Abstract
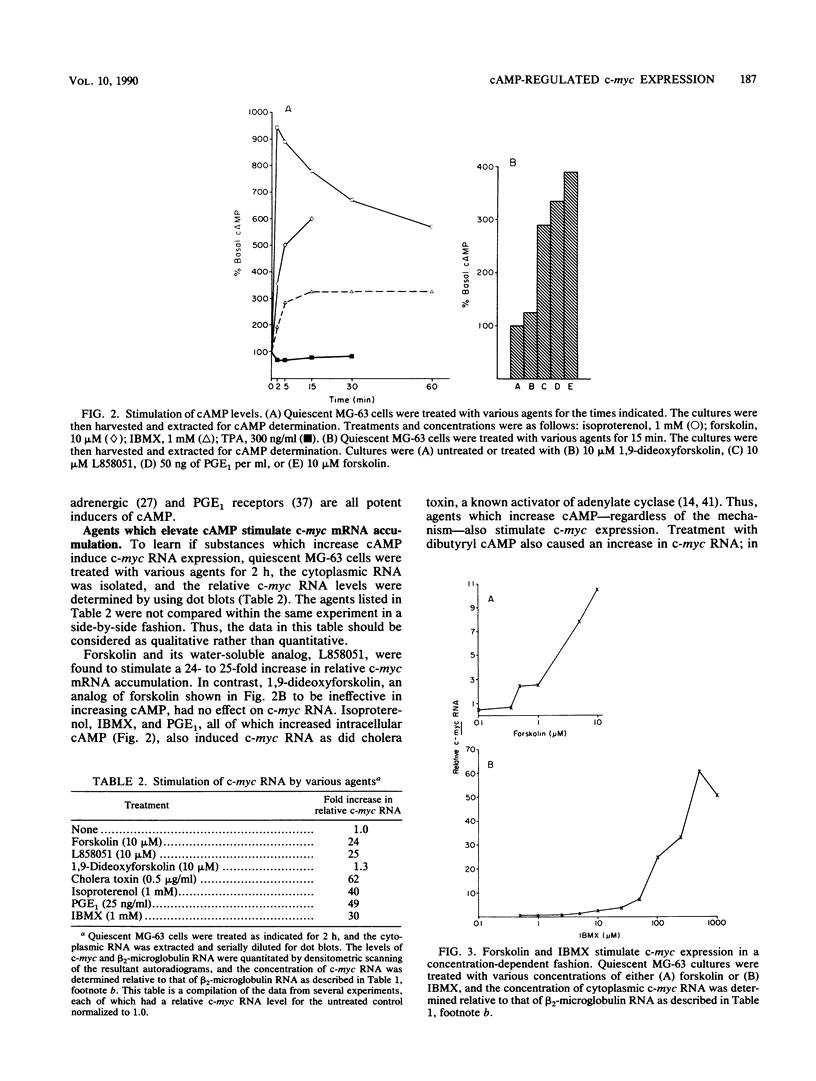
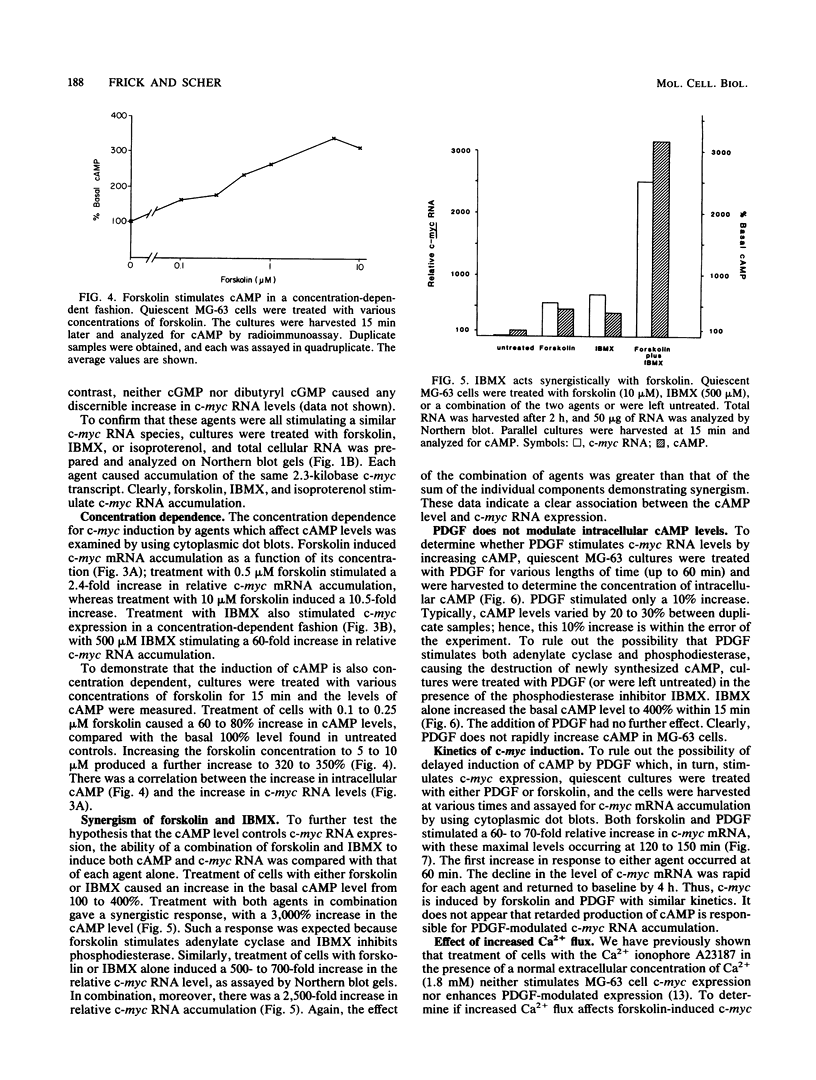
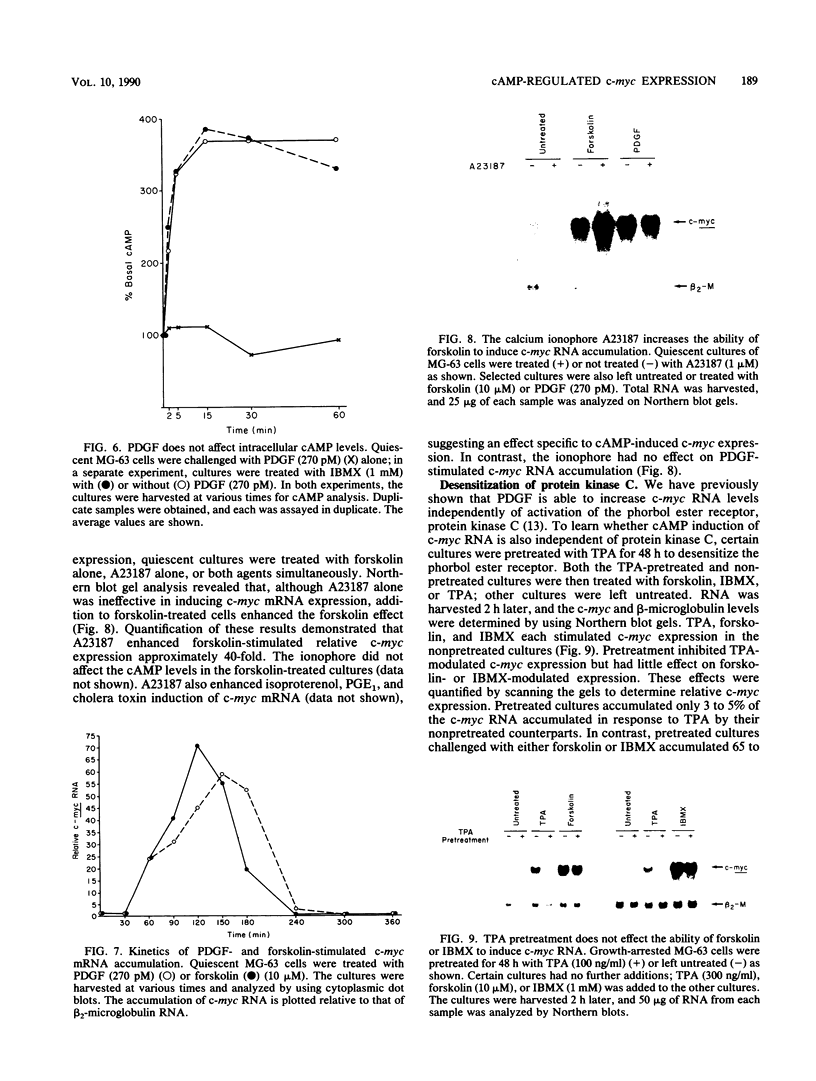
Treatment of quiescent MG-63 cells with 12-O-tetradecanoylphorbol-13-acetate (TPA) or platelet-derived growth factor (PDGF) stimulates the rapid accumulation of c-myc RNA. We have now determined that a similar effect can be induced by cAMP. Treatment with forskolin (an activator of adenylate cyclase), IBMX (a phosphodiesterase inhibitor), PGE1, and isoproterenol stimulated accumulation of both cAMP and c-myc RNA, but no increase in either cAMP or c-myc RNA was seen with the inactive forskolin analog 1,9-dideoxyforskolin. Forskolin and IBMX acted synergistically in stimulating accumulation of both cAMP and c-myc RNA. However, three lines of evidence indicated that PDGF action is not mediated by cAMP. First, PDGF treatment caused no elevation of cAMP within 1 h, even in the presence of IBMX. Second, the kinetics of c-myc RNA elevation after treatment with PDGF or forskolin were similar, ruling out delayed onset of cAMP stimulation. Finally, simultaneous treatment with forskolin and the calcium ionophore A23187 enhanced the elevation of c-myc RNA levels; no such effect was seen with PDGF. We had previously shown that PDGF action is not affected by prior treatment of MG-63 cells with TPA, a treatment which desensitizes the c-myc response to TPA. Similarly, TPA pretreatment had minimal effect on forskolin or IBMX-induced c-myc expression. These data suggest that cAMP, phorbol esters, and PDGF act independently to stimulate c-myc RNA expression in MG-63 cells. However, nuclear runoff experiments and RNA half-life measurements demonstrated that PDGF, phorbol ester, and cAMP all act to increase the transcription of the MYC gene.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Groudine M. Sequence requirements for premature termination of transcription in the human c-myc gene. Cell. 1988 Apr 22;53(2):245–256. doi: 10.1016/0092-8674(88)90386-8. