Abstract
Background
Spinal astrocytomas are rare intramedullary CNS tumors for which there is limited consensus on treatment; the importance of the extent of resection (EOR), postoperative radiotherapy, and chemotherapy remains poorly understood. We report on outcomes associated with surgery, postoperative radiotherapy, and chemotherapy in a series of patients treated at M. D. Anderson Cancer Center (MDACC) with the aim of elucidating the role of these treatments in spinal astrocytomas.
Methods
We retrospectively reviewed charts from a series of 83 patients with histologically confirmed spinal astrocytoma treated at MDACC during 1990–2011. Data collected included patient demographic characteristics, prognostic indicators, and treatment modality at diagnosis. We analyzed overall survival (OS) and progression-free survival (PFS) for pilocytic (World Health Organization [WHO] grade I) and infiltrative (WHO grades II, III, and IV) astrocytomas, separately. Multivariate analysis was performed for the infiltrative patients but not the pilocytic patients because of a limited number of cases.
Results
Higher WHO grade among all patients was associated with worse OS (P < .0001) and PFS (P = .0003). Among patients with infiltrative tumors, neither EOR nor radiotherapy was associated with a difference in outcomes in multivariate analysis; however, among patients with infiltrative astrocytomas, chemotherapy was significantly associated with improved PFS (hazard ratio = .22, P = .0075) but not OS (hazard ratio = .89, P = .83) in multivariate analysis.
Conclusion
WHO grade was the strongest prognostic indicator in patients with spinal cord astrocytomas. Our results also show that chemotherapy improved PFS in infiltrative astrocytomas in multivariate analysis, but neither EOR nor radiation therapy influenced outcomes in this group.
Keywords: chemotherapy, glioma, intramedullary, management prognosis
Intramedullary spinal cord tumors are rare, representing only 2%–4%1 of all CNS tumors, and astrocytomas represent only 6%–8%2 of these tumors. The low incidence of these malignancies has resulted in a limited number of studies of this tumor type; many studies have combined the analysis of spinal astrocytomas with other glial tumors, such as ependymomas, making it difficult to adequately interpret the data.
The existing literature on spinal astrocytoma treatment remains ambiguous in terms of prognostic factors or optimal therapy. Some studies have associated surgery with worse outcomes in low- and high-grade tumors,1–4 whereas others suggest that surgery is associated with superior outcomes5–9 in both high-grade6,7 and low-grade8 tumors. Some have reported that extent of resection does not significantly affect outcomes.7,8,10–12 Likewise, reports are inconsistent about the impact of radiotherapy, which has been associated with the spectrum of worse outcomes,9 no change in outcomes,1,7,12,13 or superior outcomes2,3,14 in different studies. Fewer studies have examined the efficacy of chemotherapy, with 2 studies suggesting no significant impact on outcomes.1,13 In addition, there is little consensus on the significance of prognostic factors. Only tumor grade has been consistently associated with outcomes among studies.2,5,12,15–19
In this retrospective study, we investigated the associations between outcomes and the treatment modalities of surgery, radiotherapy, and chemotherapy and the effect of various prognostic indicators in a cohort of 83 patients, both adults and children, with spinal astrocytomas, who were treated at MD Anderson Cancer Center (MDACC).
Patients and Methods
In this retrospective study, which was approved by the appropriate institutional review board, 83 patients with histologically confirmed spinal astrocytoma were identified from an institutional database of spinal cord gliomas treated at MDACC from 1990 through 2011. Chart review was used to collect data on patient demographic characteristics, prognostic indicators, treatment modalities, and clinical outcomes for each patient. Because MDACC serves as a referral center, 11 patients underwent operations at outside institutions before referral. Pathological slides from these patients and all other patients were reviewed by a neuropathologist at MDACC.
Prognostic factors collected included age at diagnosis, sex, World Health Organization (WHO) grade, cord location, number of segments involved, symptoms (deficits in sensation, motor function, or pain), symptom duration, and the number of neurologic deficits (i.e., a patient with both sensory and motor deficits would be considered to have 2 deficits). The treatment modalities reviewed included surgery (biopsy, subtotal, or total resection per review of operative reports), postoperative radiotherapy, and chemotherapy.
