Abstract
Background
Secreted protein acidic and rich in cysteine (SPARC) is overexpressed in astrocytomas (World Health Organization grades II–IV). We previously demonstrated that SPARC promotes glioma migration and invasion—in part, by activating the P38 mitogen-activated protein kinase (MAPK)–heat shock protein (HSP)27 signaling pathway. The commonly lost tumor suppressor phosphatase and tensin homolog (PTEN) suppresses SPARC-induced migration, which is accompanied by suppression of Shc-Ras-Raf-MEK-ERK1/2 and Akt signaling. As PTEN completely suppresses SPARC-induced migration, we proposed that PTEN must also interfere with SPARC-induced HSP27 signaling. Therefore, this study determined the effects of PTEN expression on SPARC-induced expression and phosphorylation of HSP27.
Methods
Control and SPARC-expressing clones transfected with control- or PTEN-expression plasmids were plated on fibronectin-coated tissue culture plates for 3, 6, 24, and 48 h and then lysed. Equal amounts of protein were subjected to Western blot and densitometric analyses.
Results
The results show that SPARC enhances phosphorylated (p)P38 MAPK, phosphorylated MAPK-activated protein kinase 2 (pMAPKAPK2), and serine (Ser)78 HSP27 phosphorylation relative to total HSP27. PTEN suppresses pAkt and pMAPKAPK2, suggesting that PTEN effects are downstream of pP38 MAPK. PTEN suppressed SPARC-induced sustained phosphorylation at Ser78 HSP27. As the level of total HSP27 differed based on the presence of SPARC or PTEN, the ratios of phosphorylation-specific to total HSP27 were examined. The data demonstrate that SPARC-induced phosphorylation at Ser78 remains elevated despite increasing levels of total HSP27. In contrast, PTEN inhibits SPARC-induced increases in Ser78 HSP27 phosphorylation relative to total HSP27.
Conclusion
These data describe a novel mechanism whereby PTEN inhibits SPARC-induced migration through suppression and differential regulation of pAkt and the P38 MAPK-MAPKAPK2-HSP27 signaling pathway.
Keywords: gliomas, SPARC, PTEN, HSP27, signaling
Glioblastoma multiforme (GBM) is the most aggressive primary brain tumor in humans.1 Although the majority of GBM develops de novo without evidence of lower-grade precursor tumors, ∼5%–10% progresses from low-grade astrocytoma and/or anaplastic astrocytoma and is designated as secondary GBM.2 Currently, newly diagnosed GBM patients are treated with surgery followed by temozolomide plus radiation, then 6 months of adjuvant temozolomide treatment.3,4 Although this treatment regimen increases progression-free survival at 6 months and overall survival to 14.6 months,5 the median overall survival for all patients who undergo surgery for primary GBM still ranges from 9.9 to 10.2 months.6 Therefore, additional therapies are still required.
Secreted protein acidic and rich in cysteine (SPARC) is a matricellular protein that mediates several biological functions, including cell proliferation, cell survival, cell adhesion, and cell migration, to facilitate normal development and wound healing,7,8 and its aberrant overexpression in many cancer types leads to enhanced tumor cell migration and invasion.9–11 We have previously demonstrated that SPARC is overexpressed in human astrocytomas of World Health Organization grades II–IV,12 suppresses glioma proliferation,13–15 and mediates attachment,13 migration,13,16–18 invasion,15–17,19 and survival15,20 in vitro and in vivo. Because SPARC negatively regulates cell proliferation while promoting glioma migration and invasion,15,17 we have studied its downstream signaling pathways to determine means to retain its negative effects on proliferation but block SPARC-induced migration and invasion. We have identified P38 mitogen-activated protein kinase (MAPK)–heat shock protein (HSP)27,16 Akt,17 and Shc-Raf–extracellular signal-regulated kinase (ERK)17 signaling pathways that are regulated by SPARC to induce migration and invasion. How SPARC induces these pathways is under investigation, but possible mechanisms include SPARC binding directly to integrin-linked kinase (ILK)21 or to integrin β1 to indirectly activate ILK22–24 and focal adhesion kinase,24 both known to promote glioma migration by inducing the downstream phosphorylation of the Akt25,26 and ERK27 signaling pathways, respectively.
HSP27 is a member of the family of stress response proteins that serve as molecular chaperones to offset protein misfolding, protein aggregation, or disruption of regulatory function.28 It also plays a pivotal role in mediating cell survival and death, in part by regulating survival and apoptotic pathways,29 and in stabilizing the cytoskeletal structure in times of cellular stress.30,31 Its function is dictated by its phosphorylation status,32 and aberrant expression and phosphorylation of HSP27 have been implicated in cancer progression and the malignant behavior of a number of cancer types.32 We have previously reported that SPARC promotes glioma migration and invasion by activating the P38 MAPK-HSP27 signaling pathway.16 We have demonstrated that in addition to increasing HSP27 phosphorylation, SPARC upregulates total HSP27 via increasing HSP27 transcript abundance16 and increasing HSP27 protein stability.18
Phosphatase and tensin homolog (PTEN) deleted on chromosome 10 (mutated in multiple advanced cancers [MMAC]1) is a tumor suppressor that keeps the processes of cell migration and proliferation under control. Loss of PTEN is associated with GBM development,33,34 with estimates of PTEN loss and mutation in 27%–36% of primary GBM and methylation in up to 88% of secondary gliomas.35,36 We have shown that PTEN negatively regulates SPARC-induced migration by suppressing the Shc-Ras-Raf–MAP/ERK kinase (MEK)–ERK1/2 and Akt signaling pathways.17 Interestingly, PTEN has the potential to suppress SPARC-induced P38 MAPK-HSP27 signaling via its ability to suppress activation of ILK37,38 and Akt 37 via inhibition of phosphatidyl inositol-3′ kinase (PI3K) signaling38 and/or its suppression of P38 MAPK activation.39 As PTEN completely suppressed SPARC-induced migration in our previous study,17 we propose that it must also affect the P38 MAPK-HSP27 signaling pathway. This study therefore determined whether PTEN inhibition of SPARC-induced migration is due, in part, to PTEN inhibiting or altering SPARC-induced phosphorylation of HSP27.
