Abstract
Background: Hibernation involves periods of severely depressed metabolism (torpor) and decreases in body temperature (Tb). Small arctic mammals (<5kg), in which Tb generally drop drastically, display leukopenia during hibernation. This raised the question of whether the decreased leukocyte counts in mammalian hibernators is due to torpor per se or is secondary to low Tb. The present study examined immune cell counts in brown bears (Ursus arctos), where torpor is only associated with shallow decreases in Tb. The results were compared across hibernator species for which immune and Tb data were available.
Methods and Results: The white blood cell counts were determined by flow cytometry in 13 bears captured in the field both during summer and winter over 2 years time. Tb dropped from 39.6±0.8 to 33.5±1.1°C during hibernation. Blood neutrophils and monocytes were lower during hibernation than during the active period (47%, p= 0.001; 43%, p=0.039, respectively), whereas no change in lymphocyte counts was detected (p=0.599). Further, combining our data and those from 10 studies on 9 hibernating species suggested that the decline in Tb explained the decrease in innate immune cells (R2=0.83, p<0.0001).
Conclusions: Bears have fewer innate immune cells in circulation during hibernation, which may represent a suppressed innate immune system. Across species comparison suggests that, both in small and large hibernators, Tb is the main driver of immune function regulation during winter dormancy. The lack of a difference in lymphocyte counts in this context requires further investigations.
Keywords: Brown bear, Ursus arctos, Hibernation, Innate immunity, Leukocytes, Torpor.
Introduction
The state of lowered metabolism during hibernation in mammals is a showcase of cell preservation strategies for muscle, bone, and the circulatory and innate immune systems. Hibernating mammals tolerate extremes in organ perfusion, oxygen saturation, temperature, immobilization, and calorie intake - which in combination would be lethal to humans. Hibernators may therefore act as reverse translational models for human health and disease.
In most hibernating mammals, torpor phases are interrupted by euthermic arousal phases (reviewed in: 1). During torpor, metabolism is severely depressed but hibernation also involves the inhibition of thermogenesis, leading to a considerable decrease in body temperature (Tb). The induction of torpor begins with lowering of the metabolic rate, followed by hypothermia as the Tb drifts downward (reviewed in: 1-3). Various degrees of cold torpor have been observed over a phylogenetically wide range of mammals and is most drastic in bats and rodents. The champion of all torpid mammals is the hibernating arctic ground squirrel (Urocitellus parryii, previously called Spermophilus parryii), which exhibits body temperatures as low as -2.9°C 4.
In a recent paper by Bouma et al. 5, the current knowledge of the immune system of hibernating mammals was reviewed. Although there are few data from this field, one of the most striking phenomena is the reduced number of circulating leukocytes found in all hibernating mammals studies so far. The studies reviewed by Bouma et al. 5, finding leukopenia in hibernating mammals, were all conducted on small species, including the European hamster (Cricetus cricetus), European hedgehog (Erinaceus europeaus), European ground squirrel (Spermophilus citellus), arctic ground squirrel (Urocitellus parryii), and the thirteen-lined ground squirrel (Ictidomys tridecemlineatus, previously called Spermophilus tridecemlineatus); all species in which Tb drops drastically during hibernation. The authors 5 raised the question of whether the reduced number of circulating leukocytes in mammalian hibernators is due to torpor per se or secondarily to the low Tb. Most recently, the same authors showed that, at least in small species (Syrian and Djungarian hamsters), Tb during hibernation indeed controls leukopenia 6.
The brown bear (Ursus arctos) is an exception among hibernators. The definition of hibernation as a temporary physiological state, characterized by a controlled lowering of the metabolic rate, as well as a dramatic drop in Tb 1, does not strictly apply to the brown bear. Instead, the bear has been classified as a hibernator based on the length of the torpid period (5-7 months in Scandinavia) 7-9. However, in a recent study on black bears (Ursus americanus), it was shown that bears are true hibernators; involving processes of both metabolic and thermal regulation 10. Whereas the bear's body temperature remains relatively stable during hibernation with a less dramatic drop in comparison to other hibernators (from 37°C in summer to 33-30°C in winter) 10, 11, the absolute metabolic rate in the bear is substantially reduced by 75%, and it takes several weeks after emergence for metabolic rate to return to normal levels 10. The slight Tb lowering in hibernating black bears is uncoupled from metabolic suppression 10, and, most recently, it was demonstrated that the expression of a number of genes involved in regulating the metabolism is adjusted during winter hibernation 12. By this, the bear offers a unique opportunity to further elucidate whether immune depression during hibernation is primarily caused by a lowered Tb or might be intrinsic to torpor per se.
