <45 mL/min per 1.73 m2 were ineligible. Finally, drug treatment was not standardized or described in detail in the SYMPLICITY studies.5–7 In view of the highly prevalent nonadherence among treatment-resistant patients, ideally, only long-acting, so-called forgiving, drugs,14 that is, with a slow loss of blood pressure–lowering effect during drug holidays, and single-pill combinations of various antihypertensive agents15 should have been prescribed.
Diagnosis of Secondary Hypertension
A systematic search for secondary hypertension is key in the management of treatment-resistant hypertension, because this condition is more common in patients with resistant than controlled hypertension.3,16 Treating the underlying cause in secondary hypertension allows to improve blood pressure control. Unfortunately, in both SYMPLICITY HTN studies,5,6 screening for secondary hypertension was not mandatory, and the procedures for a diagnostic workup were not standardized. In SYMPLICITY HTN-1,5 known secondary hypertension was an exclusion criterion, whereas secondary hypertension was not mentioned among the SYMPLICITY HTN-2 eligibility criteria.6
Blood Pressure Measurement
Compared with office measurement, ambulatory blood pressure monitoring removes observer bias and measurement error, minimizes the white coat effect and has greater reproducibility, and, therefore, provides a better estimate of a patient’s usual blood pressure and cardiovascular prognosis.17,18 Self-measurement of blood pressure at home offers several of the well-recognized advantages of the more complex approach of ambulatory monitoring.19,20 Current guidelines9,10,20 recommend one of these out-of-the-office modalities of automated blood pressure measurement as state-of-the-art in the management of hypertensive patents. In particular, in patients with resistant hypertension, monitoring blood pressure outside of the medical environment is essential to distinguish true resistant hypertension from white coat–resistant hypertension.21 In the Spanish Ambulatory Blood Pressure Monitoring registry,21 white coat hypertension had a prevalence of 37.5% among 8295 patients with apparently resistant hypertension. Patients with white coat–resistant hypertension have a better cardiovascular prognosis than those with truly resistant hypertension.22,23 Furthermore, in a cohort of 109 treatment-resistant hypertensive patients followed up for 4.8 years, higher ambulatory blood pressures predicted cardiovascular morbidity and mortality, whereas office blood pressure had no prognostic value.24
Notwithstanding the overwhelming evidence in favor of the superiority of out-of-the-office blood pressure measurement,17–20 in particular, in treatment-resistant patients,24 in both SYMPLICITY trials5,6 and even in the ongoing SYMPLICITY HTN-3 Study (NCT01418261),25 the primary end point rested on office blood pressure. In SYMPLICITY HTN-1,5 only 12 (27%) of 45 patients had adequate ambulatory blood pressure monitoring at baseline and >30 days after denervation. The 24-hour systolic blood pressure decreased by 11 mmHg in 9 responders according to office systolic blood pressure and by 10 mmHg in 3 nonresponders. In SYMPLICITY HTN-2,6 all of the eligible patients received an Omron HEM-705 monitor to record seated blood pressure daily during 2 weeks, 3 times in the morning and 3 times in the evening. At 6 months, the home blood pressure fell by 20/12 mmHg in 32 patients in the renal denervation group compared with a rise of 2/0 mmHg (13/7) in 40 controls, resulting in a between-group difference of 22/12 mmHg (P<0.0001); the 24-hour blood pressure decreased by 11/7 mmHg in 20 patients randomized to renal denervation and did not change (−3/−1 mmHg) in 25 controls, resulting in a between-group difference of 14/8 mmHg (P≤0.02).6 The SYMPLICITY HTN-2 investigators did not report the baseline values of the ambulatory or self-measured blood pressures, so that the prevalence of white coat hypertension at entry among the SYMPLICITY patients cannot be assessed.
Assessment of Adherence
Adherence evaluated by electronic monitoring falls from 79% in patients taking medications once daily to 51% with 4 times daily dosing.26 Approximately half of hypertensive patients do not take their medications as prescribed.27 Nonadherent patients have an increased probability of receiving add-on drug therapy while staying uncontrolled and remaining at higher cardiovascular risk than their adherent counterparts.28 Poor medication-taking behavior is a major problem among patients with hypertension and is one of the main causes of failure to achieve blood pressure control.8 More information on nonadherence is available in the online-only Data Supplement.
The prevalence of diabetes mellitus and hypercholesterolemia in SYMPLICITY HTN-2 was 34.0% and 51.9%, respectively.6 The SYMPLICITY patients were at high risk of nonadherence, because, in addition to taking 4 to 5 antihypertensive drugs, many were also on lipid-lowering, antiplatelet, and/or antidiabetic drugs. Assessment of adherence in the SYMPLICITY studies5–7 was suboptimal. SYMPLICITY HTN-1 did not report on adherence.5 In SYMPLICITY HTN-2,6 eligible patients had to comply with ≥3 drugs, including a diuretic. After the 2-week run-in period, 36 of the SYMPLICITY HTN-2 patients (19%) were excluded, because their blood pressure fell below the inclusion threshold, perhaps because of improved adherence. However, this does not mean that all of the randomized patients were adherent and even less so that they remained adherent during the entire follow-up.
Safety
Animal studies on the safety of the SYMPLICITY Catheter System are scarce. Only in 2011, after publication of SYMPLICITY HTN-26 and after the catheter had obtained a CE label in Europe,* Rippy et al29 published results obtained 4 years earlier in 7 swine. In animals euthanized 6 months after the procedure, the renal arteries showed fibrosis from 10% to 25% of the total media and the underlying adventitia, with mild disruption of the external elastic lamina.
Short-term (14–30 days) follow-up angiograms in 18 SYMPLICITY HTN-1 patients showed no evidence of renal artery stenosis or other abnormalities. Magnetic resonance angiograms in 14 patients, 6 months after the procedure, did not reveal any irregularities in any treatment locations.5 Of 49 patients who underwent renal denervation in SYMPLICITY HTN-2,6 43 had renal imaging at 6 months, including 37 renal duplex imaging, 5 MRI, and 5 computerized tomographic angiography. In the registry,7 81 of 153 patients underwent imaging of the renal arteries 6 months after renal denervation by magnetic resonance angiography, computed tomographic angiography, or renal duplex. In the SYMPLICITY studies,5–7 imaging of the renal arteries was neither standardized in terms of the technique used at baseline and follow-up nor in terms of the operators, an issue that might be most relevant for duplex imaging. Only a minority of patients were examined by magnetic resonance angiography and even less by computerized tomographic renal angiography, which is the gold standard.30–33 In view of the nonstandardized and suboptimal imaging approach in the SYMPLICITY studies,5–7 the risk of intervention-related renal artery abnormalities, stenosis or aneurysms remains a legitimate concern, in particular beyond 6 months of follow-up. The registry7 also does not provide any substantial information on renal function beyond 6 months and even suggests that, at 2 years, renal function substantially declined at least in the small subset of patients with follow-up to that time point (Figure, B).
Issues to Be Addressed
Blinded and Randomized Study Design
In the SYMPLICITY studies,5–7 the decrease in systolic blood pressure at 6 months was in the range of 25 to 30 mmHg for the office blood pressure and ≈20 mmHg for the home blood pressure, whereas, on 24-hour ambulatory monitoring, it was only ≈10 mmHg. The difference between these estimates remains to be clarified.
