Abstract
Astrocytic tumors account for 42% of childhood brain tumors, arising in all anatomical regions and associated with neurofibromatosis type 1 (NF1) in 15%. Anatomical site determines the degree and risk of resectability; the more complete resection, the better the survival rates. New biological markers and modern radiotherapy techniques are altering the risk assessments of clinical decisions for tumor resection and biopsy. The increasingly distinct pediatric neuro-oncology multidisciplinary team (PNMDT) is developing a distinct evidence base.
A multidisciplinary consensus conference on pediatric neurosurgery was held in February 2011, where 92 invited participants reviewed evidence for clinical management of hypothalamic chiasmatic glioma (HCLGG), diffuse intrinsic pontine glioma (DIPG), and high-grade glioma (HGG). Twenty-seven statements were drafted and subjected to online Delphi consensus voting by participants, seeking >70% agreement from >60% of respondents; where <70% consensus occurred, the statement was modified and resubmitted for voting.
Twenty-seven statements meeting consensus criteria are reported. For HCLGG, statements describing overall therapeutic purpose and indications for biopsy, observation, or treatment aimed at limiting the risk of visual damage and the need for on-going clinical trials were made. Primary surgical resection was not recommended. For DIPG, biopsy was recommended to ascertain biological characteristics to enhance understanding and targeting of treatments, especially in clinical trials. For HGG, biopsy is essential, the World Health Organization classification was recommended; selection of surgical strategy to achieve gross total resection in a single or multistep process should be discussed with the PNMDT and integrated with trials based drug strategies for adjuvant therapies.
Keywords: low-grade astrocytoma
Astrocytic tumors account for 42.3% of brain tumors in the childhood age range.1 The overwhelming majority are low-grade pilocytic histology (grade1), with <7% occurring as fibrillary (grade 2) tumors and 20% as high-grade tumors (malignant gliomas and diffuse intrinsic pontine glioma); oligodendroglial tumors are very rare. The association between neurofibromatosis type 1 (NF1), particularly in low-grade astrocytoma (15%), and the impact of this genetic predisposition on the clinical presentation, natural history, and response to treatment are major factors in determining treatment approaches with respect to surgery, radiotherapy, and selection of drugs.
The low-grade tumors characteristically are clustered anatomically to midline supratentorial structures in 39%, including hypothalamus, optic chiasm and nerves, ventricular system, and other midline structures; the cerebellum in 32%; the cerebral cortex in 13.1%; the brainstem in 10%; and the spinal cord in 4.5%.2 Anatomical distribution of highgrade tumors is markedly different with cortical tumors, accounting for 63%, midline supratentorial structures for 28%, and brainstem for 11%.3 Anatomical site determines resectability, which, in turn, when complete, is associated with better survival outcomes in both low-grade and high-grade tumors. Brainstem tumors are now considered to be safe targets for either stereotactic or open biopsy, with modern techniques in specialist pediatric centers. Refinement of surgical approaches to the hypothalamic tumors has highlighted the potential for surgical resection in low-grade tumors in selected cases.
International clinical trials have highlighted the importance of age as a major factor interacting with tumor behavior (progression risk) in low-grade tumors and tumor biology in high-grade tumors. Recent research identifying new biological markers of tumor classification and new druggable targets for trials has provided an opportunity to explore novel approaches to therapy. Modern radiotherapy techniques, with their enhanced capacity to limit the volume of normal brain irradiated, are reducing the risk of radiation brain injury. The experience of applying this new knowledge and refined treatment techniques is altering clinical risk assessments surrounding decisions regarding tumor resection and biopsy. Multidisciplinary pediatric neuro-oncology teams are becoming more clearly distinguished from the adult neuro-oncology teams as the evidence base for practice in childhood develops further.
For these reasons, author C.S.R. initiated an international consensus meeting to review current evidence, underpinning surgical practice in these tumors. The meeting involved 92 selected clinical and scientific specialists. The aim was to review the evidence for changes in surgical practice in astrocytomas of childhood and to establish a new consensus on surgical approaches to these tumors when managed within the context of a pediatric multidisciplinary neuro-oncology team.
Methods
Consensus Process
The second Consensus Conference on Pediatric Neurosurgery was held in Paris, France, on 11–12 February 2011 (CPN2011). During the meeting, 92 participants reviewed the evidence for clinical management and outcomes in hypothalamic chiasmatic low-grade glioma (HCLGG), diffuse intrinsic pontine glioma (DIPG), and high-grade glioma (HGG) with the intention of informing consensus discussions with a designated panel that took place at the end of each session.