In the present study, we examined immune cell counts in peripheral blood of 13 free-ranging Scandinavian brown bears during hibernation and again during the active period in summer. The results were compared to data from the literature available on different species on immune function during hibernation.
Materials & Methods
Study subjects
Blood samples were collected from 13 free-ranging brown bears, (7 females, 6 males; age 2 (n=4), 3 (n=8) and 4 (n=1) years old), during hibernation (February/March 2010 or 2011) and again from the same bears during their active period in summer (June 2010 or 2011). The bears were immobilized in the den during February/March with combination of tiletamine-zolazepam, medetomidine and ketamine and from a helicopter during June by darting with a combination of tiletamine-zolazepam and medetomidine 13, 14. Blood was collected from the jugular vein, as described previously 14, 15. An accu-temp digital thermometer (Jahpron Medical Int., Bodø, Norway), with a manufacturer reported accuracy of ±0.1ºC, was used. The temperatures reported is the temperature taken immediately on capture. All bears were clinically examined by a wildlife veterinarian, and all animals were apparently healthy with no signs of infection. None of the bears were pregnant, and since bears do not have menstruation or any other bleeding during the ovulatory cycle, we did not exclude female bears. The study was approved by the Swedish Ethical Committee on animal research (C212/9). All procedures described were in compliance with Swedish laws and regulations.
Routine blood cell count
White blood cell (WBC) count and the number of different leukocytes were determined at the accredited Clinical Chemical Laboratory at Örebro University Hospital, Sweden, by a fully automated hematology analyzer (XE-5000, Sysmex Corporation, Kobe, Japan). Blood cells were differentiated by the instruments using standardized “WBC differential analysis settings” by applying a combination of forward scatter, side scatter and fluorescence of nucleic acid material using highly specific polymethine dyes. Blood smears were prepared at the field site and stored. The leukocyte differential counts were confirmed manually, by the same lab technician for all samples, with a microscope at the Haematology and Chemistry Laboratory, University Animal Hospital, Swedish University of Agricultural Sciences, Uppsala, Sweden.
Comparative approach
A Web of Science search was performed to find studies on hibernators in which both white blood cell counts and Tb data were available. This search identified ten studies, including 9 different species6, 16-22; European hamster (Cricetus cricetus), Arctic ground squirrel (Urocitellus parryii), woodchuck (Marmota monax), 13-lined ground squirrels (Ictidomys tridecemlineatus), Syrian hamster (Mesocricetus auratus), European ground squirrel (Spermophilus cirellus), Northern leopard frog (Rana pipiens), painted turtle (Chrysemys picta) and grass snake (Natrix natrix). For the later study, no data on Tb was given in the reference 22. As the grass snake is an ectotherm, we made a search on meteorological websites. The average temperature of grass snakes, corresponding to the month the study was conducted, was assumed as Tb. Including the present study, 10 species were included in the comparative analysis.
Statistical analysis
Normality of data sets was assessed using the Shapiro-Wilk test. The effects of hibernation on innate immune cells were analyzed by using mixed linear models with time as the repeated measure (hibernation vs. active), and bears and year of capture as random effects. Further analyses were performed by adjusting on sex, age and body masses to check for possible confounding factors on cell counts. The relationship between leukopenia (expressed as a percentage of the active period) and the drop in Tb during hibernation was checked by simple regression analysis. Two-sided p-values are reported and significance was set to <0.05 (SPPS version 16).