Blood pressure–lowering effects estimated by out-of-the-office techniques amount usually to ≈60% to 70% of the effects seen on office measurement.19,34,35 There is no proof for the suggestion that renal denervation would blunt the white coat effect.36 Selection of patients for ambulatory blood pressure monitoring or the use of short-acting antihypertensive drugs14 might partially explain the discrepancy. However, the most likely explanation is that ambulatory blood pressure monitors operate in a blinded fashion. In contrast, all of the estimates of the changes in the office and home blood pressures in the SYMPLICITY studies5–7 were collected in an unblinded fashion and are vulnerable to bias introduced by physicians and patients. The SYMPLICITY protocol (version April 4, 2009) instructed investigators as follows: (1) to measure blood pressure ≥3 times; (2) to take additional measurements until they were consistent within 5 mmHg; and (3) to record 3 consistent readings on the case report forms. The number of readings required to reach consistency and those selected to be recorded on the patient forms (consecutive or not) are not in the public domain. The number of repetitions might have been different between randomized groups, particularly at the time of the assessment of the primary end point. Moreover, patients randomized to the control group in SYMPLICITY HTN-2 were offered access to renal denervation after 6 months of follow-up.6 In unblinded patients not accountable for adherence, this offer is unlikely to have stimulated controls to relentlessly take their multiple drugs for 6 months in a row.37
The nonrandomized design of SYMPLICITY HTN-15 and the registry7 also makes it likely that part of the blood pressure–lowering effect is attributed to regression to the mean,38 placebo,39–41 or nocebo42,43 effects; attenuation of the white coat reaction44; and modification of the patients’ behavior in response to the study context (Hawthorne effect). Future studies on the effects of renal denervation should not only be blinded but should have a randomized design with a control group. Unfortunately, this is not the case (Tables 1 and 2). For end points measured on a continuous scale, such as blood pressure or eGFR, reports might include a graphical representation of the distribution of the responses in the randomized groups. The Hypertension Optimal Treatment investigators45 published an example for the blood pressure changes in their trial (Figure S, available in the online-only Data Supplement). A similar approach in future studies of renal denervation might clearly illustrate the heterogeneity and overlap of the responses within and particularly between the randomized groups.
Table 1.
Randomized Controlled Clinical Trials
| SYMPLICITY HTN-3 | DEPART | ReSET | MIRT | DENER-HTN | PRAGUE-15 | INSPiRED | |
|---|---|---|---|---|---|---|---|
| Characteristic | |||||||
| NCT No. (sponsor) |
01418261 (I) | 01522430 (A) | 01459900 (A) | 01117025 (A) | 01570777 (A) | 01560312 (A) | 01505010 (A) |
| Recruiting | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Condition | HT | HT | HT | HT+AF | HT | HT | HT |
| Primary end point (mo) |
OBP (6) major AEs (1), RA stenosis (6) |
24-h ABP (6) eGFR (6) | 24-h ABP (3) | OBP (24) | Daytime ABP (6) | Office SBP (6) | Daytime ABP (36) |
| Intervention | RDN | RDN | RDN | PVI plus RDN | RDN | RDN | RDN |
| Control | RA catheterization without RDN (sham) |
RA catheterization without RDN (sham) |
RA catheterization without RDN (sham) |
PVI without RDN | Optimized AH drug treatment |
Optimized AH drug treatment |
Optimized AH drug treatment |
| Baseline AH treatment |
To be maintained | Adjustable | Adjustable | … | Adjustable | … | Adjustable |
| Special characteristics |
… | … | … | AF and supraventricular arrhythmia are secondary end points |
Standardized drug regimen; monitoring of adherence; cost-effectiveness assessment |
Follow-up of BP and cardiac events ≤5 y |
Stratification for age and adherence assessed by pill box monitoring |
| No. of patients | 530 | 120 | 70 | 150 | 120 | 150 | 230 |
| Catheter system | Symplicity | Symplicity | Symplicity | THERMOCOOL | Symplicity | Simplicity | To be determined |
| Expected completion |
2013 | 2014 | 2012 | 2012 | 2014 | 2013 | 2016 |
| Country | United States | Belgium | Denmark | Russian Federation | France | Czech Republic | Europe |
| Eligibility criteria | |||||||
| Age, y | 18–80 | 18–85 | 30–70 | 18–70 | 18–75 | ≥18 | 20–75 |
| OBP, mm Hg | SBP ≥160 | … | … | SBP >160 | ≥140/90 | SBP >140 | … |
| ABP, mm Hg | 24-h SBP≥135 | Daytime SBP ≥135 and/or 24-h DBP ≥85; patients taking ≥4 AH drugs are eligible irrespective of BP |
Daytime SBP ≥145 | … | Daytime ABP ≥135/85 on optimized treatment |
24-h SBP≥130 | 24-h SBP ≥130 and/or DBP ≥80 |
| Drug treatment | ≥3 drugs including a diuretic |
≥3 drugs including thiazide or loop diuretic; spironolactone attempted, unless contraindicated |
≥3 drugs including a diuretic |
≥3 drugs | ≥3 drugs | ≥3 drugs | ≥3 drugs including a diuretic; all major drug classes (including spironolactone) attempted |
| eGFR, mL/min per 1.73 m2 |
≥45 | ≥30 | ≥30 | ≥45 | ≥40 | … (SCrt ≤200 μmol/L) |
≥30 |
| Renal arteries | … | No renal atherosclerotic lesions; suitable anatomy; no previous intervention |
Diameter ≥4 mm; length ≥20 mm; no stenosis; no severe calcifications |
No stenosis or abnormalities; no previous intervention |
Kidneys ≥90 mm and suitable anatomy of renal arteries |
Diameter ≥4 mm; length ≥20 mm; |
Diameter ≥4 mm; length ≥20 mm; no stenosis; no previous intervention |
| Secondary hypertension |
… | Excluded | Excluded | Excluded | Excluded | Excluded | Excluded except adrenal hyperplasia |
| Safety follow-up | |||||||
| Renal function | … | mGFR, cystatin C | … | … | … | … | eGFR, mGFR |
| Imaging of renal arteries |
… | … | … | … | … | … | Arteriography (6) Angio CT (12, 24, 36) |
Trial acronyms: SYMPLICITY HTN-3, Renal Denervation in Patients with Uncontrolled Hypertension; DEPART, Catheter Based Renal Denervation Therapy in Hypertension; ReSET, Renal Sympathectomy in Treatment Resistant Essential Hypertension, a Sham Controlled Randomized Trial; MRIT, Meshalkin Research Institute Trial; DENER-HTN, Renal Denervation in Hypertension; PRAGUE-15, Renal Denervation–Hope for Patients with Refractory Hypertension; INSPiRED, Investigator Steered Project of Intravascular Renal Denervation. NCT No. is the identification No. in the trial registry (http://www.clinicaltrials.gov) of the National Institutes of Health. A (academic) and I (industry) refer to the sponsor. Numbers between parentheses indicate the timing in months after randomization when end point or measurement will be assessed. HT indicates treatment-resistant hypertension; AF, atrial fibrillation; PVI, pulmonary vein isolation; eGFR/mGFR, estimated/measured glomerular filtration rate; SCrt, serum creatinine concentration; AE, adverse events; RA, renal artery; OBP/ABP, office/ambulatory blood pressure; BP, blood pressure; RDN, renal denervation; …, unspecified information.