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Heslop J. P., Irvine R. F., Brown K. D. Inositol trisphosphate formation and calcium mobilization in Swiss 3T3 cells in response to platelet-derived growth factor. Biochem J. 1984 Aug 15;222(1):195–201. doi: 10.1042/bj2220195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiau A., Edy V. G., Heremans H., Van Damme J., Desmyter J., Georgiades J. A., De Somer P. Human interferon: mass production in a newly established cell line, MG-63. Antimicrob Agents Chemother. 1977 Jul;12(1):11–15. doi: 10.1128/aac.12.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishayee S., Ross A. H., Womer R., Scher C. D. Purified human platelet-derived growth factor receptor has ligand-stimulated tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6756–6760. doi: 10.1073/pnas.83.18.6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Witters L. A., Girard P. R., Kuo J. F., Quamo S. N. Growth factor-stimulated protein phosphorylation in 3T3-L1 cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1985 Oct 25;260(24):13304–13315. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Lee W. M., Williams P. W., Giels G. M., Williams L. T. c-myc gene expression is stimulated by agents that activate protein kinase C and does not account for the mitogenic effect of PDGF. Cell. 1985 Nov;43(1):243–251. doi: 10.1016/0092-8674(85)90029-7. [DOI] [PubMed] [Google Scholar]
- Dere W. H., Hirayu H., Rapoport B. TSH and cAMP enhance expression of the myc proto-oncogene in cultured thyroid cells. Endocrinology. 1985 Nov;117(5):2249–2251. doi: 10.1210/endo-117-5-2249. [DOI] [PubMed] [Google Scholar]
- Eick D., Berger R., Polack A., Bornkamm G. W. Transcription of c-myc in human mononuclear cells is regulated by an elongation block. Oncogene. 1987;2(1):61–65. [PubMed] [Google Scholar]
- Endo T., Nadal-Ginard B. Transcriptional and posttranscriptional control of c-myc during myogenesis: its mRNA remains inducible in differentiated cells and does not suppress the differentiated phenotype. Mol Cell Biol. 1986 May;6(5):1412–1421. doi: 10.1128/mcb.6.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frick K. K., Womer R. B., Scher C. D. Platelet-derived growth factor-induced c-myc RNA expression. Analysis of an inducible pathway independent of protein kinase C. J Biol Chem. 1988 Feb 25;263(6):2948–2952. [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Hall D. J., Stiles C. D. Platelet-derived growth factor-inducible genes respond differentially to at least two distinct intracellular second messengers. J Biol Chem. 1987 Nov 5;262(31):15302–15308. [PubMed] [Google Scholar]