Materials and Methods
Generation of PTEN-Expressing GBM Cells
Generation and characterization of SPARC-expressing (A2b2) and empty vector control–expressing (C2a2) U87 cells15,16,19 and the C2a2 and A2b2 control- and PTEN-expressing cells17 were previously reported. Briefly, the PTEN sequence was subcloned from pBP-PTEN (kindly provided by Drs. Frank Furnari and Webster Cavenee) into the pcDNA6/V5-HisB plasmid (Invitrogen) with blasticidin resistance. The PTEN-encoding pcDNA6 vector (PTEN) or pcDNA6 empty vector (EV) plasmids were transfected into the A2b2 and C2a2 cell lines, and stable transfectants were selected based on SPARC and/or PTEN expression.
Cell Culture
The 4 cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) and gentamycin (10 µg/mL), along with the selective antibiotics blasticidin (9 µg/mL) and puromycin (1 µg/mL). The selective antibiotics were removed for experimental analysis. Cell culture reagents were purchased from Invitrogen.
Western Blot Analyses
We plated 4 × 105 cells on 100-µg/mL T25 fibronectin- (Millipore) coated flasks (T25) in DMEM with 10% FBS. The cells were lysed at 3, 6, 24, and 48 h, and the protein concentrations were determined using the bicinchoninic acid protein assay (Pierce). Equal amounts of protein (14–52 µg) were loaded onto 10% or 12.5% polyacrylamide sodium dodecyl sulfate tris-glycine gels. After electrophoresis (20 mA/gel), the proteins were transferred to polyvinylidine fluoride membranes (Millipore). The membranes were blocked in 5% nonfat dry milk for 1 h prior to incubation overnight at 4°C in the primary antibodies diluted in either 5% nonfat dry milk or 5% bovine serum albumin per manufacturer's recommendations. Antibodies were diluted 1:1000 except for anti-SPARC (1:10 000), anti-PTEN, and anti–phosphorylated threonine (pThr)222 MAPK-activated protein kinase (MAPKAPK)2 (1:500) antibodies. All antibodies were then incubated with the appropriate horseradish peroxidase–conjugated secondary antibody for 1 h at room temperature. The blots were developed on X-ray film (Denville Scientific) using enhanced chemiluminescence and/or SuperSignal (both Pierce). Primary antibodies purchased were MAPKAPK2 (#3042), pAkt (#4051), Akt (#9272), phosphorylated serine (pSer)82 HSP27 (#2401), P38 MAPK (#9212), and pP38 MAPK (tyrosine [Tyr]180/Tyr182; #9216) from Cell Signaling Technology; pSer78 HSP27 (ADI-SPA-523) and pSer15 HSP27 (ADI-SPA-525) from Enzo Life Sciences; SPARC (AON-5031) from Hematologic Technologies; and actin (sc-1616), PTEN (sc-7974), pThr222 MAPKAPK2 (sc31675), and HSP27 (sc-1049) from Santa Cruz Biotechnology. Secondary antibodies purchased were goat anti-mouse (sc-2005), goat anti-rabbit (sc-2004), and donkey anti-goat (sc-2020) from Santa Cruz Biotechnology. Actin served as the loading control.
ImageJ Analysis
Films were scanned using a Hewlett-Packard 8300 series scanner, and images were captured using Adobe Photoshop CS3 software. Densitometry was measured using ImageJ software (National Institutes of Health). For all densitometric analyses, the values for phosphoprotein and total proteins were first corrected for loading by normalizing to the actin control.
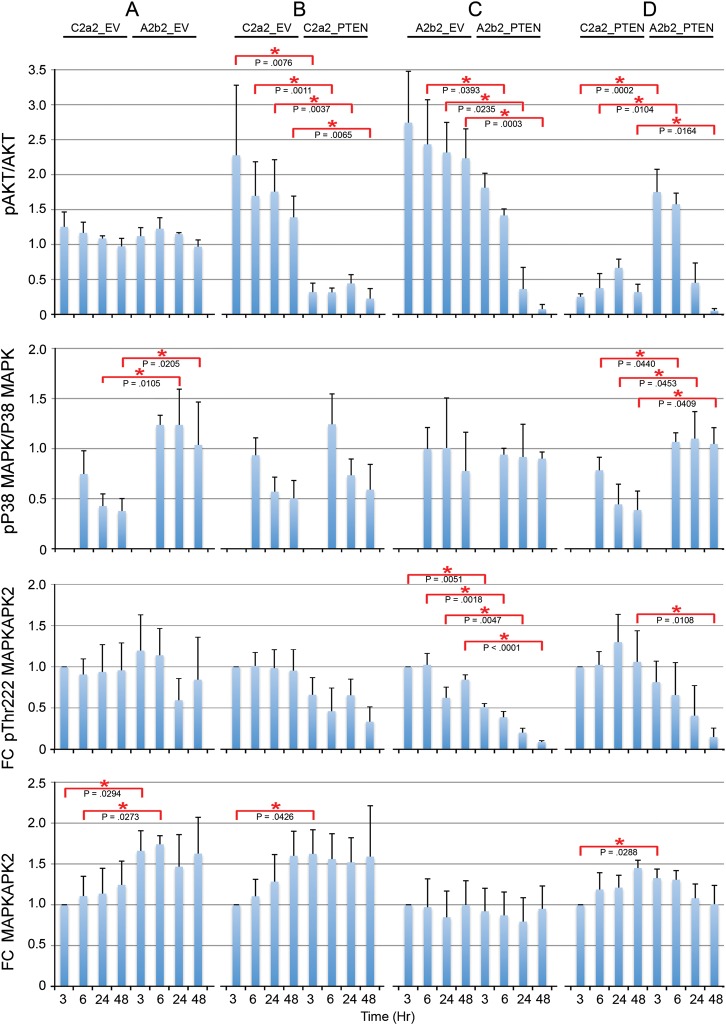