Table 2.
Nonrandomized Observational Studies
| NCT No. (Sponsor) |
Condition | Primary End Point (mo) |
No. of Patients |
Catheter System | Expected (Year of Report) |
Country |
|---|---|---|---|---|---|---|
| 01366625 (A) | HT+OSAS | BP (3) | 60 | Symplicity | 2013 | Poland |
| 01427049 (A) | HT | BP (12) | 30 | Symplicity | 2013 | Netherlands |
| 01392196 (A) | CHF (II-III) | Safety (6) | 40 | Symplicity | 2016 | International |
| 01499810 (A) | HT | BP (12) | 30 | … | 2012 | Russia |
| 01465724 (A) | HT+IFG | IR (12) | 30 | Symplicity | 2014 | Netherlands |
| 01538992 (A) | CHF (III-IV) | Safety (6) | 20 | Manrinr | 2013 | Turkey |
| 01529372 (I) | HT | Safety (12) | 20 | ReCor | 2013 | France |
| 01534299 (I) | HT | BP (6) | … | Symplicity | 2016 | Worldwide registry |
| 01390831 (A) | HT | BP (12) | 100 | THERMOCOOL | 2015 | China |
| 00664638 (A) | HT±ESRD | Safety (12) | 45 | Symplicity | 2011 | Germany |
| 01541865 (I) | HT | Safety (…) | 64 | Vessix V2 | 2014 | Austria |
| 00753116 (I) | HT+ESRD | Safety (12) | 20 | Ardian | 2009 | United States |
| 01355055 (A) | HT | SNA (…) | 26 | … | 2011 | Germany |
| 00551304 (I) | HT+ESRD | Safety (…) | … | Ardian | 2010 | Australia/Poland |
| 01442883 (A) | HT | BP (6) | 100 | Symplicity | 2012 | Germany |
| 01418560 (A) | HT+CKD | RRT (36) | 200 | THERMOCOOL | 2015 | China |
| 01417221 (A) | HT | CVD (36) | 800 | THERMOCOOL | 2016 | China |
| 01402726 (A) | HT+CHF (II-IV) | CVD (36) | 200 | THERMOCOOL | 2016 | China |
| 01417247 (A) | HT+MS | CVD (36) | 200 | THERMOCOOL | 2016 | China |
| 01438229 (I) | HT | Safety (6) | 35 | St Jude | 2012 | Australia/Greece |
| 01520506 (I) | HT | Safety (6) | 40 | Maya Medical | 2013 | Belgium/Netherlands |
NCT No. is the identification No. in the trial registry (http://www.clinicaltrials.gov) of the National Institutes of Health. A (academic) and I (industry) refer to the sponsor. Conditions: HT, treatment-resistant hypertension; OSAS, obstructive sleep apnea syndrome; CHF, heart failure (New York Heart Association class); IFG, impaired fasting glucose; ESRD, end-stage renal disease; CKD, chronic kidney disease; MS, metabolic syndrome. Primary end points (timing in months after renal denervation): BP, blood pressure; IR, insulin resistance; SNA, sympathetic nervous activity measured by microneurography; RRT, need for renal replacement therapy; CVD, cardiovascular disease. All of the catheter systems with the exception of ReCor (ultrasound) deliver radiofrequency energy to the wall of the renal artery. The Vessix V2 and Maya Medical catheters have a balloon tip and do not require the use of guiding sheet. … indicates that the information was irretrievable.
Definition of Treatment-Resistant Hypertension
In our opinion, the current definition of resistant hypertension, applied in the SYMPLICITY studies,5–7 needs revision for various reasons. First, single-pill combinations of ≥3 antihypertensive agents became available in varying dosages,15 so most hypertensive patients can be controlled with fewer pills per day in a cost-effective fashion.28,46 The number of tablets taken per day is, therefore, no longer a valid criterion to assess treatment resistance. Second, the current definition of treatment resistance does not include assessment of out-of-the-office blood pressure or adherence. Third, some patients might interpret the current definition that, once they are taking 3 antihypertensive drugs, they qualify as candidates for renal denervation. Fourth, the diagnosis of treatment-resistant hypertension implies that all lifestyle and pharmacological approaches to control blood pressure have been implemented or at least tried. Pimenta et al47 studied 12 treatment-resistant patients (≥3 medications including a diuretic) in a randomized crossover fashion on low- (50 mmol) and high- (250 mmol) sodium diets, each period lasting 7 days separated by a 2-week washout period. At baseline, office blood pressure averaged 145.8/83.9 mmHg. Mean urinary sodium excretion was 46.1 versus 252.2 mmol/24 hours during low- versus high-salt intake periods. Compared with a high-salt diet, low-salt intake entailed a fall in the office blood pressure by 22.7/9.1 mmHg.47 In a retrospective cohort study of 140126 treatment-resistant US patients (≥4 medications),48 the most frequently prescribed antihypertensive drug classes were angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers (96.2%), diuretics (93.2%), calcium channel blockers (83.6%), and β-blockers (80.0%). Long-acting chlorthalidone49 (3.0%) and aldosterone antagonists12,13,50 (5.9%)–recommended drugs in treatment-resistant hypertension- were underused, whereas dual renin system inhibition, a potential deleterious combination,51,52 was used in 15.6% of patients.
Redefinition of treatment-resistant hypertension should involve substantially more than just the number of drugs taken.26,53 The following might be accounted for: (1) a standardized diagnostic work-up to exclude secondary hypertension; (2) out-of-the-office blood pressure measurement to exclude white coat hypertension; (3) verified implementation of lifestyle recommendations; and (4) an elaborate assessment of adherence, for instance by administering a questionnaire54 but preferably by measuring biomarkers,55,56 drugs or their metabolites in biological fluids, or by the use of electronic pill boxes.57,58
Selection of Treatment-Resistant Patients for Renal Denervation
In multivariable analyses of the SYMPLICITY registry,7 significant independent predictors of greater systolic blood pressure response were higher baseline systolic blood pressure (P<0.0001) and the use of central sympatholytic agents (P=0.018). The former association is spurious,38 whereas the second is counterintuitive, because patients on sympatholytic agents were excluded from SYMPLICITY HTN-1,5 which forms the nucleus of the SYMPLICITY registry.7 None of the SYMPLICITY analyses5–7 identified conditions associated with higher sympathetic activity, such as obesity,59,60 obstructive sleep apnea,16,61 or renal dysfunction,61 as predictors of the blood pressure response to renal denervation. Age did not determine the blood pressure response, although renal sympathetic denervation might be less effective to remediate isolated systolic hypertension in the elderly, because this condition is attributed to structural changes in the large arteries.62 Identifying reliable predictors of blood pressure reduction in response to sympathetic ablation is a priority issue, should renal denervation make it to clinical practice.