The panel subsequently drafted 27 statements based on the workshop discussion for consideration using the Delphi consensus methodology by the meeting's participants with use of e-mail for distribution and a web-based survey tool for responses. Participants were asked to rate each statement as “I don't support the statement,” “I would support the statement with modification,” or “I support the statement.” A comments section was also included for each statement to permit suggested modifications. A statement was accepted if >70% of votes were in support from >60% of the attendees in each round of voting.
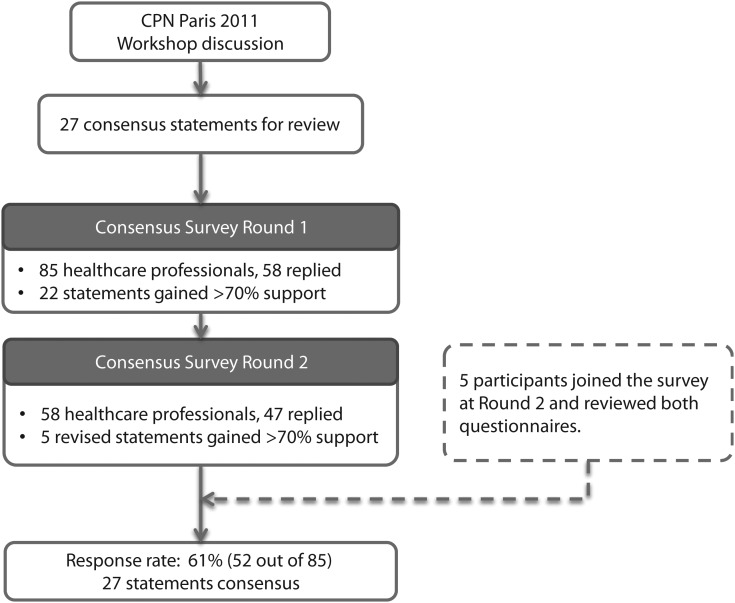
Fig. 1 shows the consensus process. The first questionnaire was sent on 03 March 2011. All 92 CPN2011 participants were invited via e-mail, but only 85 e-mail addresses were valid. Among those, 58 replied, for a response rate of ∼68%. Twenty-two of 27 questions achieved >70% support. The 5 unsuccessful statements were then revised after discussion with the consensus of panel members, and the invitation of the second round was sent on 18 May 2011. The response rate was 81% (47 of 58) in the second round, and all revised statements gained >70% support. Five participants who did not respond in the first round approached us in the second round and completed the questionnaires. They were asked to review the 22 statements from round 1 and the 5 revised statements in round 2 together, and their votes were included in the analysis (Fig. 1). A total of 52 health care professionals completed both rounds of the survey; the dropout rate was 38.8% overall. Final statements for the t3 types of pediatric brain tumors are discussed below.
Fig. 1.
Delphi consensus process.
Consensus Statements
HCLGG
- (1) Overall therapeutic purpose.
- The overall aim of therapy in childhood and adolescent HCLGG is to gain time by controlling tumor progression and to preserve function.
- Multidisciplinary discussion should be the method adopted for all patients' diagnostic assessments and clinical decision-making.
(2) Anatomical staging.
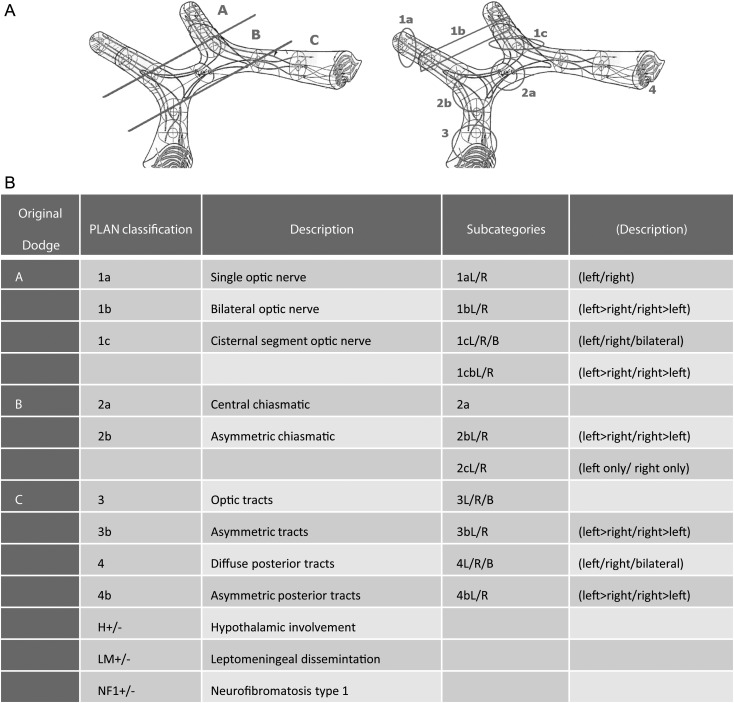
A revised anatomy-based staging system based on the current imaging techniques should be developed to assist standardized, surgical assessment of hypothalamic chiasmatic tumors that includes criteria to predict risk of severity of bilateral visual loss, surgical resectability, tumoral vascularity, tumor size, genetic status, and age at assessment. The modified Dodge PLAN classification was designed to predict visual risk.4 Development of a revision of this classification with additional surgical and clinical factors is proposed.