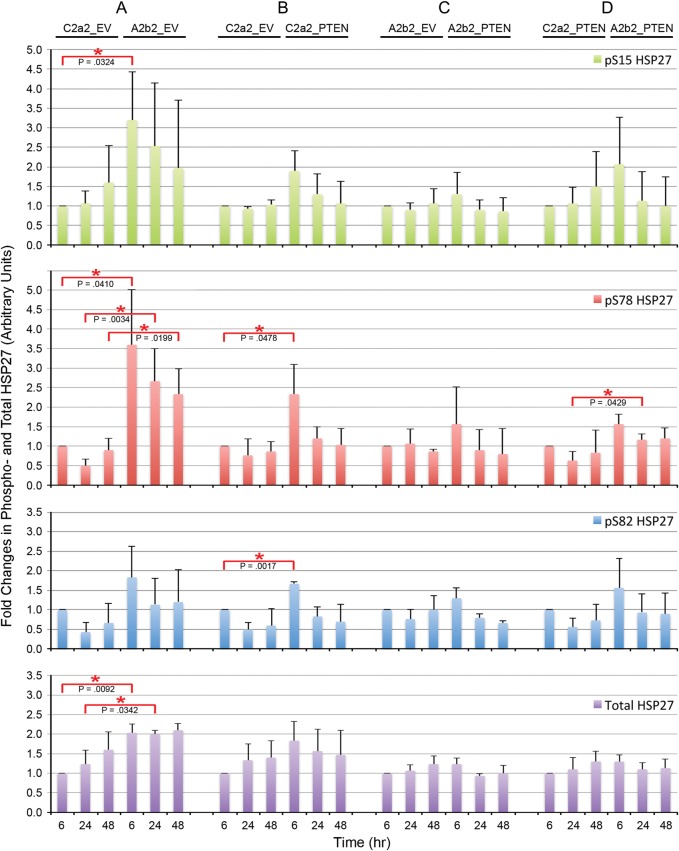
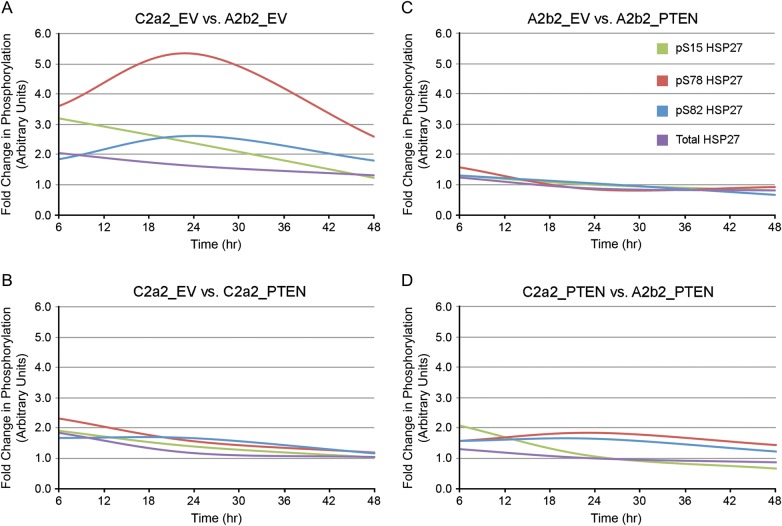
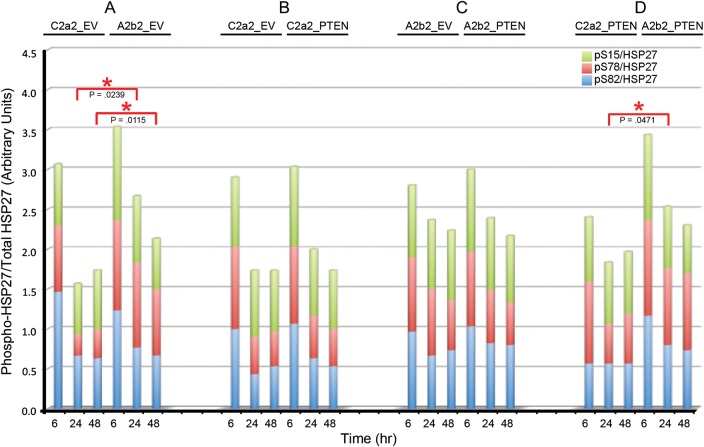
In Fig. 2, the ratios of phosphoprotein to total protein for pAkt/Akt and pP38 MAPK/P38 MAPK are calculated as the ratios of these normalized values. The average ratio is presented for 3 experiments each. In Fig. 2, the fold changes of pThr222 MAPKAPK2 and MAPKAPK2 are relative to the first 3 h (arbitrarily designated as 1) on each blot. The average fold change is presented for 3 experiments each. In Fig. 3, for all panels, the fold changes in the levels of phosphorylation or total HSP27 were calculated by normalizing to the first 6 h (arbitrarily designated as 1) in each blot. The average fold change is presented for n = 3 for phosphorylation-specific experiments and n = 9 for total HSP27. In Fig. 4, gene-specific effects over time are illustrated as smooth line plots of the fold changes in phosphorylation-specific and total HSP27 expression at matched 6, 24, and 48 h. The average fold change is presented for n = 3 for phosphorylation-specific experiments and n = 9 for total HSP27. In Fig. 5, the ratio of phosphoprotein to total protein (pHSP27/HSP27) is calculated as the ratio of the actin-normalized values. The average fold changes for each phosphoserine/total HSP27 are presented for 3 experiments.
Fig. 2.
Quantitation of SPARC-, PTEN-, and SPARC and PTEN–induced effects on pAkt, pP38 MAPK, or pMAPKAPK2 activation. Ratios of pAkt/total Akt, ratios of pP38 MAPK/total P38 MAPK, fold changes in pThr222 MAPKAPK2 and fold changes in MAPKAPK2 (doublet) due to SPARC (A), PTEN (B), PTEN in the presence of SPARC (C), and SPARC in the presence of PTEN (D) were measured. Error bars denote 1 SD. Red asterisks indicate significant differences between clones at matched time points. Average data for 3 experiments. FC, fold change.
Fig. 3.
Quantitation of SPARC-, PTEN-, and SPARC and PTEN–induced effects on HSP27 phosphorylation and expression over time. Average fold changes in phosphorylation-specific or total HSP27 due to SPARC (A) PTEN (B), PTEN in the presence of SPARC (C), and SPARC in the presence of PTEN (D) were measured. Error bars denote 1 standard deviation. Red asterisks indicate significant differences between clones at matched time points. Average data for n = 3 for phosphorylation-specific experiments and n = 9 for total HSP27.
Fig. 4.
Gene-specific effects plotted over time. Smooth line plots of the fold changes in phosphorylation-specific and total HSP27 expression at matched 6, 24, and 48 h due to SPARC (A), PTEN (B), PTEN in the presence of SPARC (C), and SPARC in the presence of PTEN (D). Average data for n = 3 for phosphorylation-specific experiments and n = 9 for total HSP27.
Fig. 5.
SPARC and PTEN effects on the levels of pHSP27 (Ser15 [green], Ser78 [red], Ser82 [blue]) relative to total HSP27. Stacked fold changes in phosphorylation-specific relative to total HSP27 due to SPARC (A), PTEN (B), PTEN in the presence of SPARC (C), and SPARC in the presence of PTEN (D) were measured. Red asterisks indicate significant differences between time points for Ser78. No significant differences were detected for Ser15 and Ser82. Average ratios for 3 experiments.