- Heremans H., Billiau A., Cassiman J. J., Mulier J. C., de Somer P. In vitro cultivation of human tumor tissues. II. Morphological and virological characterization of three cell lines. Oncology. 1978;35(6):246–252. doi: 10.1159/000225298. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Garber S. S., Aldrich R. W. Effect of forskolin on voltage-gated K+ channels is independent of adenylate cyclase activation. Science. 1988 Jun 17;240(4859):1652–1655. doi: 10.1126/science.2454506. [DOI] [PubMed] [Google Scholar]
- Kacich R. L., Williams L. T., Coughlin S. R. Arachidonic acid and cyclic adenosine monophosphate stimulation of c-fos expression by a pathway independent of phorbol ester-sensitive protein kinase C. Mol Endocrinol. 1988 Jan;2(1):73–77. doi: 10.1210/mend-2-1-73. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L., Hyland J. K., Watt R., Rosenberg M., Baserga R. Microinjected c-myc as a competence factor. Science. 1985 Jun 14;228(4705):1313–1315. doi: 10.1126/science.4001943. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Nishizuka Y. Cooperative roles of various membrane phospholipids in the activation of calcium-activated, phospholipid-dependent protein kinase. J Biol Chem. 1981 Jul 25;256(14):7146–7149. [PubMed] [Google Scholar]
- Kaibuchi K., Tsuda T., Kikuchi A., Tanimoto T., Yamashita T., Takai Y. Possible involvement of protein kinase C and calcium ion in growth factor-induced expression of c-myc oncogene in Swiss 3T3 fibroblasts. J Biol Chem. 1986 Jan 25;261(3):1187–1192. [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Khalili K., Weinmann R. Shut-off of actin biosynthesis in adenovirus serotype-2-infected cells. J Mol Biol. 1984 Jun 5;175(4):453–468. doi: 10.1016/0022-2836(84)90179-7. [DOI] [PubMed] [Google Scholar]
- Laurenza A., Khandelwal Y., De Souza N. J., Rupp R. H., Metzger H., Seamon K. B. Stimulation of adenylate cyclase by water-soluble analogues of forskolin. Mol Pharmacol. 1987 Jul;32(1):133–139. [PubMed] [Google Scholar]