Tumors were graded according to the WHO grade, as recorded in the latest pathological report. If pathology reported a low-grade malignancy, the presence of cysts or contrast enhancement on imaging was used to further classify the tumor as pilocytic, because these tumors tend to enhance with contrast.20 Operative and radiological reports were used to determine tumor location, number of spinal segments involved, and surgeon-defined extent of resection. Outcomes were correlated with the initial treatment modality. Progression-free survival (PFS) time was calculated as the time from treatment to evidence of recurrence on imaging or death. Overall survival (OS) was calculated as the time from diagnosis to death. For those patients discharged to hospice care and subsequently lost to follow-up, date of death was collected from the Social Security Index provided by the US Social Security Administration.
Univariate Kaplan-Meier survival analysis was performed with Wilcoxon and log-rank tests, and the Cox proportional hazards regression analysis was used for multivariate analysis.
Multivariate analysis was performed on the infiltrative tumors but not pilocytic tumors because of the small number of pilocytic cases (n = 31). The covariates selected included the 3 treatment modalities, age at diagnosis (per year), WHO grade, number of spinal segments involved (≥3 or <3), number of neurological deficits (≤1 or >1), and symptom duration (median of ≥4.6 months). Both the number of spinal segments involved and the number of neurological deficits were considered as relating to the extent of tumor infiltration and, thus, were potentially good candidates as prognostic variables; symptom duration was included because it was a significant prognostic indicator for survival on univariate analysis.
The variable gross total resection (GTR), compared with less than GTR, was used in univariate analysis. However, 4 patients with infiltrative tumors had a resection that could not be further classified as either subtotal or total based on operative reports or imaging. To include these patients in the multivariate analysis, the variable more than biopsy, compared with biopsy only, was used instead.
Pearson's χ2 tests were used to compare demographic variables. P values <.05 were considered to be statistically significant.
Results
Patient Characteristics
The patient characteristics are summarized in Table 1. Median age at diagnosis was 28.3 years, with a range of 3 months through 77 years; 31 patients had WHO grade I tumors, 14 had grade II, 18 had grade III, and 18 had grade IV; 2 patients were classified as having high-grade tumors, indicating either grade III or IV. Chemotherapy was administered to 41.8% of patients, radiotherapy to 69.5%, and surgery (subtotal or total resection) to 55.5%. The most commonly administered chemotherapeutic agent was temozolomide, which was used in 17 cases; carmustine (BCNU) was the second most common agent and was administered in 4 cases. Other agents used included VP-16, combined vincristine and carboplatin, and the 6-thioguanine, procarbazine, lomustine, and hydroxyurea; procarbazine, lomustine, and vincristine; and nitrogen mustard, vincristine, and procarbazine prednisone regimens. Median follow-up time of patients alive at the time of analysis was 8.4 years, and median follow-up time of all patients was 4.1 years. Because of the known distinct biological and clinical behaviors of these subtypes, the overall cohort was subdivided into a pilocytic group (WHO grade I) and an infiltrative group (WHO grade II, III, and IV) for analysis of outcomes.
Table 1.
Patient characteristics