Statistical Analysis
We used t-tests to assess mean differences in fold change (log2) from the reference level of 0 (log2 [1]) (1-sample t-test) and between time points (2-sample t-test) between clones (see Figs 2, 3, and 5). All comparisons were considered significant at P < .05.
Results
Confirmation of SPARC and PTEN Expression in U87-Transfected Cells
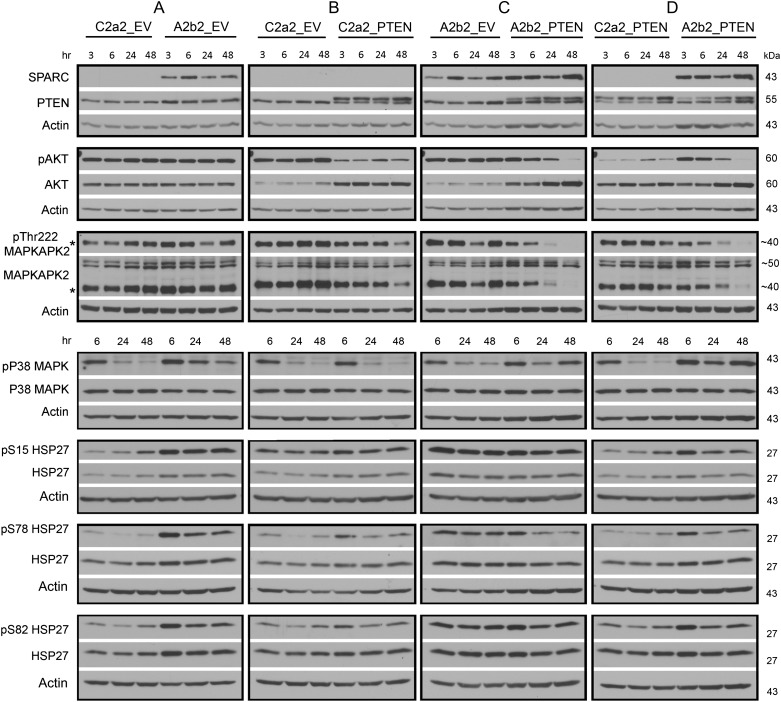
Generation and characterization of A2b2 (SPARC-expressing) and C2a2 (empty vector control–expressing) cells have previously been reported.15,16,19 These U87 malignant glioma (MG)–derived cell lines were transfected with a PTEN-encoding pcDNA6 vector (PTEN) or pcDNA6 empty vector (EV), and blasticidin-resistant stable clones were selected, as previously reported.17 SPARC and PTEN expression were confirmed by Western blot analysis (Fig. 1). PTEN expression is indicated by the higher molecular weight band. The lower molecular weight PTEN band is the mutant form of PTEN expressed by U87MG cells, which contain a deletion that includes exon 31.33,34 To determine the effects of PTEN expression on SPARC-induced signaling, cells were plated on fibronectin for 3, 6, 24, and 48 h and then lysed, and protein expression was assessed using Western blot analyses (Fig. 1).
Fig. 1.
Effects of SPARC and/or PTEN on Akt, MAPKAPK2, P38 MAPK, and HSP27 phosphorylation over time. Equal numbers of cells were plated on fibronectin for 3, 6, 24, or 48 h. Lysates were analyzed by Western blot for SPARC and PTEN to ensure appropriate expression, and for their effects on phosphorylated and total Akt, MAPKAPK2, P38 MAPK, and HSP27 expression, as indicated. For PTEN: the higher band is the transfected wild-type PTEN. The lower band is the endogenous mutant PTEN. *The Thr222 phosphorylated MAPKAPK2 is detected by both the phosphorylation-specific and total MAPKAPK2 antibodies. Molecular weights (in kDa) are shown to the right of each blot. Representative image for 3 experiments.
SPARC Increases pTyr180/Tyr182 P38 MAPK, pMAPKAPK2, pSer15, and pSer78 HSP27 Phosphorylation, Increases HSP27, and Increases pSer78 HSP27/Total HSP27 Ratio
We previously reported that SPARC induces the pP38 MAPK–HSP27 signaling pathway.16 The involvement of MAPKAPK2 was inferred, as the inhibitor of P38 MAPK used in that study inhibited the ability of P38 to activate MAPKAPK2, a kinase that directly phosphorylates HSP27. Here we further investigate this signaling pathway induced by SPARC over time and specifically examine MAPKAPK2 expression (Fig. 1). We show that compared with control cells, SPARC increases pP38 MAPK at 24 and 48 h (Fig. 2A). Because activated P38 MAPK promotes phosphorylation of MAPKAPK2 at Thr222, we assessed the effects of SPARC on pThr222 MAPKAPK2 and total MAPKAPK2. We found that SPARC did not significantly increase phosphorylation at Thr222 (Fig. 2A). Using an antibody that detects total MAPKAPK2, we found that this antibody detects a higher molecular weight doublet as well as the Thr222 MAPKAPK2 (in Fig. 1, the lower band in the MAPKAPK2 panel is the same shown for the specific anti-Thr222 MAPKAPK2 species). As the doublet signal detected by the total MAPKAPK2 antibody was a higher molecular weight than Thr222 MAPKAPK2, we conclude that all the MAPKAPK2 is phosphorylated. This means that SPARC induces phosphorylation at a different residue(s) of MAPKAPK2 (Fig. 2A). Further investigation will define the sites, but induction is early at 3 and 6 h and remains high. The earlier and sustained elevation of pMAPKAPK2 suggests that activation by pP38 MAPK may occur at the later time points, although we cannot exclude regulation at 3 h.
We further investigated SPARC effects on HSP27 phosphorylation. In agreement with previous results, representative Western blots show that SPARC increases total HSP27 and phosphorylation of HSP27 at all 3 serines (Fig. 1). Densitometric analyses (Fig. 3A) reveal statistically significant increases in pSer15 at 6 h, in pSer78 at 6, 24, and 48 h, and in total HSP27 at 6 and 24 h, with levels remaining elevated at 48 h. Figure 4A shows the fold changes in phosphorylated and total HSP27 induced by SPARC relative to control levels at the specific times are illustrated in Figure 4A. Although total HSP27 is also elevated by SPARC as early as 6 h (Fig. 3A), the levels of pSer78 relative to total HSP27 are elevated by SPARC at 24 and 48 h (Fig. 5A).