Nervous and Hemodynamic Mechanisms Underlying the Antihypertensive Effect
Inhibition of the sympathetic nervous system might not be the only mechanism underlying the antihypertensive effect of renal denervation. The only direct measurements available came from 10 SYMPLICITY HTN-15 patients, in whom renal norepinephrine spillover decreased by 47% (95% CI, 28% to 65%) from baseline to 15 to 30 days after the procedure. In a single patient, Krum et al5 observed a decrease in whole-body spillover of norepinephrine and muscle sympathetic nerve activity 1 month after the procedure. Future trials should encompass a comprehensive evaluation in a randomized and blinded fashion of the sympathetic nervous activity, for instance by measuring changes in circulating or urinary catecholamines, microneurography,63 quantifying the renal spillover of catecholamines,5 or by assessing heart rate variability,64,65 which is easily feasible.
In the SYMPLICITY patients,5–7 the decline in blood pressure was progressive. Future studies should clarify to what extent changes in the circulating volume and sodium and fluid homeostasis play a role and identify the hemodynamic mechanisms underlying the blood pressure reduction, such as changes in peripheral resistance attributed to peripheral arteriolar vasodilatation (functional) or remodeling (structural). As highlighted by the SYMPLICITY HTN-1 investigators,7 an outstanding question with regard to renal denervation in general and the radiofrequency approach taken in particular regards the durability of the blood pressure–lowering effect. Efferent nerves anatomically regrow over a period of months to years, however, without consistent demonstration of functional reinnervation.63,66
A Holistic and Comprehensive Assessment of Long-Term Outcomes
Renal denervation as a treatment option for resistant hypertension still awaits a balanced evaluation of its potential benefits and harms over a time period of 3 to 5 years. Such assessment should include blood pressure control in adherent and nonadherent patients, residual need of antihypertensive medications, quality of life, the incidence of cardiovascular morbidity and mortality, and a cost-effectiveness analysis based on state-of-the-art methodology.67 In the SYMPLICITY registry7 (Figure, B), eGFR declined faster than in recent trials of hypertensive and/or high-risk patients, such as those enrolled in the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET),68 in the Telmisartan Randomised Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND),69 or in the Avoiding Cardiovascular Events Through Combination Therapy In Patients Living With Systolic Hypertension trial (ACCOMPLISH).70 If patients with more severe renal dysfunction than in the SYMPLICITY studies5–7 would become eligible for enrollment in future trials, the long-term assessment of renal function and alterations in the structure of the renal arteries subjected to radiofrequency energy should move to the forefront of research.
Novel Renal Denervation Systems
The evidence available from the SYMPLICITY studies5–7 was obtained with the first-generation 8F-compatible Ardian catheter, which had a design different from the currently marketed 6F devices. Newer ablation systems are being tested and will soon be released into the market, among them the St Jude Medical Renal Artery Ablation System, the Maya Medical One Shot Ablation System, the THERMOCOOL Irrigated Tip Catheter, the Integrated Ablation System, the ReCor Paradise System, and the VESSIX V2 renal denervation system (Table 2). Differences in design characteristics among catheters encompass71 the following: (1) the need for using a guiding sheet versus balloon-steered catheters; (2) the application of radiofrequency versus ultrasound energy; (3) single versus multiple radiofrequency electrodes; (4) single-shot versus repeat energy delivery systems with or without adjustable energy delivery; (5) the possibility to stabilize and center the catheter in the renal artery by balloon inflation or expanding the electrodes; and (6) the possibility of controlling temperature by cooling. The St Jude catheter has an expandable basket of electrodes allowing fixation in the renal artery. The Maya approach allows radiofrequency energy application with a single device placement, reproducible electrode apposition using a balloon-guided technique, and includes a helical ablation pattern for more complete denervation. The THERMOCOOL catheter is already approved for ablation in atrial ablation. It is an open-loop irrigated catheter designed to maintain lower tip-to-tissue temperatures and to deliver a constant preset radiofrequency energy regardless of local blood flow cooling. The Paradise catheter is balloon guided, emits ultrasound energy circumferentially, and allows for cooling of the endothelium. The VESSIX V2 system is a balloon catheter with electrodes mounted on the exterior of the balloon to facilitate delivery of radiofrequency energy. Trials comparing these different approaches should focus on safety and the measurement of the activity of the sympathetic nervous system.
Position Statement on Renal Denervation
The message promulgated by manufacturers of renal denervation systems in the indication of treatment-resistant hypertension is as follows:
“This technology could potentially help alleviate some of the $500 billion impact that hypertension has on our health care systems by reducing or eliminating costly and lifelong medication use. Patients could potentially benefit through an overall reduction in risks for cardiovascular complications of hypertension, including death.”72
There are no data to support such contentions. Moreover, marketers within these companies, with the help of invasive radiologists and cardiologists, are searching for new indications for renal sympathetic nervous denervation in patients with heart failure (Table 2), the metabolic syndrome or impaired glucose tolerance (Table 2), hypertension combined with atrial fibrillation (Table 1), obstructive sleep apnea73 (Table 2), left ventricular hypertrophy and diastolic dysfunction,74 or polycystic ovary syndrome.75 Despite the rationale underlying some of these indications, it is unsure whether the expectations raised mainly from uncontrolled observational studies, that one size might fit all, will materialize in properly powered randomized controlled trials.
Renal denervation is not a panacea, even in resistant hypertensive patients. Nowadays, it should not be considered as an alternative to well-conducted drug treatment, which includes documentation of adherence to and persistence of antihypertensive drugs and the use of recommended combinations of antihypertensive agents at the highest tolerated daily doses. The US Food and Drug Administration found the evidence summarized in this review too light to allow commercialization of the SYMPLICITY Catheter System. Medtronic Inc is, therefore, sponsoring the SYMPLICITY HTN-3 Trial,25 in which, at 27 locations, 530 patients will be randomized to renal denervation or a sham procedure (Table 1). Hypertension management before study enrollment will be more intensive as compared with the previous SYMPLICITY studies5–7 and involve the use of spironolactone.76,77 In both treatment groups, antihypertensive drugs will be continued throughout follow-up, which is limited to 6 months. Although a 24-hour blood pressure <135 mmHg is an exclusion criterion, the primary end point is still the baseline-adjusted, between-group difference in the office systolic blood pressure, like in the SYMPLICITY HTN-2 Trial.6 The investigators are not blinded. Masking patients to randomization, as stated in the protocol,25 will be difficult, if not impossible. Table 1 summarizes the design characteristics of 4 other randomized controlled trials of renal denervation in the indication of treatment-resistant hypertension. At the time of writing of this review, ≥2 other randomized controlled trials were being set up or starting enrollment in Denmark (M.H. Olsen, Odense) and Norway (S.E. Kjeldsen, Oslo).