| Variable | All (n = 83) | Pilocytic (n = 31) | Infiltrative (n = 52) | Pa |
|---|---|---|---|---|
| Age (years) (median) | 28.7 ± 18.1 | 26.6 ± 16.6 | 30.0 ± 18.9 | 0.41 |
| <28.3 | 42 (50.6%) | 16 (51.6%) | 26 (50.0%) | 0.89 |
| ≥28.3 | 41 (49.4%) | 15 (48.4%) | 26 (50.0%) | |
| Sex | ||||
| Male | 42 (50.6%) | 17 (54.8%) | 25 (48.1%) | 0.55 |
| Female | 41 (49.4%) | 14 (45.2%) | 27 (51.9%) | |
| WHO Grade | ||||
| I | 31 (37.4%) | 31 (100%) | - | |
| II | 14 (16.9%) | - | 14 (26.9%) | |
| III or IV | 38 (45.8%) | - | 38 (73.1%) | |
| Symptomsb | ||||
| Sensory Deficit | 47 (59.5%) | 18 (62.1%) | 29 (58.0%) | 0.72 |
| Motor Deficit | 46 (58.2%) | 11 (37.9%) | 35 (70.0%) | 0.0053 |
| Pain | 44 (56.4%) | 18 (62.1%) | 26 (53.1%) | 0.44 |
| Symptom Duration (months) (n =68) | 12.3 ± 27.7 | 15.2 ± 40.3 | 10.9 ± 19.4 | 0.44 |
| <4 | 32 (47.1%) | 12 (54.6%) | 20 (43.5%) | 0.39 |
| ≥4 | 36 (52.9%) | 10 (45.5%) | 26 (56.5%) | |
| Spinal Level Involvedb | ||||
| Cervical | 47 (56.1%) | 20 (64.5%) | 26 (51.0%) | 0.23 |
| Thoracic | 57 (69.5%) | 20 (64.5%) | 37 (72.6%) | 0.44 |
| Lumbar | 12 (14.6%) | 3 (9.7%) | 9 (17.7%) | 0.32 |
| Number of Segments (n =80) | ||||
| <3 segments | 20 (25.0%) | 7 (23.3%) | 13 (26.0%) | 0.79 |
| ≥3 segments | 60 (75.0%) | 23 (76.7%) | 37 (74.0%) | |
| Treatment | ||||
| Surgery (n = 77)c | 0.23 | |||
| Biopsy Only | 31 (37.4%) | 9 (29.0%) | 22 (42.3%) | |
| Subtotal Resection | 31 (37.4%) | 11 (35.5%) | 20 (38.5%) | |
| Total Resection | 15 (18.1%) | 9 (29.0%) | 6 (11.5%) | |
| Radiotherapy Used | 57 (69.5%) | 15 (48.4%) | 42 (82.4%) | 0.0012 |
| Chemotherapy Used | 33 (41.8%) | 5 (16.1%) | 28 (58.3%) | 0.0002 |
Data presented as number of patients, with percentages in parentheses or mean ± standard deviation.
aPearson's χ2 test or Fischer's exact test.
bPercentages do not sum to 100% because some patients were tabulated more than once.
cSix patients has surgery that was documented as subtotal or total and were not tabulated but were included in other analysis.
Extent of resection was not significantly different (P = .23) between the infiltrative and pilocytic tumors; however, patients with infiltrative tumors were more likely to have only a biopsy (42.3% vs 29.0%) and less likely to have a gross total resection (GTR; 11.5% vs 29.0%). Infiltrative tumors were significantly more likely to be treated with radiotherapy (82.4% vs 48.4%; P = .0012) and chemotherapy (58.3% vs 16.1%; P = .0002). Patients with infiltrative tumors were also more likely to present with motor deficits (70.0% vs 37.9%; P = .0053).
Univariate Analysis
Univariate survival analyses of OS and PFS are summarized in Tables 2 and 3. Kaplan-Meier plots of OS and PFS are shown in Fig. 1.
Table 2.
Univariate survival analysis for pilocytic patients
| Variable | OS |
PFS |
||
|---|---|---|---|---|
| Meanb (years) | P | Median (years) | P | |
| Surgery: GTR | ||||
| GTR | 4.58 | 0.35 | 6.09 | 0.070c |
| Less than GTR | 15.0 | 3.04 | ||
| Radiotherapy | ||||
| Yes | 15.0 | 0.32 | 2.54 | 0.047a |
| No | 4.51 | 13.2 | ||
| Chemotherapy | ||||
| Yes | 2.92 | 0.032a,c | 1.59 | 0.023a |
| No | 19.4 | 6.09 | ||
| Symptom Duration (months) (median) | ||||
| ≥3.2 | 6.54 | 0.80 | 5.32 | 0.67 |
| <3.2 | 4.47 | 2.71 | ||
| Deficits: Sensory | ||||
| Yes | 6.23 | 0.057c | 2.71 | 0.58 |
| No | 19.4 | 5.32 | ||
| Deficits: Motor | ||||
| Yes | 4.49 | 0.53 | 8.19 | 0.040a |
| No | 16.3 | 1.92 | ||
| Deficits: | ||||
| More than 1 | 5.25 | 0.96 | 3.99b | 0.36 |
| 1 or none | 6.40 | 2.71 | ||
aP ≤0.05.
bMean reported in place of median because survival >50%.
cWilcoxon P value reported; log-rank value reported otherwise.