Results & Discussion
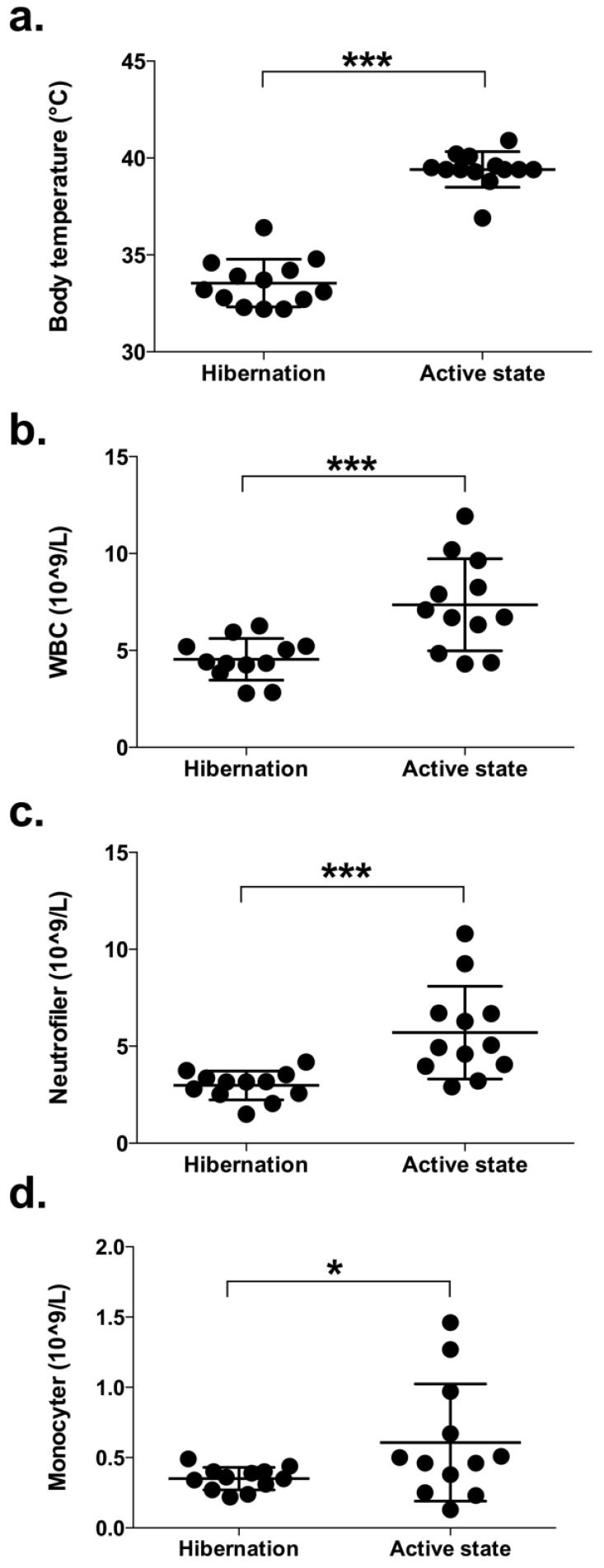
Blood samples were collected from all 13 bears during hibernation and again in summer. During winter, the rectal temperature, recorded with a digital thermometer, was 33.5 ± 1.1 (32.2-35.4) ˚C and during summer 39.6 ± 0.8 (38.8-40.9) °C (Figure 1a). Rectal temperatures seen during summer captures were typical for bears immobilized during the active period 23. WBC count and the presence of different types of leukocytes were investigated. Bears had significantly lower counts of peripheral blood leukocytes during hibernation compared to the active period (4.5 ± 1.1 vs. 7.4 ± 2.4 x109/L, p = 0.001; Figure 1b). These data are in agreement with a previous study showing lower leukocyte counts in captive brown bears during denning compared to non-hibernating animals 11. In addition, we found that the bears had significantly fewer neutrophils (3.0 ± 0.7 vs. 5.7 ± 2.4 x109/L, p = 0.001; Figure 1c) and monocytes (0.35 ± 0.08 vs. 0.61 ± 0.4, p = 0.039; Figure 1d) during hibernation. These findings are consistent with the results for small hibernating animals 5. On the other hand, we could not detect any change in lymphocyte counts between hibernation and active periods (1.1 ± 0.4 vs. 1.1 ± 0.4 x109/L, p = 0.599; data not shown). In one of the bears, WBC was 21.9 x109/L during hibernation, which is 4-5 times higher compared to the other bears (Figure 1). Neutrophils, monocytes and lymphocytes were slightly elevated (4.3, 1.1 and 2.0 x 109/L, respectively) compared to the other bears (Figure 1). However, the number of eosinophils was extremely high in this bear compared to the other bears (14.9 vs. 0.22 ± 0.11 x109/L). C-reactive protein (CRP) was not elevated (0.085 vs. 0.046 ± 0.04 mg/L for the other bears). The leukocyte count of this bear was similar to the other bears when examined in the summer. Still, based on the haematology analyses, this bear was excluded from the study.