(3) Combined histopathological and biological/genetic classification.
Rapid developments in this field means that combined histopathological and biological assessment of tumors can be imminently anticipated as an essential requirement of future clinical trials.
- (4) Tumor biopsy: at diagnosis,
- Typical intrinsic optic pathway tumors in association with NF1 do not require biopsy at the time of diagnosis.
- For typical intrinsic optic pathway tumors in association with NF1, it is acceptable to perform a biopsy after multidisciplinary discussion by a specialist pediatric neuro-oncology team (including a pediatric neurosurgeon) before offering a clinical intervention when they are being managed in a clinical trial that involves a relevant biological stratification or question.
- (5) Tumor biopsy: before nonsurgical treatment in sporadic tumor
-
NF1-negative/sporadic:It is acceptable to perform a biopsy after multidisciplinary discussion by a specialist pediatric neuro-oncology team (including a pediatric neurosurgeon) before offering nonsurgical treatment (chemotherapy or biological therapy) in a clinical trial that involves a relevant biological stratification or question.
-
NF1-positive:At the planning of nonsurgical treatment (biological therapy, chemotherapy, or radiotherapy), multidisciplinary discussion should recommend biopsy of tumors with any atypical features and justify any decision not to biopsy such tumors at this stage.
-
- (6) Initiating nonsurgical treatment because of threat to vision at diagnosis:
- Threat to vision is high if there is clear evidence of visual deterioration in the history before diagnosis.
- Threat to vision is high if there is clinical evidence of bilateral vision loss, as judged by visual acuity or visual fields.
- Bilateral optic atrophy is evidence of bilateral visual loss but is also indicative of irreversible optic nerve damage.
- In a child with unilateral visual loss, as judged by reduced visual acuity, visual fields, or presence of optic atrophy, and in the absence of a clear history of visual deterioration, who can reliably cooperate with detailed vision testing, can be observed closely (every 1-2 months) for evidence of continued visual change before initiating nonsurgical treatment (chemotherapy andbiologic therapy).
- (7) Primary tumor resection/debulking:
- Attempted primary resection of HCLGG is not the current recommended standard of care.
- Multidisciplinary discussion identifies some patients with characteristics (e.g., cystic tumors, large tumors, and exophytic or hypothalamic tumors) when early surgical debulking is possible with acceptable risk. The proposed anatomical classification will be designed to assist with selection of these cases.
- In view of the risk or early postoperative tumor regrowth, close observation with 2 monthly scans for at least 6 months is recommended to select patients for follow-up nonsurgical therapy to control subsequent recurrence.
- (8) Clinical trials:
- The selection and testing of surgical and nonsurgical treatments requires an active international trials program, including patients with newly diagnosed cases and patients requiring second- and third-line or subsequent therapies.
These trials will specify criteria for selecting treatment modalities according to anatomical stage, genetic status, age, and symptomatology to increasingly standardize indications for treatment.
DIPG
(9) Typical DIPG:
Biopsy of a typical DIPG (defined by a short history and typical imaging findings) is justified when the patient is part of an ethically approved clinical study in which the tissue obtained will be used to investigate or inform the role of biological markers after treatment selection or molecular tumor grading.
- (10) Atypical pontine region tumors:
- Biopsy by an experienced pediatric neurosurgeon is indicated to confirm the diagnosis and guide therapy.
- An atypical pontine region tumor would be considered separately from classic DIPG for therapy or research purposes.
HGG
- (11) Surgical biopsy in HGG is necessary:
- to ensure comprehensive diagnosis,
- for ethically approved clinical and biological research, and
- to help understand why conventional therapies are effective in a subset of patients and are not in others.
- (12) Decision making:
- (a) A multidisciplinary team approach is favored for the management of hemispheric and thalamic tumors, suspicious of HGGs.
- (b) Multidisciplinary preoperative discussion would help determine whether gross total resection can be achieved without major morbidity.