Table 3:
Univariate survival analysis for infiltrative patients
| Variable | OS |
PFS |
||
|---|---|---|---|---|
| Median (years) | P | Median (years) | P | |
| Surgery: GTR | ||||
| GTR | 1.35 | 0.15 | 0.76 | 0.22 |
| Less than GTR | 2.86 | 0.89 | ||
| Radiotherapy | ||||
| Yes | 2.82 | 0.30 | 0.84 | 0.21 |
| No | 4.37 | 1.46 | ||
| Chemotherapy | ||||
| Yes | 2.18 | 0.13a | 0.89 | 0.71 |
| No | 4.37 | 1.06 | ||
| Symptom Duration (months) (median) | ||||
| ≥4.6 | 2.86 | 0.027a,c | 0.76 | 0.67 |
| <4.6 | 1.37 | 0.67 | ||
| Deficits: Sensory | ||||
| Yes | 2.82 | 0.56 | 0.70 | 0.92 |
| No | 2.86 | 1.06 | ||
| Deficits: Motor | ||||
| Yes | 2.81 | 0.21 | 1.01 | 0.21 |
| No | 2.85 | 0.24 | ||
| Deficits: | ||||
| More than 1 | 2.18 | 0.10 | 0.76 | 0.46 |
| Only 1 | 6.38 | 1.06 | ||
| WHO Grade | 0.0004a | 0.036a | ||
| II | 7.61b | 1.48 | ||
| III | 2.82 | 0.84 | ||
| IV | 1.39 | 0.55 | ||
aP ≤.05
bMean reported in place of median because survival >50%.
cWilcoxon P value reported; log-rank value reported otherwise.
Fig. 1.
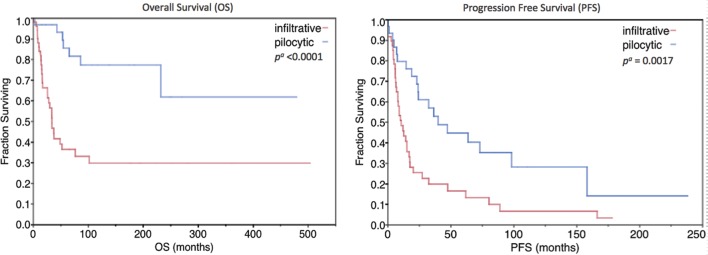
Kaplan-Meier plot of overall survival and progression-free survival among patients with pilocytic and infiltrative tumors. aLog-rank test.
Table 4.
Multivariate survival analysis for infiltrative patients
| Variable | OS |
PFS |
||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P | Hazard Ratio (95% CI) | P | |
| Surgery: | ||||
| More than biopsy | 0.56 (0.21–1.57) | 0.27 | 0.38 (0.12–1.13) | 0.080 |
| Radiotherapy | 0.81 (0.17–4.18) | 0.79 | 0.56 (0.14–2.82) | 0.45 |
| Chemotherapy | 0.89 (0.31–2.79) | 0.83 | 0.22 (0.068–0.66) | 0.0075a |
| #of Segments (≥3) | 3.00 (0.71–14.20) | 0.14 | 0.80 (0.16–3.96) | 0.79 |
| Age (per year) | 1.03 (1.00–1.055) | 0.085 | 1.01 (0.98–1.04) | 0.57 |
| #of Deficits >1 | 1.77 (0.56–5.92) | 0.33 | 3.35 (1.00–13.10) | 0.0496a |
| Symptoms | 0.61 (0.21–1.82) | 0.36 | 0.89 (0.35–2.28) | 0.80 |
| ≥4.6 months | ||||
| WHO Grade | 0.014a | 0.018a | ||
| Grade 2 | Ref. | Ref. | ||
| Grade 3 | 6.56 (0.92–137) | 4.56 (0.93–28.7) | ||
| Grade 4 | 14.7 (2.15–305) | 8.14 (1.80–48.7) | ||
Cox proportional hazards model.
aP ≤ .05.
The 1-, 2-, and 5-year OS rates of the pilocytic group were 97%, 97%, and 85.4%, respectively. The 1-, 2-, and 5-year OS rates of the infiltrative group were 84%, 66.3%, and 36.4%, respectively.
Pilocytic histology was a significant prognostic indicator for improved OS (mean, 16.0 vs 4.23 years; P < .0001) and PFS (median, 3.33 vs 0.89 years; P = .0017). Among the pilocytic group, chemotherapy was associated with worse OS (mean, 2.92 vs 19.4 years; P = .032) and worse PFS (median, 1.59 vs 6.09 years; P = .023). Radiotherapy was also significantly associated with worse PFS (median, 2.54 vs 13.2 years; P = .047). Although not statistically significant, GTR was associated with better PFS among pilocytic patients (median, 6.09 vs 3.04 years; P = .070). Sensory deficits were nonsignificantly correlated with worse OS (mean, 6.23 vs 19.4 years; P = .057), and motor deficits were associated with improved PFS (median, 8.19 vs 1.92 years; P = .040).