PTEN Suppression of SPARC-Induced pSer78 HSP27 Phosphorylation Occurs Downstream of P38 MAPK and Is Accompanied by Suppressed pAkt and pMAPKAPK2
Densitometric analyses (Fig. 2B–D) demonstrate that, as expected, PTEN suppressed the phosphorylation of Akt in the absence (Fig. 2B) or presence (Fig. 2C) of SPARC. Interestingly, pAkt was suppressed by PTEN earlier, by 3 h, in the control cells (Fig. 2B) but only by 24 and 48 h in the SPARC-expressing cells (Fig. 2C).
PTEN had no effect on pP38 MAPK in control cells (Fig. 2B) and did not suppress pP38 MAPK in the SPARC-expressing cells (Fig. 2C). Looking downstream of P38 MAPK, we found that PTEN increased the MAPKAPK2 doublet at 3 h, and levels remained high (Fig. 2B), but PTEN had no effect in the presence of SPARC (Fig. 2C). Comparing the effects of SPARC in the absence of PTEN (Fig. 2A) versus the presence of PTEN (Fig. 2D), we found that SPARC in the presence of PTEN could not induce the doublet activity as efficiently as in the absence of PTEN. Interestingly, PTEN significantly reduced the phosphorylation at Thr222 MAPKAPK2, specifically in the presence of SPARC (Fig. 2C). The inability of SPARC to increase the pMAPKAPK2 doublet in the presence of PTEN (Fig. 2D) to levels seen in the absence of PTEN (Fig. 2A) corresponds to the reduction in pAkt to control levels by 48 h and the suppression of pThr222 MAPKAPK2 by 48 h (Fig. 2D).
Compared with SPARC, PTEN differentially regulated HSP27 phosphorylation and expression (Fig. 1). Densitometric analyses (Fig. 3B) show that PTEN did not significantly increase pSer15 HSP27 or total HSP27 but did increase phosphorylation of HSP27 at Ser78 and Ser82, although only at 6 h (Fig. 3B). However, the fold change in pSer78 HSP27 induced by PTEN at 6 h (Fig. 4B) was less than that induced by SPARC (Fig. 4A). Surprisingly, the presence of PTEN had no effect in the presence of SPARC (Figs 3C and 4C). A comparison of SPARC effects in the absence of PTEN (Figs 3A and 4A) versus in the presence of PTEN (Figs 3D and 4D) indicates that PTEN suppresses the ability of SPARC to induce phosphorylation on Ser15 and Ser78. Because SPARC increased total HSP27 and PTEN did not, we examined the effects of SPARC versus PTEN expression on the ratios of pHSP27 to total HSP27 (Fig. 5). The results indicate that SPARC upregulates pSer78 HSP27 relative to total HSP27 in the absence of PTEN (Fig. 5A), but PTEN limits the ability of SPARC to increase this phosphorylation (Fig. 5D).
Discussion
We have previously demonstrated that SPARC promotes migration, in part, via the P38 MAPK–HSP27 signaling pathway.16 We also demonstrated that reconstitution of PTEN expression in SPARC-expressing cells prevents SPARC-induced migration in vitro and invasion in vivo.17 This PTEN-induced inhibition of migration was accompanied by suppression of Akt and Shc-Raf-ERK signaling.17 As PTEN is capable of completely suppressing SPARC-induced migration in vitro and in vivo in our model, we hypothesized that PTEN must also negatively affect SPARC–P38 MAPK–HSP27 signaling.
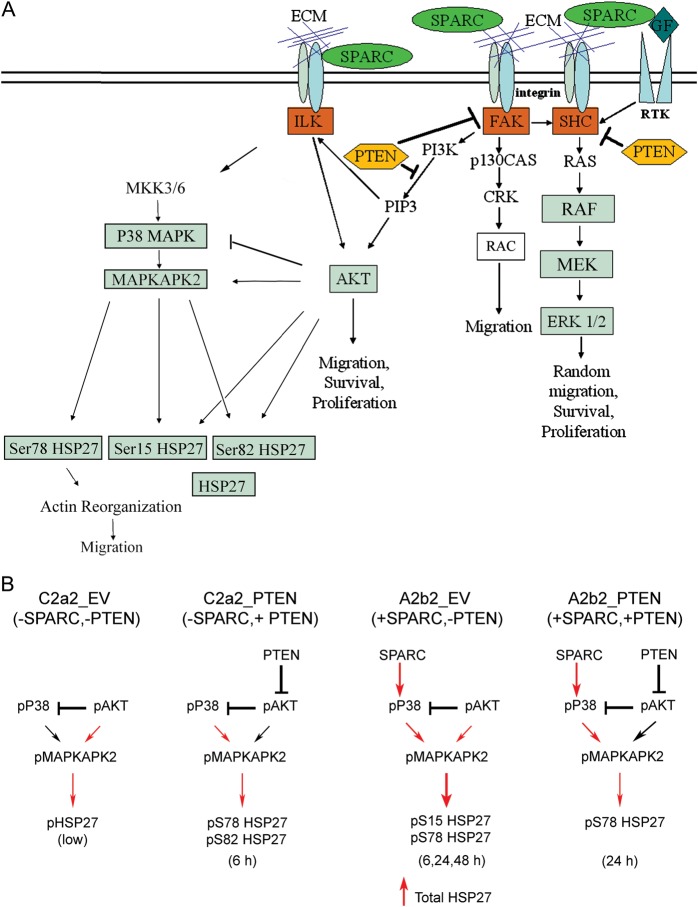
Figure 6A illustrates our proposed SPARC-mediated signaling pathways based on our previous reports, and we now add the proposed signaling whereby PTEN can suppress SPARC-induced signaling through HSP27. Figure 6B summarizes the proposed signaling based on the present report. We propose that in control cells (C2a2_EV), pAkt suppresses pP38 MAPK, and baseline levels of pAkt maintain baseline levels of pMAPKAPK2, which in turn maintain low levels of pHSP27. The effect of PTEN (C2a2_PTEN) is to suppress pAkt. This suppression releases the negative inhibition of P38 MAPK, to induce a transient increase in the pMAPKAPK2 doublet at 3 h and pSer78 and Ser82 HSP27. SPARC (A2b2_EV) promoted the upregulation of pP38 MAPK that overcomes pAkt inhibition of P38 MAPK. SPARC expression leads to a transient increase in pSer15 and sustained pSer78 HSP27. PTEN expression in SPARC-expressing cells (A2b2_PTEN) suppresses pAkt. This suppression acts downstream of SPARC-induced pP38 MAPK and greatly suppresses pMAPKAKP2 by 48 h, leading to decreased Ser78 HSP27. In the presence of SPARC, the changes in Ser78 phosphorylation relative to total HSP27 lead to sustained pSer78 HSP27. We therefore propose that SPARC-induced phosphorylation on Ser78 of HSP27 prevents unphosphorylated HSP27 from acting as an actin capping protein to prevent actin polymerization and migration and that PTEN-induced suppression of pAkt and pMAPKAPK2 thwarts SPARC-induced signaling to prevent migration.