- (c) If initial gross total resection cannot be achieved safely, a stepwise approach with initial biopsy or subtotal resection, followed by trials-based chemotherapy to improve resectability (reduce vascularity or tumor volume), then reconsideration of further surgery, is the preferred strategy.
(13) Pathology classification:
The World Health Organization neuropathology classification is the current international standard. Research aimed at exploring alternative neuropathology classification should be promoted.
Discussion
Previous reports of consensus meetings in the field are minimal,5,6 although the use of consensus methodologies for creating clinical guidelines is well described in the literature. This meeting was initiated to review evidence justifying a review of surgical practice in pediatric neuro-oncology by inviting established experts to review evidence and promote consensus discussions with the help of an expert panel. The draft statements, generated by discussion during the conference, were submitted for subsequent consensus voting and refinement by the conference participants with use of web-based questionnaires, under the supervision of the expert panel, who discussed results of voting and suggested amendments to statements after reviewing feedback.7–9 Preset consensus rate of 70% and response rate of >60% meant that agreement was measurable and representative.
This method for reaching consensus by structured presentation of evidence discussions, drafting of statements, and subsequent rounds of voting seeking consensus is an established method for clinical guideline development, which is based on a number of assumptions about decision-making in groups. It assumes that safety lies in numbers, as opposed to individual decision-making, because several people are less likely to arrive at the wrong conclusion. It lends authority to the product of the process, especially if the individuals are established experts in the field. The use of reasoned argument in a group promotes a situation in which assumptions are challenged and participants are forced to justify views, thereby enhancing rationality. The organization and structure of the process enhances control, and its formality enhances scientific credibility. It is for these reasons that the Delphi method was suggested and adopted for this process by the expert group and the participants consented to the subsequent voting process at the end of the conference discussions. The potential limitations are determined by the structure and organization of the process and the constitution of the participants and expert group. These aspects have been described in this article.
An attempt to seek additional consensus from members of a subsequent Société Internationale d'Oncologie Pediatrique (Europe) Brain Tumor Committee meeting group in Liverpool in 2011 did not generate sufficient response rate from participants for the votes to be included; furthermore, their lack of involvement of the majority in the discussions at the Paris Conference undermined their contribution as informed participants, despite their special interest status as SIOPE Brain Tumor Committee members.
The meeting was structured to consider the needs of HCLGG, DIPG, and HGG.
HCLGGs are subcategorized by their association with NF1 in up to 30%, their age at presentation, and their threat to vision or other neurological functions. These factors have been used to select patients, either for observation, attempted resection, or adjuvant nonsurgical therapy in clinical trials. The NF1-associated tumors are often multifocal, involving the optic nerve(s) chiasm and/or posterior optic radiations; their growth involves the optic nerves in the pathways intimately. They almost never present in persons <1 year of age. In contrast, sporadic HCLGGs frequently present during infancy (age, <1 year) with large tumors. Sporadic HCLGGs are commonly smaller when presenting later in childhood (age, >18 months). Their anatomical location threatens the visual pathway by compression and/or invasion and threatens other adjacent structures similarly. Their tendency to progress reduces as the children grow older. It is common for tumor growth to arrest later in childhood, leaving significant residual tumor. Life is threatened by rapidly growing, large tumors during infancy; hydrocephalus can be a complicating factor; and vision is threatened at all ages. They are chronically relapsing tumors with a tendency to burnout into a static state. It is for these reasons that the consensus states that the overall purpose is “to gain time by controlling tumor progression,” justifying clinical strategies aimed at minimizing harm and containing tumor progression. Such approaches have been established in recent trials. The consensus statements therefore recognize the need for a precautionary, multidisciplinary strategy directed at the overall therapeutic purpose (statements 1a and b).
Surgical consideration of risks for vision, endocrine functions, homeostasis, emotion, memory, and central neuronal connections must be considered. The anatomical relationships of the structures were considered in detail, as well as the association with NF1. A review of the consensus regarding selection of cases for biopsy, particularly within the framework of clinical trials, based on these factors and current techniques, was discussed and refined (statements 4a and b, 5a and b).
The detailed discussion of visual threat was informed primarily by a pediatric oncologist expert in the consent process with such patients. The professional group not represented was pediatric ophthalmology. Although this may be a weakness of the group's constitution in this area, the current clinical practice is that ophthalmologists assist with diagnosis and visual assessment but are not directly involved in nonsurgical treatment consent or sight-saving operative interventions directed at the tumor. Future consensus discussion in this area would be enhanced by involvement of pediatric ophthalmologists to inform the group on the interpretation of visual assessment and prediction of visual disability. The strong association with visual threat in HCLGG justified reappraisal of which cases were suitable for immediate intervention with nonsurgical treatments and which were considered to be suitable for careful observation and deferred treatments (statements 6a–d).