Among the infiltrative group, WHO grade was a strong prognostic indicator for improved OS (P = .0004) and PFS (P = .036). Grade II patients had a mean survival (OS remained >50%) of 7.61 years, grade III had a 2.82 year median survival, and grade IV had a 1.39 year median survival. Chemotherapy had a nonsignificant association with worse OS (median, 2.18 vs 4.37 years; P = .13). A comparison of PFS and OS among patients treated with temozolomide compared with other agents was not significant. Although not statistically significant, GTR was associated with worse OS (median, 1.35 vs 2.86 years; P = .15). Having >1 type of neurologic deficit (pain, motor, or sensory) was nonsignificantly associated with worse OS (median, 2.18 vs 6.38 years; P = .10), and a brief (<4.6 month median) symptom duration had a significant association with worse OS (median, 1.37 vs 2.86 years; P = .027).
Multivariate
The results of multivariate analysis for infiltrative patients are presented in Table 4. WHO grade was associated with significantly worse OS (P = .014) and PFS (P = .018), and chemotherapy was associated with significantly improved PFS (hazard ratio = .22; P = .0075) among infiltrative patients. Patients with >1 neurologic deficit had significantly worse PFS (hazard ratio = 3.35; P = .0496).
Discussion
The results of our study confirm the importance of WHO grade as the most significant prognostic variable in patients with spinal cord astrocytomas. In addition, they also demonstrate the distinctly better prognosis of patients with pilocytic subtype, compared with infiltrative tumors. Our reported 5-year survival times for pilocytic and infiltrative patients (85.4% vs 36.4%) are similar to those reported by Minnehan et al.2 From a therapeutic perspective, the follow-up time of 8.4 years in living patients and 4.1 years in all patients allows for clinically relevant interpretation of data. In this context, we did not observe a significant difference in outcomes with more extensive surgery or with radiotherapy. However, we identified an association between significantly improved PFS and chemotherapy among patients with infiltrative subtype of cord astrocytomas. This study is one of the few to report on the administration of chemotherapy in spinal astrocytomas; the potential benefit seen in the infiltrative subtype may suggest a role for this modality in this subgroup that will need to be confirmed in prospective studies.
Characteristics
The more prevalent use of radiotherapy in infiltrative tumors, compared with pilocytic tumors (48.4% vs 82.4%; P = .0012), reflects the need to address residual disease after surgery in infiltrative tumors and, likely, the preference for more aggressive treatment for infiltrative tumors, given their worse prognosis. For similar reasons, chemotherapy (16.1% vs 58.3%; P = .0002) was more often used in infiltrative tumors. The nonsignificant (P = .23) difference in extent of resection between infiltrative and pilocytic tumors reflects the technical difficulty of removing infiltrative tumors, which had fewer GTRs (11.5% vs 29.0%).
Treatment
GTR showed a trend toward improved PFS among pilocytic patients (P = .070) but was not associated with improved OS; this may be related to the overall slower growth rate of these tumors or the use of adjuvant therapies in subtotally resected tumors. However, this result could also have been influenced by the small numbers of patients and the loss to follow-up of several patients after having local failure, resulting in a decrease in the power of the OS analysis.
Surgery classified as more than biopsy in infiltrative astrocytomas was not associated with a significant difference in PFS (hazard ratio = 0.38, P = .080) in multivariate analysis, likely because of the high frequency of local failure of residual disease. Extent of resection in infiltrative tumors in univariate analysis showed a trend toward worse OS (median 1.35 vs 2.86 years; P = .15) with GTR. This may represent the correlation between more aggressive surgery in patients with higher-grade astrocytomas in this study, rather than worse outcomes because of the surgery.