- Lefkowitz R. J., Stadel J. M., Caron M. G. Adenylate cyclase-coupled beta-adrenergic receptors: structure and mechanisms of activation and desensitization. Annu Rev Biochem. 1983;52:159–186. doi: 10.1146/annurev.bi.52.070183.001111. [DOI] [PubMed] [Google Scholar]
- Lindsten T., June C. H., Thompson C. B. Multiple mechanisms regulate c-myc gene expression during normal T cell activation. EMBO J. 1988 Sep;7(9):2787–2794. doi: 10.1002/j.1460-2075.1988.tb03133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechti N., Piechaczyk M., Blanchard J. M., Marty L., Bonnieu A., Jeanteur P., Lebleu B. Transcriptional and post-transcriptional regulation of c-myc expression during the differentiation of murine erythroleukemia Friend cells. Nucleic Acids Res. 1986 Dec 22;14(24):9653–9666. doi: 10.1093/nar/14.24.9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmet H., Sinnett-Smith J., Moore J. P., Evan G. I., Rozengurt E. Differential induction of c-fos and c-myc by cyclic AMP in Swiss 3T3 cells: significance for the mitogenic response. Oncogene Res. 1988;3(3):281–286. [PubMed] [Google Scholar]
- Nepveu A., Levine R. A., Campisi J., Greenberg M. E., Ziff E. B., Marcu K. B. Alternative modes of c-myc regulation in growth factor-stimulated and differentiating cells. Oncogene. 1987;1(3):243–250. [PubMed] [Google Scholar]
- Nepveu A., Marcu K. B., Skoultchi A. I., Lachman H. M. Contributions of transcriptional and post-transcriptional mechanisms to the regulation of c-myc expression in mouse erythroleukemia cells. Genes Dev. 1987 Nov;1(9):938–945. doi: 10.1101/gad.1.9.938. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuse S., Roger P. P., Vassart G., Dumont J. E. Enhancement of cmyc mRNA concentration in dog thyrocytes initiating DNA synthesis in response to thyrotropin, forskolin, epidermal growth factor and phorbol myristate ester. Biochem Biophys Res Commun. 1986 Dec 30;141(3):1066–1076. doi: 10.1016/s0006-291x(86)80152-8. [DOI] [PubMed] [Google Scholar]
- Ricci F., Stein E. M. Oscillatory singular integrals and harmonic analysis on nilpotent groups. Proc Natl Acad Sci U S A. 1986 Jan;83(1):1–3. doi: 10.1073/pnas.83.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Stroobant P., Waterfield M. D., Deuel T. F., Keehan M. Platelet-derived growth factor elicits cyclic AMP accumulation in Swiss 3T3 cells: role of prostaglandin production. Cell. 1983 Aug;34(1):265–272. doi: 10.1016/0092-8674(83)90157-5. [DOI] [PubMed] [Google Scholar]