Fig. 6.
Proposed signaling mechanism for SPARC ± PTEN. (A) Summary of SPARC-mediated signaling pathways that impact glioma migration. We have previously demonstrated that SPARC induces glioma migration. This migration is accompanied by increases in pP38 MAPK and pHSP27 and can be suppressed with the inhibition of HSP27 expression.16 In addition, we have demonstrated that PTEN suppresses pAkt, and together with SPARC, suppresses the Raf-MEK-ERK signaling pathway.17 PTEN also inhibits SPARC-induced migration.17 Data presented in this manuscript show that PTEN suppresses pAkt, which correlates with suppression of pMAPKAPK2, which in turn limits the increase in phosphorylation of Ser78 relative to total HSP27. This decrease in pSer78 HSP27/total HSP27 correlates with suppressed migration. (B) Proposed mechanisms for SPARC and PTEN regulation of HSP27 expression and phosphorylation. In the absence of SPARC and PTEN (C2a2_EV), pAkt inhibits P38 MAPK but activates a low level of pHSP27 via direct activation of pMAPKAPK2. In the absence of SPARC, PTEN (C2a2_PTEN) inhibits Akt activation, thereby suppressing the inhibition of pP38 MAPK, which activates MAPKAPK2 to phosphorylate Ser78 and Ser82 of HSP27, but only at 6 h. In the absence of PTEN, SPARC (A2b2_EV) upregulates pP38 MAPK, which activates MAPKAPK2 to enhance Ser15 HSP27 at 6 h and sustained Ser78 HSP27 at 6, 24, and 48 h. SPARC also increases total HSP27 expression. PTEN in the presence of SPARC (A2b2_PTEN) suppresses pAkt, which limits the activation of SPARC-induced MAPKAPK2, thereby suppressing SPARC-induced pHSP27. We propose that the overall increase in pSer78 HSP27 relative to total HSP27 in SPARC-expressing cells promotes uncapping of unphosphorylated HSP27 from actin filaments to promote actin polymerization that promotes migration, whereas PTEN suppresses these SPARC-induced changes. Red arrows: positive signaling; black arrows: suppressed signaling. Abbreviation: ECM, extracellular matrix.
SPARC is a secreted protein that regulates many cellular functions, including cell adhesion, migration, survival, and proliferation, by mediating signaling mechanisms induced by the integrins and growth factor receptors stimulated by extracellular matrix and growth factors present in the microenvironment.10,11 It is interesting to note that many of the SPARC-induced signaling mechanisms are pathways that are negatively regulated by PTEN.17 As SPARC is increased in the majority of GBM,12,40 and PTEN mutation and loss or promoter methylation occurs frequently in primary GBM,35,36,41,42 it is expected that SPARC-induced effects would be greater in PTEN-null gliomas. These studies suggest that targeting SPARC-induced signaling pathways that are negatively regulated by PTEN is a promising strategy to suppress glioma invasion.
In addition, unphosphorylated P38 MAPK, MAPKAPK2, and HSP27 exist in a complex along with Akt.30 The stimulation of P38 MAPK activates MAPKAPK2, which can phosphorylate HSP27.43 HSP27 binding to Akt facilitates MAPKAPK2 activation of Akt, which in turn can also phosphorylate HSP27,30 which disrupts the complex to release pHSP27. Activated Akt can also provide negative feedback regulation of P38 MAPK.44–46 Therefore, the strength of signal activation through P38 MAPK versus Akt may dictate the effects of MAPKAPK2 activation.
Although the exact mechanism remains to be elucidated, it is proposed that both unphosphorylated and phosphorylated HSP27 bind to actin filaments but that unphosphorylated HSP27 stabilizes the filaments by binding to the actin filament47 and/or acting as an actin capping protein,31 whereas pHSP27 induces actin filament reorganization, which promotes migration.30 Therefore, signals that promote P38 MAPK and Akt activation may influence migration by their actions on HSP27. We and others have demonstrated that SPARC increases pAkt under stress conditions,20,48 and we have demonstrated that SPARC upregulates pP38 MAPK signaling.16 We have proposed that SPARC induces these changes by binding to β1 integrin and the activation of ILK,21 which in turn is sufficient to induce pAkt25,26 and pP38 MAPK activation.49 PTEN can inhibit the activation of both ILK and Akt by suppressing PI3K activation, suggesting at least 2 pathways whereby PTEN could suppress SPARC-induced pHSP27. Our results show that reconstitution of PTEN into SPARC-expressing U87 cells negatively impacts both MAPKAPK2 and Akt signaling and alters the ratio of phosphorylated to total HSP27. Future experiments are needed to determine the role of ILK signaling in regulating these changes.
In addition, we show that it is not phosphorylation levels alone that are important but also the level of phosphorylation relative to total HSP27. We show that PTEN does not inhibit HSP27 phosphorylation at all serines, but rather also influences its function by inhibiting or preventing the increase in total HSP27 induced by SPARC. We propose that by altering the ratio of phosphorylated to total HSP27, PTEN promotes the likelihood that unphosphorylated HSP27 binds to the actin filament to stabilize it and thereby inhibits the binding of phosphorylated HSP27 to promote actin filament reorganization to facilitate migration. We have shown that SPARC increases HSP27 expression by increasing HSP27 transcript abundance16 and stabilizing HSP27 protein.18 However, further studies are necessary to determine the mechanism whereby PTEN prevents increased HSP27 protein levels.
HSP27 is a multifunctional protein.28 Its function in regulating migration is dictated by its phosphorylation status, but it is not clear whether the timing of phosphorylation, the number of phosphorylated sites, or the combinations of phosphorylated sites play a role in the execution of its specific functions. In addition, different kinases induce serine-specific phosphorylation.32 For example, in leukemia cells, apigenin treatment induces an early response (15 min) in smooth muscle cells, eliciting phosphorylation of Ser78 and Ser82, which is followed by a late response (6 h) with phosphorylation of Ser15.50 In smooth muscle cells, unphosphorylated HSP27 acts as an actin capping protein to stabilize actin filaments, and stimulation of HSP27 phosphorylation at Ser82 followed by phosphorylation at Ser15 promotes actin polymerization.31 Other studies suggest that the order of phosphorylation is not crucial.51 However, in breast cancer, specific Ser78 phosphorylation correlates with tumor progression.52 In addition, STAT3, the signal transducer and activator of transcription 3 factor, can physically interact with HSP27 and enhance expression and phosphorylation of Ser78.53 In our study, we see an early (6 h) Ser15 phosphorylation, followed by a late (24 and 48 h) phosphorylation of Ser78 of HSP27 in response to SPARC expression. Our studies would suggest that the timing and specificity of phosphorylation relative to total HSP27 levels are important in dictating its migration-inducing function.