Radiotherapy among pilocytic patients was not associated with a survival difference, but was unexpectedly associated with worse PFS (median, 2.54 vs 13.2 years; P = .047) in univariate analysis. This result is most likely attributable to an association with worse prognosis and the decision to administer radiotherapy. We did not find a significant association between radiotherapy and outcomes in multivariate analysis of infiltrative tumors. This unexpected lack of improvement in PFS with radiotherapy may partially have occurred as the result of patients with less favorable prognosis at diagnosis being disproportionately treated with postoperative radiation. However, we did not find an association between WHO grade in infiltrative tumors and the frequency of postoperative radiotherapy (data not shown), suggesting that other prognostic factors and clinical judgment may likely influence the decision to administer radiotherapy in cases with less favorable prognosis. The only prognostic indicator for OS among pilocytic patients was sensory deficits, and we likewise did not find a significant association between radiotherapy administration and sensory deficits (data not shown).
Patients with pilocytic tumors treated with chemotherapy had worse OS and PFS in univariate analysis. Again, this is most likely attributable to an association between chemotherapy administration and poor initial prognosis, as described previously. Among patients with infiltrative tumors, univariate analysis showed no difference in outcomes with chemotherapy; however, chemotherapy was significantly associated with improved PFS (hazard ratio = .22; P = .0075) in multivariate analysis. This result likely demonstrates a real improvement in PFS with chemotherapy when various prognostic factors were accounted for in the multivariate analysis. We note that there was not a significant improvement in OS (hazard ratio = 0.89; P = .83), and this may partially be explained by the observation that some patients failed locally and were subsequently lost to follow-up, resulting in a decrease in the power of the OS analysis.
The most commonly administered chemotherapeutic agent was temozolomide. A comparison of PFS and OS among patients who received temozolomide, compared with other agents, was not significant. Because of the relatively smaller number of patients treated with chemotherapy and regimen changes for some patients because of toxicities, this result was not surprising. We cannot draw conclusions regarding the use of specific agents from this smaller subset of the data.
Prognostic Indicators
Among patients with pilocytic tumors, univariate analysis showed an association between worse OS with sensory deficits but improved PFS with motor deficits. Bouffet et al.15 similarly found an association between sensory deficits and worse outcomes; perhaps, sensory deficits are less likely to prompt patients to seek medical care, compared with motor deficits, potentially delaying diagnosis and treatment and resulting in worse outcomes. As expected, WHO grade among infiltrative tumors was a significant prognostic indicator for OS and PFS in both univariate and multivariate analyses. WHO grade remains the most consistent and effective prognostic indicator in the literature, and our study confirms this. Long symptom duration (median, >4.6 months) had a significant association (P = .027) with increased OS in univariate analysis and is consistent with findings by Minnehan et al.2 However, symptom duration did not maintain significance in multivariate analysis. Presence of >1 neurologic deficit was a significant indicator of worse PFS in multivariate analysis, and we speculate that this may correspond to the extent of tumor infiltration.
The limitations of our study include its retrospective nature, which may introduce selection bias when interpreting results; the small number of patients in the pilocytic subgroup, which precluded a multivariate analysis; and the potential confounding effects of changes in diagnostic and therapeutic modalities over the period studied. We opted not to perform a multivariate analysis of a combined cohort, which would have been less useful in studying the efficacy of different treatment modalities, because treatment decisions should take into account whether a tumor is pilocytic or infiltrative.
Conclusion
The results of our study confirm the importance of WHO grade as the most significant prognostic variable in patients with spinal cord astrocytomas. Our findings suggest that chemotherapy confers significant improvement in PFS among patients with infiltrative astrocytomas.