Unfortunately, manufacturers overtook European regulators in speed. CE-label certification of electric safety currently permits producers to sell catheter systems for renal denervation for routine clinical use to any interventional facility, whereas the procedure should only be executed by experienced interventionists in tertiary referral centers after careful selection of truly refractory hypertensive patients. Another major issue is that renal denervation is costly. If, as in the SYMPLICITY studies5–7 and several ongoing or planned studies (Tables 1 and 2), antihypertensive drug treatment must be continued, the procedure only inflates the costs of treatment-resistant hypertension without any proof of long-term benefit. In most countries, health care insurers do not reimburse the procedure. Inequality between patients in the possibility of accessing this new treatment modality is an additional argument to ban the procedure from regular hospital care until new evidence consolidates the initial claims of benefit.5–7
At this point in time, one can only hope that solid evidence from randomized clinical trials (Table 1) will not challenge the credibility of the large number of observational studies on renal denervation (Table 2). Such evidence should prevent that a promising technique will undergo the demise, as happened recently with devices for closure of the foramen ovale.78,79 Closing devices, not approved by the US Food and Drug Administration, were available for prevention of recurrent stroke,80 but the evidence rested only on small and poorly controlled observational studies.79 CLOSURE I (Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack Due to Presumed Paradoxical Embolism Through Patent Foramen Ovale; NCT00201461)78 was a large randomized clinical trial involving 909 patients. It showed no benefit of closure with a device compared with medical therapy alone in terms of recurrent stroke or transient ischemic attack but instead increased risks of major vascular events and atrial fibrillation.78 The suggestion of the editorial, “Closing the Door Except for Trials, ” might also be applicable for the legions of renal denervation systems (Table 2).80
Conclusions and Perspectives
Renal denervation for the management of treatment-resistant hypertension represents a more targeted approach for interfering with the sympathetic nervous system81 than the unselective sympatholytic surgery as practiced from the 1960s until the 1980s.82 Assuming that renal denervation would be efficacious in a large number of patients with a variety of conditions may be overly optimistic. Hypertension is not a single disease entity, as postulated by Platt.83 The evidence collected over the past 50 years demonstrated that Page84 and Pickering85 were right, respectively, in stating that hypertension is a mosaic of conditions and that arterial pressure is a continuous trait without dividing the line between normal and abnormal. Models of essential hypertension share the characteristic that renal sodium excretion is impaired at any degree of blood pressure.86–88 In rats, transplantation of a kidney from a hypertensive to a normotensive animal produces hypertension,89 although by definition the transplanted kidney is not innervated. Moreover, essential hypertension is characterized by generalized membrane abnormalities, which could affect the function of the vasculature and many organs in various ways.90,91 Isolated systolic hypertension in the elderly is caused by stiffening of the large arteries and not by an increased sympathetic tone.62
Future research on renal denervation as a way to treat hypertension should address unresolved issues, such as the size and durability of the antihypertensive, renal, and sympatholytic effects; long-term safety; quality of life; possibility to relax antihypertensive drug treatment after the procedure; cost-effectiveness; and, above all, the long-term benefit in terms of hard cardiovascular-renal outcomes. For now, renal denervation should remain the ultima ratio in adherent and truly resistant patients with severe hypertension, in whom all other efforts to reduce blood pressure have failed. The intervention should only be offered to patients within a context of clinical research in highly skilled tertiary referral centers that participate in international registries constructed independent of the manufacturers (Table 3). Consensus along these lines is rapidly growing, at least in Europe.92–94
Table 3.
Center Requirements for the Application of Renal Denervation in Treatment-Resistant Hypertension
| Characteristic | Specifications |
|---|---|
| Experience | Management of resistant hypertension High-volume interventional cardiology/radiology |
| Protocol | Written protocol for diagnostic work-up, procedure, and follow-up Written informed consent Ethical approval Contingency plans for the management of complication Insurance/business plan |
| Infrastructure | Availability of high-quality computerized tomographic/MRI Catheter laboratory |
| Multidisciplinary team |
Hypertension specialists with experience in managing resistant hypertension and interventional cardiologists/radiologists with experience of the renal denervation procedure Access to nephrologists and vascular surgery |
| Transparency | Participation in registration program |
Modified according to the Joint United Kingdom Societies Consensus on Renal Denervation for Treatment-Resistant Hypertension (http://www.bhsoc.org/docs/The-Joint-UK-Societies’-Consensus-on-Renal-Denervation-for-resistant-hypertension.pdf).
Supplementary Material
Acknowledgments
We gratefully acknowledge Mrs Sandra Covens and Mrs Sonja Zuba for their help in preparing this article.
Sources of Funding The European Union (grants IC15-CT98-0329-EPOGH, LSHM-CT-2006-037093-InGenious HyperCare, HEALTH-2007-2.1.1-2-HyperGenes, HEALTH-2011.2.4.2-2-EU-MASCARA, and the ERC Advanced Researcher grant 2011-294713-EPLORE); the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Brussels, Belgium (grants G.0575.06 and G.0734.09); and the Katholieke Universiteit Leuven, Leuven, Belgium (grants OT/04/34 and OT/05/49) gave support to the Studies Coordinating Centre. The authors did not receive any funding for writing this review.
Footnotes
CE stands for Conformité Européenne, meaning European Conformity (http://icqc.eu/userfiles/File/DECISION-768-2008-EC.pdf). The CE label ascertains that a product conforms with all applicable EC directives. Medical devices must not only be safe, but also function in a medical-technical way as described in the manufacturer’s intended purpose (http://ec.europa.eu/health/medical-devices/files/meddev/2_4_1_rev_9_classification_en.pdf).
Disclosures A.P. and J.R. were investigators in the SYMPLICITY HTN-2 Trial. A.P. organized a symposium on renal denervation (March 17, 2012, Diegem, Brussels) with nonbinding financial support from Medtronic Inc.
J.A.S. had full access to all of the data and had final responsibility for the decision to submit the article for publication.
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA. 112.195263/-/DC1.