In conclusion, we propose that PTEN inhibits SPARC-induced increases in Ser78 HSP27 phosphorylation relative to total HSP27. These data describe a novel mechanism whereby PTEN inhibits SPARC-induced migration through suppression and differential regulation of the P38 MAPK–MAPKAPK2-HSP27 pathway. These results reinforce the targeting of HSP27 as therapeutic in the inhibition of SPARC-induced glioma invasion, especially for patients having PTEN mutant tumors.
Funding
This research was supported by the National Institutes of Health/National Cancer Institute (grant no. R01CA86997 to S. A. R) and Wayne State University (two Undergraduate Research and Creative Projects Awards to R. A.). The authors are grateful to the Barbara Jane Levy family for their continued support.
Acknowledgments
We thank Drs Frank Furnari and Webster Cavenee for the pBP-PTEN plasmid and thank Dr Stacey Thomas for critical review of the manuscript.
Conflict of interest statement. The authors have no conflicts of interest to declare.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 5.Polley MY, Lamborn KR, Chang SM, Butowski N, Clarke JL, Prados M. Six-month progression-free survival as an alternative primary efficacy endpoint to overall survival in newly diagnosed glioblastoma patients receiving temozolomide. Neuro Oncol. 2010;3:274–382. doi: 10.1093/neuonc/nop034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helseth R, Helseth E, Johannesen TB, et al. Overall survival, prognostic factors, and repeated surgery in a consecutive series of 516 patients with glioblastoma multiforme. Acta Neurol Scand. 2010;122:159–167. doi: 10.1111/j.1600-0404.2010.01350.x. [DOI] [PubMed] [Google Scholar]
- 7.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 8.Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107:1049–1054. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark CJ, Sage EH. A prototypic matricellular protein in the tumor microenvironment—where there's SPARC, there's fire. J Cell Biochem. 2008;104:721–732. doi: 10.1002/jcb.21688. [DOI] [PubMed] [Google Scholar]
- 10.Arnold SA, Brekken RA. SPARC: a matricellular regulator of tumorigenesis. J Cell Commun Signal. 2009;3–4:255–273. doi: 10.1007/s12079-009-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas S, Rempel SA. The role of SPARC in tumor-stromal interactions. In: Mueller MM, Fusenig NE, editors. Vol 4. New York, NY:: Springer; 2011. pp. 301–346. Tumor-Associated Fibroblasts and Their Matrix, series The Tumor Microenvironment. [Google Scholar]
- 12.Rempel SA, Golembieski WA, Ge S, et al. SPARC: a signal of astrocytic neoplastic transformation and reactive response in human primary and xenograft gliomas. J Neuropathol Exp Neurol. 1998;57:1112–1121. doi: 10.1097/00005072-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Rempel SA, Golembieski WA, Fisher JL, et al. SPARC modulates cell growth, attachment and migration of U87 glioma cells on brain extracellular matrix proteins. J Neurooncol. 2001;53:149–160. doi: 10.1023/a:1012201300188. [DOI] [PubMed] [Google Scholar]
- 14.Vadlamuri SV, Media J, Sankey SS, Nakeff A, Divine G, Rempel SA. SPARC affects glioma cell growth differently when grown on brain ECM proteins in vitro under standard versus reduced-serum stress conditions. Neuro Oncol. 2003;54:244–254. doi: 10.1093/neuonc/5.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultz C, Lemke N, Ge S, et al. Secreted protein acidic and rich in cysteine promotes glioma invasion and delays tumor growth in vivo. Cancer Res. 2002;62:6270–6277. [PubMed] [Google Scholar]
- 16.Golembieski WA, Thomas SL, Schultz CR, et al. HSP27 mediates SPARC-induced changes in glioma morphology, migration, and invasion. Glia. 2008;56:1061–1075. doi: 10.1002/glia.20679. [DOI] [PubMed] [Google Scholar]
- 17.Thomas SL, Alam R, Lemke N, Schultz LR, Gutiérrez JA, Rempel SA. PTEN augments SPARC suppression of proliferation and inhibits SPARC-induced migration by suppressing SHC-RAF-ERK and AKT signaling. Neuro Oncol. 2010;12:941–955. doi: 10.1093/neuonc/noq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClung HM, Golembieski W, Schultz CR, Rempel SA. Deletion of the SPARC acidic domain or EGF-like module reduces SPARC-induced migration and signaling through p38 MAPK/HSP27 in glioma. Carcinogenesis. 2012;33:275–84. doi: 10.1093/carcin/bgr276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golembieski WA, Ge S, Nelson K, et al. Increased SPARC expression promotes U87 glioblastoma invasion in vitro. Int J Dev Neurosci. 1999;17:463–472. doi: 10.1016/s0736-5748(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 20.Schultz CR, Golembieski WA, King DA, Brown SL, Brodie C, Rempel SA. Inhibition of HSP27 alone, or in combination with pAKT inhibition as therapeutic approaches to target SPARC-induced glioma survival. Mol. Cancer. 2012;11:20. doi: 10.1186/1476-4598-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barker TH, Baneyx G, Cardo-Vila M, et al. SPARC regulates extracellular matrix organization through its modulation of integrin-linked kinase activity. J Biol Chem. 2005;280:36483–36493. doi: 10.1074/jbc.M504663200. [DOI] [PubMed] [Google Scholar]
- 22.Nie J, Chang B, Traktuev DO, et al. IFATS collection: combinatorial peptides identify alpha5beta1 integrin as a receptor for the matricellular protein SPARC on adipose stromal cells. Stem Cells. 2008;26:2735–2745. doi: 10.1634/stemcells.2008-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weaver MS, Workman G, Sage EH. The copper binding domain of SPARC mediates cell survival in vitro via interaction with integrin beta1 and activation of integrin-linked kinase. J Biol Chem. 2008;283:22826–22837. doi: 10.1074/jbc.M706563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Q, Bao S, Song L, et al. Targeting SPARC expression decreases glioma cellular survival and invasion associated with reduced activities of FAK and ILK kinases. Oncogene. 2007;26:4084–4094. doi: 10.1038/sj.onc.1210181. [DOI] [PubMed] [Google Scholar]