Funding
None declared.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Raco A, Piccirilli M, Landi A, et al. High-grade intramedullary astrocytomas: 30 years’ experience at the Neurosurgery Department of the University of Rome “Sapienza”. J Neurosurg Spine. 2010;12(2):144–153. doi: 10.3171/2009.6.SPINE08910. [DOI] [PubMed] [Google Scholar]
- 2.Minehan KJ, Brown PD, Scheithauer BW, et al. Prognosis and treatment of spinal cord astrocytoma. Int J Radiat Oncol Biol Phys. 2009;73(3):727–733. doi: 10.1016/j.ijrobp.2008.04.060. [DOI] [PubMed] [Google Scholar]
- 3.Minehan KJ, Shaw EG, Scheithauer BW, et al. Spinal cord astrocytoma: pathological and treatment considerations. J Neurosurg. 1995;83(4):590–595. doi: 10.3171/jns.1995.83.4.0590. [DOI] [PubMed] [Google Scholar]
- 4.Constantini S, Miller DC, Allen JC, et al. Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg. 2000;93(2 Suppl):83–193. doi: 10.3171/spi.2000.93.2.0183. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura M, Chiba K, Ishii K, et al. Surgical outcomes of spinal cord astrocytomas. Spinal Cord. 2006;44(12):740–745. doi: 10.1038/sj.sc.3101932. [DOI] [PubMed] [Google Scholar]
- 6.Adams H, Avendano J, Raza SM, et al. Prognostic factors and survival in primary malignant astrocytomas of the spinal cord: a population-based analysis from 1973 to 2007. Spine (Phila Pa 1976) 2012;37(12):E727–735. doi: 10.1097/BRS.0b013e31824584c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGirt MJ, Goldstein IM, Chaichana KL, et al. Extent of surgical resection of malignant astrocytomas of the spinal cord: outcome analysis of 35 patients. Neurosurgery. 2008;63(1):55–60. doi: 10.1227/01.NEU.0000335070.37943.09. discussion 60–51. [DOI] [PubMed] [Google Scholar]
- 8.Epstein FJ, Farmer JP, Freed D. Adult intramedullary astrocytomas of the spinal cord. J Neurosurg. 1992;77(3):355–359. doi: 10.3171/jns.1992.77.3.0355. [DOI] [PubMed] [Google Scholar]
- 9.Przybylski GJ, Albright AL, Martinez AJ. Spinal cord astrocytomas: long-term results comparing treatments in children. Childs Nerv Syst. 1997;13(7):375–382. doi: 10.1007/s003810050103. [DOI] [PubMed] [Google Scholar]
- 10.Huddart R, Traish D, Ashley S, et al. Management of spinal astrocytoma with conservative surgery and radiotherapy. Br J Neurosurg. 1993;7(5):473–481. doi: 10.3109/02688699308995069. [DOI] [PubMed] [Google Scholar]
- 11.Sandler HM, Papadopoulos SM, Thornton AF, Jr, et al. Spinal cord astrocytomas: results of therapy. Neurosurgery. 1992;30(4):490–493. doi: 10.1227/00006123-199204000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Kim MS, Chung CK, Choe G, et al. Intramedullary spinal cord astrocytoma in adults: postoperative outcome. J Neurooncol. 2001;52(1):85–94. doi: 10.1023/a:1010680924975. [DOI] [PubMed] [Google Scholar]
- 13.Townsend N, Handler M, Fleitz J, et al. Intramedullary spinal cord astrocytomas in children. Pediatr Blood Cancer. 2004;43(6):629–632. doi: 10.1002/pbc.20082. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Wahab M, Etuk B, Palermo J, et al. Spinal cord gliomas: A multi-institutional retrospective analysis. Int J Radiat Oncol Biol Phys. 2006;64(4):1060–1071. doi: 10.1016/j.ijrobp.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 15.Bouffet E, Pierre-Kahn A, Marchal JC, et al. Prognostic factors in pediatric spinal cord astrocytoma. Cancer. 1998;83(11):2391–2399. doi: 10.1002/(sici)1097-0142(19981201)83:11<2391::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Benes V, 3rd, Barsa P, Benes V, Jr., et al. Prognostic factors in intramedullary astrocytomas: a literature review. Eur Spine J. 2009;18(10):1397–1422. doi: 10.1007/s00586-009-1076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Innocenzi G, Salvati M, Cervoni L, et al. Prognostic factors in intramedullary astrocytomas. Clin Neurol Neurosurg. 1997;99(1):1–5. doi: 10.1016/s0303-8467(96)00555-0. [DOI] [PubMed] [Google Scholar]
- 18.Reimer R, Onofrio BM. Astrocytomas of the spinal cord in children and adolescents. J Neurosurg. 1985;63(5):669–675. doi: 10.3171/jns.1985.63.5.0669. [DOI] [PubMed] [Google Scholar]
- 19.Robinson CG, Prayson RA, Hahn JF, et al. Long-term survival and functional status of patients with low-grade astrocytoma of spinal cord. Int J Radiat Oncol Biol Phys. 2005;63(1):91–100. doi: 10.1016/j.ijrobp.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Rossitch E, Jr., Zeidman SM, Burger PC, et al. Clinical and pathological analysis of spinal cord astrocytomas in children. Neurosurgery. 1990;27(2):193–196. doi: 10.1097/00006123-199008000-00003. [DOI] [PubMed] [Google Scholar]