References
- 1.Staessen JA, Kuznetsova T, Stolarz K. Hypertension prevalence and stroke mortality across populations. JAMA. 2003;289:2420–2422. doi: 10.1001/jama.289.18.2420. [DOI] [PubMed] [Google Scholar]
- 2.Weinehall L, Öhgren B, Persson M, Stegmayr B, Boman K, Hallmans G, Lindholm LH. High remaining risk in poorly treated hypertension: the ‘rule of halves’ still exist. J Hypertens. 2002;20:2081–2088. doi: 10.1097/00004872-200210000-00029. [DOI] [PubMed] [Google Scholar]
- 3.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 4.Fagard RH. Resistant hypertension. Heart. 2012;98:254–261. doi: 10.1136/heartjnl-2011-300741. [DOI] [PubMed] [Google Scholar]
- 5.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 6.Symplicity HTN-2 Investigators Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 7.Symplicity HTN-1 Investigators Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911–917. doi: 10.1161/HYPERTENSIONAHA.110.163014. [DOI] [PubMed] [Google Scholar]
- 8.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 9.Chobanian AV, Bakris GL, Black BK, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, the National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 10.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HAJ, Zanchetti A. 2007 guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 11.Turner MJ, van Schalkwyk JM. Is it ethical to perform irreversible renal denervation before a trial low sodium intake for treatment-resistant hypertension? Hypertension. 2011;58:1–9. doi: 10.1161/HYPERTENSIONAHA.111.176297. [DOI] [PubMed] [Google Scholar]
- 12.Calhoun DA, White WB. Effectiveness of the selective aldosterone blocker, eplerenone, in patients with resistant hypertension. J Am Soc Hypertens. 2008;2:462–468. doi: 10.1016/j.jash.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Eide IK, Torjesen PA, Drolsum A, Babovic A, Lilledahl NP. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22:2217–2226. doi: 10.1097/00004872-200411000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Osterberg LG, Urquhart J, Blaschke TF. Understanding forgiveness: minding and mining the gaps between pharmacokinetics and therapeutics. Clin Pharmacol Ther. 2010;88:457–459. doi: 10.1038/clpt.2010.171. [DOI] [PubMed] [Google Scholar]
- 15.Black HR. Triple fixed-dose combination therapy: back to the past. Hypertension. 2009;54:19–22. doi: 10.1161/HYPERTENSIONAHA.109.132688. [DOI] [PubMed] [Google Scholar]
- 16.Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, Paula LKG, Amaro ACS, Amodeo C, Bortolotto LA, Krieger EM, Bradley TD, Lorenzi-Filho G. The most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811–817. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- 17.Kikuya M, Hansen TW, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, Richart T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA, on behalf of the International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcome (IDACO) investigators Diagnostic thresholds for ambulatory blood pressure monitoring based on 10-year cardiovascular risk. Circulation. 2007;115:2145–2152. doi: 10.1161/CIRCULATIONAHA.106.662254. [DOI] [PubMed] [Google Scholar]
- 18.Hansen TW, Kikuya M, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, Richart T, Torp-Pedersen C, Lind L, Jeppesen J, Ibsen H, Imai Y, Staessen JA, on behalf of the IDACO Investigators Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analasis of 7030 individuals. J Hypertens. 2007;25:1554–1564. doi: 10.1097/HJH.0b013e3281c49da5. [DOI] [PubMed] [Google Scholar]
- 19.Staessen JA, Thijs L, Ohkubo T, Kikuya M, Richart T, Boggia J, Adiyaman A, Dechering DG, Kuznetsova T, Thien T, de Leeuw P, Imai Y, O’Brien E, Parati G. Thirty years of research on diagnostic and therapeutic thresholds for the self-measured blood pressure at home. Blood Press Monit. 2008;13:352–365. doi: 10.1097/MBP.0b013e3283108f93. [DOI] [PubMed] [Google Scholar]
- 20.Pickering TG, Houston Miller N, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardio-vascular Nurses Association. Hypertension. 2008;52:10–29. doi: 10.1161/HYPERTENSIONAHA.107.189010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Oliveras A, Ruilope LM. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:889–890. doi: 10.1161/HYPERTENSIONAHA.110.168948. [DOI] [PubMed] [Google Scholar]
- 22.Redon J, Campos C, Narciso ML, Rodicio JL, Pascual JM, Ruilope LM. Prognostic value of ambulatory blood pressure monitoring in refractory hypertension: a prospective study. Hypertension. 1998;31:712–718. doi: 10.1161/01.hyp.31.2.712. [DOI] [PubMed] [Google Scholar]
- 23.Pierdomenico SD, Lapenna D, Bucci A, Di Tommaso R, Di Mascio R, Manente BM, Caldarella MP, Neri M, Cuccurullo F, Mezzetti A. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens. 2005;18:1422–1428. doi: 10.1016/j.amjhyper.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Salles GF, Cardoso CR, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168:2340–2346. doi: 10.1001/archinte.168.21.2340. [DOI] [PubMed] [Google Scholar]
- 25.Kandzari DE, Bhatt DL, Sobotka PA, O’Neill WW, Esler M, Flack JM, Katzen BT, Leon MB, Massaro JM, Negoita M, Oparil S, Rocha-Singh K, Straley C, Townsend RR, Bakris G. [Accessed July 16, 2012];Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol. 2012 May 9; doi: 10.1002/clc.22008. DOI: 10.1002/clc.22008. http://onlinelibrary.wiley.com/doi/10.1002/clc.22008/full. [DOI] [PMC free article] [PubMed]
- 26.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 27.De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nursing. 2003;2:323. doi: 10.1016/S1474-5151(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 28.Sherill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens. 2011;13:898–909. doi: 10.1111/j.1751-7176.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rippy MK, Zarins D, Barman NC, Wu A, Duncan KL, Zarins CK. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol. 2011;100:1095–1101. doi: 10.1007/s00392-011-0346-8. [DOI] [PubMed] [Google Scholar]
- 30.Patel ST, Mills JL, Sr, Tynan-Cuisinier G, Goshima KR, Westerband A, Hughes JD. The limitations of magnetic resonance angiography in the diagnosis of renal artery stenosis: comparative analysis with conventional arteriography. J Vasc Surg. 2005;41:462–468. doi: 10.1016/j.jvs.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 31.Willmann JK, Baumert B, Schertler T, Wildermuth S, Pfammatter T, Verdun FR, Seifert B, Marincek B, Böhm T. Aortoiliac and lower extremity arteries assessed with 16-detector row CT angiography: prospective comparison with digital substraction angiography. Radiology. 2005;236:1083–1093. doi: 10.1148/radiol.2362040895. [DOI] [PubMed] [Google Scholar]
- 32.Turba UC, Uflacker R, Bozlar U, Hagspiel KD. Normal renal arterial anatomy assessed by multidetector CT angiography: are there differences between men and women? Clin Anat. 2009;22:236–242. doi: 10.1002/ca.20748. [DOI] [PubMed] [Google Scholar]
- 33.Liu PS, Platt JF. CT angiography of the renal circulation. Radiol Clin North Am. 2010;48:347–365. doi: 10.1016/j.rcl.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Staessen JA, Byttebier G, Buntinx F, Celis H, O’Brien ET, Fagard R, for the Ambulatory Blood Pressure Monitoring and Treatment of Hypertension Investigators Antihypertensive treatment based on conventional or ambulatory blood pressure measurement: a randomized controlled trial. JAMA. 1997;278:1065–1072. [PubMed] [Google Scholar]