Figure 1.
Body temperature and the number of leukocytes in peripheral blood of brown bears during hibernation and during their active period in summer. (a) The rectal temperature, recorded with a digital thermometer, was taken from 13 free-ranging brown bears immediately on capture during winter and again in the summer. (b-d) Blood was withdrawn from 12 free-ranging brown bears during hibernation and again during their active period. The number of peripheral white blood cells (WBC; b), neutrophils (c), and monocytes (d) were determined by flow cytometry. (* and *** represents significant difference: p < 0.05 and < 0.001, respectively.
Our data support the view that the innate immune system is suppressed during hibernation. As discussed by Bouma et al. 5, this could affect the defence towards infections, as illustrated by the massive death of hibernating bats caused by a fungus 24 that has an optimal growth temperature of 12.5-15.8°C but grows well at 3-6°C 25. Interestingly, it has been suggested that one reason why animals arouse periodically from torpor is to reactivate a dormant immune system to combat pathogens that enter the body during the torpor bouts 26. Although arousals in bears show very different patterns as compared to small-bodied hibernators 10, further investigation is required to find out whether or not hibernating bears exhibit increased cell counts upon arousal from torpor.
In contrast to the extreme decrease in peripheral blood lymphocytes in small hibernating animals 5, 6, our data suggest that cells of the adaptive immune system are not affected in bears during hibernation. However, given that many acquired immune responses are stimulated by innate cells, there may still be an effect on the functioning of this immunological aspect. During arousal bouts in small hibernating animals, there is a rapid (within a few hours) reappearance of retained lymphocytes probably from the gut and spleen to the blood 6, 27, 28. Hibernating black bears do not show spontaneous arousals to normothermic levels of Tb 10, as do small hibernators. Instead, they change position on a daily basis and display multiday cycles of Tb between 30-36˚C 10. Still, bears can be aroused relatively easily, and one could speculate that the short awaken period before we anesthetized the bears could have influenced the number of lymphocytes. However, this period of time was very short (approx. 15 min), and, in addition, the number of circulation lymphocytes during arousal found in small hibernators is still only half of the number found in active animals during summer 29, suggesting that this was not a major factor influencing our results. Another explanation of depressed neutrophils and monocytes during torpor could be that longer-lived lymphocytes would remain in circulation longer than the short-lived neutrophils. On the other hand, this explanation requires a decreased rate of granulopoiesis or a shorter life span of granulocytes. If bear leukocytes are sequestered in peripheral tissue during hibernation as found in small hibernators 6, 27, 28, this phenomenon might explain why monocytes are depressed with torpor due to their ability to leave the circulation. Also, further studies should investigate the hibernating effect of lymphocytes in sub-groups to see if there are different patterns between, for instance, B- and T-lymphocytes, and if this could explain the numbers of lymphocytes found in hibernating vs. active bears. Although the length of time and body temperature history during hibernation could play a role in determining the leukocyte profile at the particular time of sampling, this is unlikely based on work done in ground squirrels 19 that found that the numbers of leukocytes decreased by 90% within 24 hours of torpor and remained unchanged during the remainder of the torpor-period. Although this indicates that the length of time hibernating prior to sampling may not have an impact on the results, further studies including the temperature history (using biologging techniques) would be necessary to determine if these results in ground squirrels apply to the brown bear.