- 25.Hannigan GE, McDonald PC, Walsh MP, Dedhar S. Integrin-linked kinase: not so ‘pseudo’ after all. Oncogene. 2011;30:4375–4385. doi: 10.1038/onc.2011.177. [DOI] [PubMed] [Google Scholar]
- 26.McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase—essential roles in physiology and cancer biology. J Cell Sci. 2008;121:3121–3132. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- 27.Natarajan M, Hecker TP, Gladson CL. FAK signaling in anaplastic astrocytoma and glioblastoma tumors. Cancer J. 2003;9:126–33. doi: 10.1097/00130404-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 29.Arya R, Mallik M, Lakhotia SC. Heat shock genes—integrating cell survival and death. J Biosci. 2007;32:595–610. doi: 10.1007/s12038-007-0059-3. [DOI] [PubMed] [Google Scholar]
- 30.Zheng C, Lin Z, Zhao ZJ, Yang Y, Niu H, Shen X. MAPK-activated protein kinase-2 (MK2)–mediated formation and phosphorylation-regulated dissociation of the signal complex consisting of p38, MK2, Akt, and Hsp27. J Biol Chem. 2006;281:37215–3726. doi: 10.1074/jbc.M603622200. [DOI] [PubMed] [Google Scholar]
- 31.McGregor E, Kempster L, Wait R, Gosling M, Dunn MJ, Powell JT. F-actin capping (CapZ) and other contractile saphenous vein smooth muscle proteins are altered by hemodynamic stress: a proteonomic approach. Mol Cell Proteomics. 2004;3:115–124. doi: 10.1074/mcp.M300046-MCP200. [DOI] [PubMed] [Google Scholar]
- 32.Kostenko S, Moens U. Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell Mol Life Sci. 2009;66:3289–3307. doi: 10.1007/s00018-009-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Yen C, Liaw D, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 34.Steck PA, Pershouse MA, Jasser SA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 35.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morimoto AM, Tomlinson MG, Nakatani K, Bolen JB, Roth RA, Herbst R. The MMAC1 tumor suppressor phosphatase inhibits phospholipase C and integrin-linked kinase activity. Oncogene. 2000;19:200–209. doi: 10.1038/sj.onc.1203288. [DOI] [PubMed] [Google Scholar]
- 38.Attwell S, Mills J, Troussard A, Wu C, Dedhar S. Integration of cell attachment, cytoskeletal localization, and signaling by integrin-linked kinase (ILK), CH-ILKBP, and the tumor suppressor PTEN. Mol Biol Cell. 2003;14:4813–4825. doi: 10.1091/mbc.E03-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao L, Yang YB, Li XM, Xu CF, Li T, Wang XY. Differential sensitivity of human endometrial carcinoma cells with different PTEN expression to mitogen-activated protein kinase signaling inhibits and implications for therapy. J Cancer Res Clin Oncol. 2010;136:1089–1099. doi: 10.1007/s00432-009-0756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Capper D, Mittelbronn M, Goeppert B, Meyermann R, Schittenhelm J. Secreted protein, acidic and rich in cysteine (SPARC) expression in astrocytic tumour cells negatively correlates with proliferation, while vascular SPARC expression is associated with patient survival. Neuropathol Appl Neurobiol. 2010;36:183–197. doi: 10.1111/j.1365-2990.2010.01072.x. [DOI] [PubMed] [Google Scholar]
- 41.Ermoian RP, Furniss CS, Lamborn KR, et al. Dysregulation of PTEN and protein kinase B is associated with glioma histology and patient survival. Clin Cancer Res. 2002;8:1100–1106. [PubMed] [Google Scholar]
- 42.Wiencke JK, Zheng S, Jelluma N, et al. Methylation of the PTEN promoter defines low-grade gliomas and secondary glioblastoma. Neuro Oncol. 2007;9:271–279. doi: 10.1215/15228517-2007-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Gratton JP, Morales-Ruiz M, Kureishi Y, Fulton D, Walsh K, Sessa WC. Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. J Biol Chem. 2001;276:30359–65. doi: 10.1074/jbc.M009698200. [DOI] [PubMed] [Google Scholar]
- 45.Widenmaier SB, Ao Z, Kim SJ, Warnock G, McIntosh CH. Suppression of p38 MAPK and JNK via Akt-mediated inhibition of apoptosis signal-regulating kinase 1 constitutes a core component of the beta-cell pro-survival effects of glucose-dependent insulinotropic polypeptide. J Biol Chem. 2009;284:30372–82. doi: 10.1074/jbc.M109.060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rane MJ, Song Y, Jin S, et al. Interplay between Akt and p38 MAPK pathways in the regulation of renal tubular cell apoptosis associated with diabetic nephropathy. Am J Physiol Renal Physiol. 2010;298:F49–61. doi: 10.1152/ajprenal.00032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graceffa P. Hsp27-actin interaction. Biochem Res Int. 2011;2011:901572. doi: 10.1155/2011/901572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi Q, Bao S, Maxwell JA, et al. Secreted protein acidic, rich in cysteine (SPARC), mediates cellular survival of gliomas through AKT activation. J Biol Chem. 2004;279:52200–52209. doi: 10.1074/jbc.M409630200. [DOI] [PubMed] [Google Scholar]
- 49.Ishii T, Satoh E, Nishimura M. Integrin-linked kinase controls neurite outgrowth in N1E-115 neuroblastoma cells. J Biol Chem. 2001;276:42994–43003. doi: 10.1074/jbc.M105198200. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez-Mejia ME, Voss OH, Murnan EJ, Doseff AI. Apigenin-induced apoptosis of leukemia cells is mediated by a bimodal and differentially regulated residue-specific phosphorylation of heat-shock protein-27. Cell Death Dis. 2010;1:e64. doi: 10.1038/cddis.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landry J, Lambert H, Zhou M, et al. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem. 1992;267:794–803. [PubMed] [Google Scholar]
- 52.Zhang D, Wong LL, Koay ES. Phosphorylation of Ser78 of Hsp27 correlated with HER-2/neu status and lymph node positivity in breast cancer. Mol Cancer. 2007;6:52. doi: 10.1186/1476-4598-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song H, Ethier SP, Dziubinski ML, Lin J. Stat3 modulates heat shock 27kDa protein expression in breast epithelial cells. Biochem Biophys Res Commun. 2004;314:143–150. doi: 10.1016/j.bbrc.2003.12.048. [DOI] [PubMed] [Google Scholar]