- 35.Staessen JA, Den Hond E, Celis H, Fagard R, Keary L, Vandenhoven G, O’Brien ET, for the Treatment of Hypertension Based on Home or Office Blood Pressure (THOP) Trial investigators Antihypertensive treatment based on blood pressure measurement at home or in the physician’s office: a randomized controlled trial. JAMA. 2004;291:955–964. doi: 10.1001/jama.291.8.955. [DOI] [PubMed] [Google Scholar]
- 36.Doumas M, Anyfanti P, Bakris G. Should ambulatory blood pressure monitoring be mandatory for future studies in resistant hypertension: a perspective. J Hypertens. 2012;30:874–876. doi: 10.1097/HJH.0b013e328352c3c7. [DOI] [PubMed] [Google Scholar]
- 37.Azizi M, Steichen O, Frank M, Bobrie G, Plouin PF, Sapoval M. Catheter-based radiofrequency renal ablation in patients with resistant hypertension. Eur J Vasc Endovasc Surg. 2012;43:293–299. doi: 10.1016/j.ejvs.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 38.Bland JM, Altman DG. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet. 1995;346:1085–1087. doi: 10.1016/s0140-6736(95)91748-9. [DOI] [PubMed] [Google Scholar]
- 39.Staessen JA, Thijs L, Bieniaszewski L, O’Brien ET, Palatini P, Davidson C, Dobovisek J, Jääskivi M, Laks T, Lehtonen A, Vanhanen H, Webster J, Fagard R, on behalf of the Systolic Hypertension in Europe (SYST-EUR) Trial investigators Ambulatory monitoring uncorrected for placebo overestimates long-term antihypertensive action. Hypertension. 1996;27:414–420. doi: 10.1161/01.hyp.27.3.414. [DOI] [PubMed] [Google Scholar]
- 40.Benedetti F. Mechanisms of placebo and placebo-related effects across diseases and treatments. Annu Rev Pharmacol Toxicol. 2008;48:33–60. doi: 10.1146/annurev.pharmtox.48.113006.094711. [DOI] [PubMed] [Google Scholar]
- 41.Preston RA, Materson BJ, Reda DJ, Williams DW. Placebo-associated blood pressure response and adverse effects in the treatment of hypertension: observations from a Department of Veterans Affairs Cooperative Study. Arch Intern Med. 2000;160:1449–1454. doi: 10.1001/archinte.160.10.1449. [DOI] [PubMed] [Google Scholar]
- 42.Colloca L, Miller FG. The nocebo effect and its relevance for clinical practice. Psychosom Med. 2011;73:598–603. doi: 10.1097/PSY.0b013e3182294a50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colloca L, Finniss D. Nocebo effects, patient-clinician communication, an therapeutic outcomes. JAMA. 2012;307:567–568. doi: 10.1001/jama.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mancia G, Bertinieri G, Grassi G, Parati G, Pomidossi G, Ferrari A, Gregorini L, Zanchetti A. Effects of blood pressure measurement by the doctor on patient’s blood pressure and heart rate. Lancet. 1983;II:695–698. doi: 10.1016/s0140-6736(83)92244-4. [DOI] [PubMed] [Google Scholar]
- 45.Hansson L, Zanchetti A, Carruthers SG, Dahlöf B, Elmfeldt D, Julius S, Ménard J, Rahn KH, Wedel H, Westerling S, for the HOT Study group Effects of intensive blood pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 46.Mahmud A, Feely J. Low-dose quadruple antihypertensive combination: more efficacious than individual agents–a preliminary report. Hypertension. 2007;49:272–275. doi: 10.1161/01.HYP.0000254479.66645.a3. [DOI] [PubMed] [Google Scholar]
- 47.Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell’Italia LJ, Calhoun DA. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2008;54:475–481. doi: 10.1161/HYPERTENSIONAHA.109.131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanselin MR, Saseen JJ, Allen RR, Marrs JC, Nair KV. Description of antihypertensive use in patients with resistant hypertension prescribed four or more agents. Hypertension. 2011;58:1008–1013. doi: 10.1161/HYPERTENSIONAHA.111.180497. [DOI] [PubMed] [Google Scholar]
- 49.Ernst ME, Carter BC, Goerdt CJ, Steffensmeier JJG, Bryles Phillips B, Zimmerman MB, Bergus GR. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47:352–358. doi: 10.1161/01.HYP.0000203309.07140.d3. [DOI] [PubMed] [Google Scholar]
- 50.Ojji DB, Alfa J, Ajayi SO, Mamven MH, Falase AO. Pattern of heart failure in Abuja, Nigeria: an echocardiographic study. Cardiovasc J Afr. 2009;20:349–352. [PMC free article] [PubMed] [Google Scholar]
- 51.The ONTARGET Investigators Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 52.Birkenhäger WH, Staessen JA. Dual inhibition of the renin system by aliskiren and valsartan. Lancet. 2007;270:195–196. doi: 10.1016/S0140-6736(07)61099-X. [DOI] [PubMed] [Google Scholar]
- 53.Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. Am J Hypertens. 2006;19:1190–1196. doi: 10.1016/j.amjhyper.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Young LM, Haakenson CM, Lee KK, van Eeckhout JP. Riboflavin use as a drug marker in Veterans Administration cooperative studies. Control Clin Trials. 1984;5(suppl 4):497–504. doi: 10.1016/0197-2456(84)90010-2. [DOI] [PubMed] [Google Scholar]
- 56.Azizi M, Ménard J, Peyrard S, Lièvre M, Marre M, Chatellier G. Assessment of patients’ and physicians’ compliance to an ACE inhibitor treatment based on urinary N-Acetyl Ser-Asp-Lys-Pro determination in the Noninsulin-Dependent Diabetes, Hypertension, Microalbuminuria, Proteinuria, Cardiovascular Events and Ramipril (DIABHYCAR) Study. Diabet Care. 2006;29:1331–1336. doi: 10.2337/dc06-0255. [DOI] [PubMed] [Google Scholar]
- 57.Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336:1114–1117. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Onzenoort HAW, Verberk WJ, Kroon AA, Kessels AGH, Neef C, van der Kuy PHM, de Leeuw PW. Electronic monitoring of adhrence, treatment of hypertension, and blood pressure control. Am J Hypertens. 2012;25:54–59. doi: 10.1038/ajh.2011.153. [DOI] [PubMed] [Google Scholar]
- 59.Esler M, Rumantir M, Kaye D, Lambert G. The sympathetic neurobiology of essential hypertension: disparate influences of obesity, stress, and noradrenaline transporter dysfunction. Am J Hypertens. 2001;14:139s–146s. doi: 10.1016/s0895-7061(01)02081-7. [DOI] [PubMed] [Google Scholar]
- 60.Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens. 2001;14:304s–309s. doi: 10.1016/s0895-7061(01)02236-1. [DOI] [PubMed] [Google Scholar]
- 61.Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension. Hypertension. 2009;54:690–697. doi: 10.1161/HYPERTENSIONAHA.108.119883. [DOI] [PubMed] [Google Scholar]
- 62.Staessen J, Amery A, Fagard R. Editorial review: isolated systolic hypertension in the elderly. J Hypertens. 1990;8:393–405. doi: 10.1097/00004872-199005000-00001. [DOI] [PubMed] [Google Scholar]
- 63.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Eng J Med. 2009;361:932–934. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- 64.Kingwell BA, Thompson JM, Kaye DM, McPherson GA, Jennings GL, Esler MD. Heart rate spectral analysis, cardiac norepinephrine spillover, and muscle sympathtic nerve activity during human sympathetic nervous activation and failure. Circulation. 1994;90:234–240. doi: 10.1161/01.cir.90.1.234. [DOI] [PubMed] [Google Scholar]