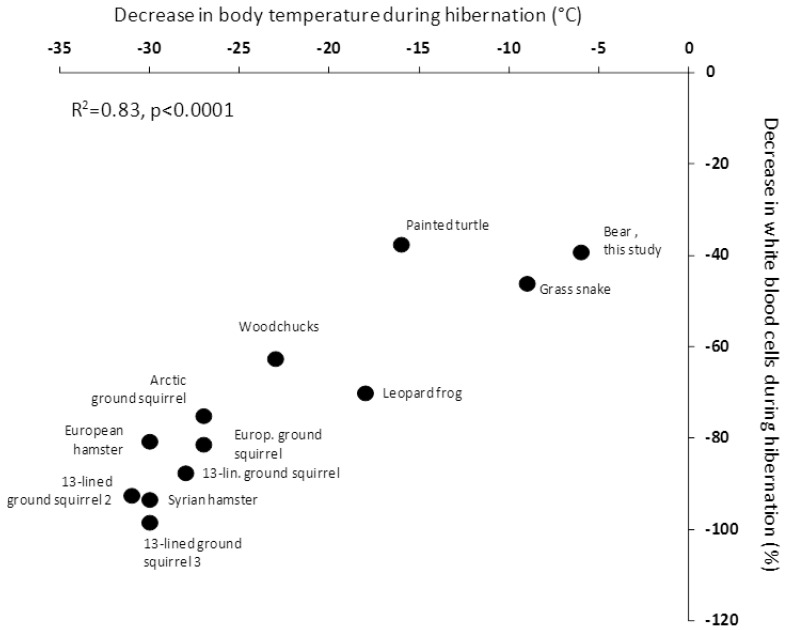
Our study thus supports the concept that hibernation has significant effects on the immune system. Whether or not the hibernation-induced leukopenia is a general phenomenon of hibernation, independently of body mass, resulting from a direct body temperature effect, is an important question from an evolutionary point of view. In small hibernators, the number of circulating white blood cells drops dramatically when the body temperature decreases to 5˚C, and it was thus recently argued that the hibernation induced immune depression is a temperature-dependent mechanism 6. Even more recently, metabolic suppression in bears during hibernation was suggested to be independent of Tb 10; indicating that processes of hibernation may occur in a temperature-independent matter. The direct corollary is to question whether the hibernation-induced leukopenia is independent of Tb when body size is large or whether the temperature effect is a general feature of hibernation. Clearly, this would bring lights on many questions of the above discussion. The between-species-comparison we performed shows that, across taxa, the depression of the immune function appears indeed as a direct function of the temperature reached during torpor (Figure 2). The intercept is slightly off zero, indicating that we cannot rule out the contribution of other mechanisms. However, given the strength of the relationship with Tb explaining 83% of the relationship, it is likely that any alternative mechanisms are of modest importance, but further studies on large animals, such as different bear species, are needed. Taken together, our results suggest that the drop in Tb, reached during the torpor bout, is the main drive of the process of leukopenia observed during hibernation and this may be a general mechanism in hibernators. We think that similar approaches are needed to investigate the relationship between metabolic depression and hypothermia. Indeed, we find it hard to explain how such an independency is possible given the first law of thermodynamics. We rather think that further studies are clearly needed to look at regional/organ body temperature regulations.
Figure 2.
Relationship between leukopenia and the drop in Tb during hibernation across hetherothermic species. European hamster (Cricetus cricetus; 16), Arctic ground squirrel (Urocitellus parryii; 17, woodchuck (Marmota monax; 18, 13-lined ground squirrels 1 (Citrellus tricemlineatus; 18, 13-lined ground squirrels 2 (Ictidomys tridecemlineatus; 38, 13-lined ground squirrels 3 (Ictidomys tridecemlineatus; 39, Syrian hamster (Mesocricetus auratus; 6), European ground squirrel (Spermophilus citrellus; 19, Northern leopard frog (Rana pipiens; 20, painted turtle (Chrysemys picta; 21, and grass snake (Natrix natrix; 22 . The bear data are from the present study.
In conclusion, the brown bear offers novel information on the innate immune system during hibernation. The present study supports the hypothesis that the reduced number of white blood cells during hibernation is a temperature-dependent phenomenon conserved across evolution. Future studies are needed to understand the mechanisms regulating the innate immune system during hibernation. We hypothesize that the reduced number of neutrophils during hibernation is associated with increased survival. One explanation could be that the suppression in immune function, as a function of body temperature, is proportional to the decrease in virulence of any pathogens present in the hibernator. Whether this is due to energy benefits of a reduced immune function 6 or other mechanisms must be determined in future studies.