- Schramm M., Neufeld G., Citri Y., Kirilovsky J., Steiner S. Reconstitution of partial reactions requiring the beta-adrenergic receptor and the specific response to binding of the agonist. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:29–35. [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W., Metzger H., de Souza N. J., Reden J. Structure-activity relationships for activation of adenylate cyclase by the diterpene forskolin and its derivatives. J Med Chem. 1983 Mar;26(3):436–439. doi: 10.1021/jm00357a021. [DOI] [PubMed] [Google Scholar]
- Siegel L. I., Bresnick E. Northern hybridization analysis of RNA using diethylpyrocarbonate-treated nonfat milk. Anal Biochem. 1986 Nov 15;159(1):82–87. doi: 10.1016/0003-2697(86)90310-6. [DOI] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontano D., Chin W. W., Moses A. C., Ingbar S. H. Thyrotropin and dibutyryl cyclic AMP increase levels of c-myc and c-fos mRNAs in cultured rat thyroid cells. J Biol Chem. 1986 Mar 25;261(9):3919–3922. [PubMed] [Google Scholar]
- Wagoner P. K., Pallotta B. S. Modulation of acetylcholine receptor desensitization by forskolin is independent of cAMP. Science. 1988 Jun 17;240(4859):1655–1657. doi: 10.1126/science.2454507. [DOI] [PubMed] [Google Scholar]
- Watt R., Stanton L. W., Marcu K. B., Gallo R. C., Croce C. M., Rovera G. Nucleotide sequence of cloned cDNA of human c-myc oncogene. Nature. 1983 Jun 23;303(5919):725–728. doi: 10.1038/303725a0. [DOI] [PubMed] [Google Scholar]
- Wharton W., Leof E. B., Olashaw N., Earp H. S., Pledger W. J. Increases in cyclic AMP potentiate competence formation in BALB/c-3T3 cells. J Cell Physiol. 1982 May;111(2):201–206. doi: 10.1002/jcp.1041110212. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Womer R. B., Frick K., Mitchell C. D., Ross A. H., Bishayee S., Scher C. D. PDGF induces c-myc mRNA expression in MG-63 human osteosarcoma cells but does not stimulate cell replication. J Cell Physiol. 1987 Jul;132(1):65–72. doi: 10.1002/jcp.1041320109. [DOI] [PubMed] [Google Scholar]