- 65.Stolarz K, Staessen JA, Kuznetsova T, Tikhonoff V, State D, Babeanu S, Casiglia E, Fagard RH, Kawecka-Jaszcz K, Nikitin Y, on behalf of the European Project on Genes in Hypertension (EPOGH) investigators Host and environmental determinants of heart rate and heart rate variability in four European populations. J Hypertens. 2003;21:525–535. doi: 10.1097/00004872-200303000-00018. [DOI] [PubMed] [Google Scholar]
- 66.Kaye DM, Esler M, Kingwell B, McPherson G, Esmore D, Jennings G. Functional and neurochemical evidence for partial cardiac sympathetic reinnervation after cardiac transplantation in humans. Circulation. 1993;88:1110–1118. doi: 10.1161/01.cir.88.3.1110. [DOI] [PubMed] [Google Scholar]
- 67.Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17:479–500. doi: 10.2165/00019053-200017050-00006. [DOI] [PubMed] [Google Scholar]
- 68.Mann JFE, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S, on behalf of the ONTARGET investigators Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 69.The Telmisartan Randomised Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease (TRANSCEND) investigators Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174–1183. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- 70.Jamerson K, Weber MA, Bakris GL, Dahlöf B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ, ACCOMPLISH Trial Investigators Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–28. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 71.Rocha-Singh KJ. Renal artery denervation: a brave new frontier. Endovascular Today. 2012;11:45–52. [Google Scholar]
- 72.Callaghan F. [Accessed June 21, 2012];St Jude Medical announces first use of renal denervation technology. http://www.medlatest.com/2011/10/19/st-jude-medical-announces-first-use-of-renal-denervation-technology/
- 73.Witkowski A, Prejbisz A, Florczak E, Kadziela J, Sliwinsky P, Bielen P, Michalowska I, Kabat M, Warchol E, Januszewicz M, Narkiewicz K, Somers VK, Sobotka PA, Januszewics A. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension. 2011;58:559–565. doi: 10.1161/HYPERTENSIONAHA.111.173799. [DOI] [PubMed] [Google Scholar]
- 74.Brandt MC, Mahfoud F, Reda S, Schirmer SH, Erdmann E, Böhm M, Hoppe UC. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59:901–909. doi: 10.1016/j.jacc.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 75.Schlaich MP, Straznicky N, Grima M, Ika-Sari C, Dawood T, Mahfoud F, Lambert E, Chapra R, Socratous F, Hennebry S, Eikelis N, Böhm M, Krum H, Lambert G, Esler MD, Sobotka PA. Renal denervation: a potential new treatment modality for polycystic ovary syndrome? J Hypertens. 2011;29:991–996. doi: 10.1097/HJH.0b013e328344db3a. [DOI] [PubMed] [Google Scholar]
- 76.Bakris GL. New insights: from risk factors to treatment implications. Nat Rev Cardiol. 2012;9:75–77. doi: 10.1038/nrcardio.2011.202. [DOI] [PubMed] [Google Scholar]
- 77.Václavík J, Sedlák R, Planchý M, Navrátil K, Plášek J, Jarkovský J, Václavík T, Husár R, Kociánová E, Táborský M. Addition of Spironolactone in Patients With Resistant Arterial Hypertension (ASPIRANT): a randomized, double-blind, placebo-controlled trial. Hypertension. 2011;57:1069–1075. doi: 10.1161/HYPERTENSIONAHA.111.169961. [DOI] [PubMed] [Google Scholar]
- 78.Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, Raizner A, Wechsler L, for the CLOSURE I investigators Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Eng J Med. 2012;366:991–999. doi: 10.1056/NEJMoa1009639. [DOI] [PubMed] [Google Scholar]
- 79.Kitsios GD, Dahabreh IJ, Abu Dabrh AM, Thaler DE, Kent DM. Patent forman ovale closure and medical treatments for secondary stroke prevention. Stroke. 2012;43:422–431. doi: 10.1161/STROKEAHA.111.631648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnston SC. Patent foramen ovale closure: closing the door except for trial. N Engl J Med. 2012;366:1048–1050. doi: 10.1056/NEJMe1201173. [DOI] [PubMed] [Google Scholar]
- 81.Schlaich MP, Sobotka PA, Krum H, Whitbourn R, Walton A, Esler MD. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension. 2009;54:1195–1201. doi: 10.1161/HYPERTENSIONAHA.109.138610. [DOI] [PubMed] [Google Scholar]
- 82.Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. JAMA. 1953;152:1501–1504. doi: 10.1001/jama.1953.03690160001001. [DOI] [PubMed] [Google Scholar]
- 83.Platt R. The nature of essential hypertension. Lancet. 1959;274:55–57. doi: 10.1016/s0140-6736(59)90512-4. [DOI] [PubMed] [Google Scholar]
- 84.Page IH. The mosaic theory 32 years later. Hypertension. 1982;4:177. doi: 10.1161/01.hyp.4.2.177. [DOI] [PubMed] [Google Scholar]
- 85.Pickering G. The Nature of Essential Hypertension. JA Churchill Ltd; London, United Kingdom: 1961. [Google Scholar]
- 86.Hall JE, Guyton AC, Coleman TG, Leland Mizelle H, Woods LL. Regulation of arterial pressure: role of pressure natriuresis and diuresis. Fed Proc. 1986;45:2897–2903. [PubMed] [Google Scholar]
- 87.Guyton AC. Long-term arterial pressure control: an analysis from animal experiments and computer and graphic models. Am J Physiol. 1990;259:R865–R877. doi: 10.1152/ajpregu.1990.259.5.R865. [DOI] [PubMed] [Google Scholar]
- 88.Bianchi G, Ferrari P. Renal factors involved in the pathogenesis of genetic forms of hypertension. In: Sassard J, editor. Genetic Hypertension. Colloque INSERM. John Libbey Eurotext Ltd; Montrouge, France: 1992. pp. 447–458. [Google Scholar]
- 89.Bianchi G, Fox U, DiFrancesco GF, Giovanetti AM, Pagetti D. Blood pressure changes by kidney cross-transplantation between spontaneously hypertensive rats and normotensive rats. Clin Sci Mol Med. 1974;47:435–448. doi: 10.1042/cs0470435. [DOI] [PubMed] [Google Scholar]
- 90.Staessen JA, Wang J, Bianchi G, Birkenhäger WH. Essential hypertension. Lancet. 2003;361:1629–1641. doi: 10.1016/S0140-6736(03)13302-8. [DOI] [PubMed] [Google Scholar]
- 91.Citterio L, Simonini M, Zagato L, Salvi L, Delli Carpini S, Lanzani C, Messaggio E, Casamassima N, Frau F, D’Avila F, Cusi D, Barlassina C, Manunta P. Genes involved in vasoconstriction and vasodilation affect salt-sensitive hypertension. PLoS One. 2011;6:e19620. doi: 10.1371/journal.pone.0019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mahfoud F, Vonend O, Bruck H, Clasen W, Eckert W, Frye B, Haller H, Hausberg M, Hoppe UC, Hoyer J, Hahn K, Keller T, Krämer BK, Kreutz R, Potthoff SA, Reinecke H, Schmieder R, Schwenger V, Kintscher U, Böhm M, Rump LC. Interventionelle renale Sympathikusdenervation zur Behandlung der therapieresistenten Hypertonie: expert consensus statement on interventional renal sympathetic denervation for hypertension treatment. Dtsch Med Wschr. 2011:2418–2224. doi: 10.1055/s-0031-1272580. [DOI] [PubMed] [Google Scholar]
- 93.Schmieder RE, Redon J, Grassi G, Kjeldsen JE, Mancia G, Narkiewicz K, Parati G, Ruilope L, van de Borne P, Tsioufis C. ESH position paper: renal denervation–an interventional therapy of resistant hypertension. J Hypertens. 2012;30:387–841. doi: 10.1097/HJH.0b013e328352ce78. [DOI] [PubMed] [Google Scholar]
- 94.Pathak A, Girerd X, Azizi M, Benamer H, Halimi JM, Lantelme P, Lefevre T, Sapoval M. Société Française d’Hypertension Artérielle, Société Française de Cardiologie, Groupe Athérome Coronaire et Interventionnel, Société Française de Radiologie. Expert consensus: renal denervation for the treatment of hypertension. Diagn Interv Imaging. 2012;93:386–394. doi: 10.1016/j.diii.2012.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.