In perspective, our findings of a hibernation-associated drop in white blood cells in brown bears could inspire research in human pathophysiological states and increase the understanding of hibernation physiology in general and aspects of ecological immunology. High numbers of neutrophils are associated with disorders, including ischemic heart disease 30, complications of metabolic syndrome 31 and hypertension 32. Patients with coronary artery disease display dysregulated neutrophils 33, 34, and activated neutrophils play a role in morbid obesity 35, rheumatoid arthritis 36 and bronchial asthma 37. If the regulatory mechanisms behind white blood cell lowering in bears could be worked out, this might have therapeutic potential. Understanding the mechanisms of specific physiological alterations involved in hibernation is of relevance for pharmacologically induced suspended animation - a therapeutic option in patients with acute severe cerebral or cardiovascular disease 6.
Author Contributions
Study design: Sahdo, Evans, Arnemo, Fröbert, Särndahl.
Acquisition of data: Sahdo, Evans, Blanc, Fröbert.
Analysis and Interpretation of data: Sahdo, Evans, Arnemo, Blanc, Fröbert, Särndahl.
Preparation of manuscript: Sahdo, Evans, Arnemo, Blanc, Fröbert, Särndahl.
All authors were involved in revising the manuscript for important intellectual content and approved the final version.
Acknowledgments
We thank Sven Brunberg and the research personnel in the Scandinavian Brown Bear Research Project for their assistance in the field. Rita Lind, Clinical Chemical Laboratory at Örebro University Hospital, Sweden is acknowledged for excellent technical assistance.
Funding sources: The Scandinavian Brown Bear Research Project is funded by the Swedish Environmental Protection Agency, the Norwegian Directorate for Nature Management, the Swedish Association for Hunting and Wildlife Management, WWF Sweden and the Research Council of Norway.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Statement of Responsibility: The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Abbreviations
- Tb
body temperature
- WBC
white blood cells.
References
- 1.Storey KB. Out cold: biochemical regulation of mammalian hibernation - a mini-review. Gerontology. 2010;56(2):220–30. doi: 10.1159/000228829. [DOI] [PubMed] [Google Scholar]
- 2.Lyman CP. Oxygen consumption, body temperature and heart rate of woodchucks entering hibernation. Am J Physiol. 1958;194(1):83–91. doi: 10.1152/ajplegacy.1958.194.1.83. [DOI] [PubMed] [Google Scholar]
- 3.Andrews MT. Advances in molecular biology of hibernation in mammals. Bioessays. 2007;29(5):431–40. doi: 10.1002/bies.20560. [DOI] [PubMed] [Google Scholar]
- 4.Barnes BM. Freeze avoidance in a mammal: body temperatures below 0 degree C in an Arctic hibernator. Science. 1989;244(4912):1593–5. doi: 10.1126/science.2740905. [DOI] [PubMed] [Google Scholar]
- 5.Bouma HR, Carey HV, Kroese FG. Hibernation: the immune system at rest? J Leukoc Biol. 2010;88(4):619–24. doi: 10.1189/jlb.0310174. [DOI] [PubMed] [Google Scholar]
- 6.Bouma HR, Kroese FG, Kok JW, Talaei F, Boerema AS, Herwig A. et al. Low body temperature governs the decline of circulating lymphocytes during hibernation through sphingosine-1-phosphate. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(5):2052–7. doi: 10.1073/pnas.1008823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friebe A, Jon ES, Sandegren F. Denning Chronology of Female Brown Bears in Central Sweden. Ursus. 2001;12:37–45. [Google Scholar]
- 8.Manchi S, Swenson JE. Denning behaviour of Scandinavian brown bears Ursus arctos. Wildlife Biology. 2005;11(2):123–32. [Google Scholar]
- 9.Geiser F, Ruf T. Hibernation versus Daily Torpor in Mammals and Birds: Physiological Variables and Classification of Torpor Patterns. Physiological Zoology. 1995;68(6):935–66. [Google Scholar]
- 10.Toien O, Blake J, Edgar DM, Grahn DA, Heller HC, Barnes BM. Hibernation in black bears: independence of metabolic suppression from body temperature. Science. 2011;331(6019):906–9. doi: 10.1126/science.1199435. [DOI] [PubMed] [Google Scholar]
- 11.Hissa R, Siekkinen J, Hohtola E, Saarela S, Hakala A, Pudas J. Seasonal patterns in the physiology of the European brown bear (Ursus arctos arctos) in Finland. Comp Biochem Physiol A Physiol. 1994;109(3):781–91. doi: 10.1016/0300-9629(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 12.Fedorov VB, Goropashnaya AV, Toien O, Stewart NC, Chang C, Wang H. et al. Modulation of gene expression in heart and liver of hibernating black bears (Ursus americanus) BMC Genomics. 2011;12:171. doi: 10.1186/1471-2164-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreeger TJ, Arnemo JM. Handbook of Wildlife Chemical Immobilization. Laramie: International Wildlife Veterinary Services; 2007. [Google Scholar]
- 14.Evans A, Sahlén V. et al. Capture, Anesthesia, and Disturbance of Free-Ranging Brown Bears (Ursus arctos) During Hibernation. PLoS One. 2011 doi: 10.1371/journal.pone.0040520. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frobert O, Christensen K, Fahlman A, Brunberg S, Josefsson J, Sarndahl E. et al. Platelet function in brown bear (Ursus arctos) compared to man. Thromb J. 2010;8:11. doi: 10.1186/1477-9560-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reznik G, Reznik-Schuller H, Emminger A, Mohr U. Comparative studies of blood from hibernating and nonhibernating European hamsters (Cricetus cricetus L) Laboratory Animal Science. 1975;25(2):210–5. [PubMed] [Google Scholar]
- 17.Toien O, Drew KL, Chao ML, Rice ME. Ascorbate dynamics and oxygen consumption during arousal from hibernation in Arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2001;281(2):R572–83. doi: 10.1152/ajpregu.2001.281.2.R572. [DOI] [PubMed] [Google Scholar]
- 18.Szilagyi JE, Senturia JB. A comparison of bone marrow leukocytes in hibernating and nonhibernating woodchucks and ground squirrels. Cryobiology. 1972;9(4):257–61. doi: 10.1016/0011-2240(72)90044-2. [DOI] [PubMed] [Google Scholar]
- 19.Bouma HR, Strijkstra AM, Boerema AS, Deelman LE, Epema AH, Hut RA. et al. Blood cell dynamics during hibernation in the European Ground Squirrel. Veterinary Immunology and Immunopathology. 2010;136(3-4):319–23. doi: 10.1016/j.vetimm.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Cooper EL, Wright RK, Klempau AE, Smith CT. Hibernation alters the frog's immune system. Cryobiology. 1992;29(5):616–31. doi: 10.1016/0011-2240(92)90066-b. [DOI] [PubMed] [Google Scholar]
- 21.Schwanz L, Warner DA, McGaugh S, Di Terlizzi R, Bronikowski A. State-dependent physiological maintenance in a long-lived ectotherm, the painted turtle (Chrysemys picta) Journal of Experimental Biology. 2011;214(Pt 1):88–97. doi: 10.1242/jeb.046813. [DOI] [PubMed] [Google Scholar]
- 22.Wojtaszek JS. Haematology of the grass snake Natrix natrix natrix L. Comp Biochem Physiol A Comp Physiol. 1991;100(4):805–12. doi: 10.1016/0300-9629(91)90296-o. [DOI] [PubMed] [Google Scholar]
- 23.Fahlman Å, Arnemo JM, Swenson JE, Pringle J, Brunberg S, Nyman G. Physiologic Evaluation of Capture and Anesthesia with Medetomidine-Zolazepam-Tiletamine in Brown Bears (Ursus arctos) Journal of Zoo and Wildlife Medicine. 2011;42(1):1–11. doi: 10.1638/2008-0117.1. [DOI] [PubMed] [Google Scholar]
- 24.Cryan PM, Meteyer CU, Boyles JG, Blehert DS. Wing pathology of white-nose syndrome in bats suggests life-threatening disruption of physiology. BMC Biol. 2010;8:135. doi: 10.1186/1741-7007-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verant ML, Boyles JG, Waldrep W Jr, Wibbelt G, Blehert DS. Temperature-dependent growth of Geomyces destructans, the fungus that causes bat white-nose syndrome. PLoS One. 2012;7(9):e46280. doi: 10.1371/journal.pone.0046280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prendergast BJ, Freeman DA, Zucker I, Nelson RJ. Periodic arousal from hibernation is necessary for initiation of immune responses in ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2002;282(4):R1054–62. doi: 10.1152/ajpregu.00562.2001. [DOI] [PubMed] [Google Scholar]
- 27.Inkovaara P, Suomalainen P. Studies on the physiology of the hibernating hedgehog. 18. On the leukocyte counts in the hedgehog's intestine and lungs. Ann Acad Sci Fenn Biol. 1973;200:1–21. [PubMed] [Google Scholar]
- 28.Kurtz CC, Carey HV. Seasonal changes in the intestinal immune system of hibernating ground squirrels. Dev Comp Immunol. 2007;31(4):415–28. doi: 10.1016/j.dci.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Suomalainen P, Rosokivi V. Studies on the physiology of the hibernating hedgehog. 17. The blood cell count of the hedgehog at different times of the year and in different phases of the hibernating cycle. Ann Acad Sci Fenn Biol. 1973;198:1–8. [PubMed] [Google Scholar]
- 30.Pinto EM, Huppert FA, Morgan K, Mrc C, Brayne C. Neutrophil counts, monocyte counts and cardiovascular disease in the elderly. Exp Gerontol. 2004;39(4):615–9. doi: 10.1016/j.exger.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Tsai JC, Sheu SH, Chiu HC, Chung FM, Chang DM, Chen MP. et al. Association of peripheral total and differential leukocyte counts with metabolic syndrome and risk of ischemic cardiovascular diseases in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2007;23(2):111–8. doi: 10.1002/dmrr.647. [DOI] [PubMed] [Google Scholar]
- 32.Shankar A, Klein BE, Klein R. Relationship between white blood cell count and incident hypertension. Am J Hypertens. 2004;17(3):233–9. doi: 10.1016/j.amjhyper.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Sarndahl E, Bergstrom I, Brodin VP, Nijm J, Lundqvist Setterud H, Jonasson L. Neutrophil activation status in stable coronary artery disease. PLoS One. 2007;2(10):e1056. doi: 10.1371/journal.pone.0001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarndahl E, Bergstrom I, Nijm J, Forslund T, Perretti M, Jonasson L. Enhanced neutrophil expression of annexin-1 in coronary artery disease. Metabolism. 2010;59(3):433–40. doi: 10.1016/j.metabol.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 35.Nijhuis J, Rensen SS, Slaats Y, van Dielen FM, Buurman WA, Greve JW. Neutrophil activation in morbid obesity, chronic activation of acute inflammation. Obesity (Silver Spring) 2009;17(11):2014–8. doi: 10.1038/oby.2009.113. [DOI] [PubMed] [Google Scholar]
- 36.Weissmann G, Korchak H. Rheumatoid arthritis. The role of neutrophil activation. Inflammation. 1984;8(Suppl):S3–14. doi: 10.1007/BF00915708. [DOI] [PubMed] [Google Scholar]
- 37.Mann BS, Chung KF. Blood neutrophil activation markers in severe asthma: lack of inhibition by prednisolone therapy. Respir Res. 2006;7:59. doi: 10.1186/1465-9921-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spurrier WA, Dawe AR. Several blood and circulatory changes in the hibernation of the 13-lined ground squirrel, Citellus tridecemlineatus. Comp Biochem Physiol A Comp Physiol. 1973;44(2):267–82. doi: 10.1016/0300-9629(73)90479-9. [DOI] [PubMed] [Google Scholar]
- 39.Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM. Local cerebral blood flow during hibernation, a model of natural tolerance to "cerebral ischemia". Journal of Cerebral Blood Flow and Metabolism. 1994;14(2):193–205. doi: 10.1038/jcbfm.1994.26. [DOI] [PubMed] [Google Scholar]