It was agreed that primary attempted surgical resection of HCLGG is not the current recommended standard of care. However, it was also acknowledged that selected cases were amenable to attempted resection or debulking with low risk. The risk of visual damage with such surgery was acknowledged, and it was proposed that a modification of the Dodge/PLAN classification by incorporation of surgical, genetic, and visual risk into multidisciplinary planning of surgical approaches would assist with standardized reporting and, therefore, formal evaluation in clinical trials.4 When resection occurs in a tumor that is growing, careful follow-up imaging is recommended: 2 monthly scans for 6 months (n = 3), based on a precautionary approach to monitor early progression is recommended (statement 2 and 7a–c) (Fig. 2). The requirement for ongoing study through trials of multidisciplinary approaches to treat these tumors in children and young persons was reinforced and further endorsed as a key consensus statement (Statement 8).
Fig. 2.
Modified Dodge classification of optic pathway tumors, identifying anatomical sites, hypothalamic involvement, metastasis and NF status.
DIPG
This brainstem tumor is radiologically distinct from other brain stem tumors and is associated with a particularly poor prognosis, which has shown no signs of improvement in the past 30 years, despite considerable efforts to test new treatments. A previous neuro-surgical consensus5 concluded that biopsy was not indicated, because histological grading did not alter therapy or outcome. This view has prevailed until recently, when a number of reports on the safety of biopsy using a variety of minimally invasive techniques have been presented and published and the capacity to explore the biological nature of these uniquely located, diffuse tumors has expanded. This change in clinical view is driven by the opportunity to include these patients in trials of novel therapies. Obtaining tissue samples under such circumstances ensures that the biological observations can be interpreted in the light of complete information about patient, tumor, treatment, and outcome (statement 9). After consideration of DIPG, the other brainstem tumor types were also considered to require biopsy after assessment by the pediatric multidisciplinary neuro-oncology team (statement 10). The selection of targets for biopsy was not the focus of consensus discussion. For HGG at other sites, contrast-enhancing areas are preferentially targeted.
HGG
HGGs include grades 3 (anaplastic astrocytoma) and 4 (glioblastoma multiforme [GBM]) gliomas according to World Health Organization classification; they constitute <10% of CNS tumors in childhood, classically occurring in cortical regions and basal ganglia. The World Health Organization classification is currently the histological classification most commonly applied. Their rarity means that relatively few studies have been completed, and frequently, trials have combined grade 3 and 4 histologies. Pediatric studies have identified children's GBMs, in particular, as being biologically distinct, being predominantly primary (de novo) GBM rather than secondary GBM, and arising from prior low-grade gliomas (grade 2). Biological differences are also seen between adult and paediatric HGGs, with differences in epidermal growth factor, platelet-derived growth factor, IDH-1, and CDKN2A being the most significant. Characteristic chromosomal abnormalities are also reported with different frequencies between childhood and adult tumors, specifically 1q gain being more common and chromosome 7 gain and 10q loss being less common in children.
Developments in imaging characterization can now group these tumors using MR perfusion, which measures relative blood volume in areas of brain and tumor; higher grade tumors have increased vascularity. MR spectroscopic profiles are also characteristic in particular tumor types; diffusion-weighted MR can grade the level of cellularity, and diffusion tensor imaging may assess tumor infiltration of the surrounding brain. These advances in biological and anatomical characterization of HGGs are contributing to more comprehensive, preoperative characterization of these tumors, influencing the surgical risk assessment of resectability. These new factors require histological correlation, with World Health Organization classification as the preferred system, justifying biopsy for both clinical management and clinical trials and in the persistent need to validate emerging technologies (statements 11 and 13). When neurological risks are high, it was agreed that cases be selected through multidisciplinary assessment for a staged approach combining surgery with chemotherapy in sequence, to enhance complete resection rates and minimize neurological risks. The selection of chemotherapy would depend on either current best practice based on evidence or a trial of novel treatment for which the patient is formally eligible (statement 12a–c).
Conclusions
These consensus statements have been reported to act as a basis for further advancing clinical practice in pediatric neuro-oncology and represent a quantified, contemporary statement of representative opinion by a meeting of international clinical leaders in the field.
Acknowledgments
We thank all CPN2011 consensus survey participants, in particular those who completed both rounds of the surveys:
Antoinette Schouten Van Meeteren, Alice Carvalho, An Van Damme, Bart Depreitere, Bengt Gustavsson, Bernt J Due Tonnessen, Anne Isabelle Bertozzi-Salamon, Angela Brentrup, Charles Raybaud, Chris Jones, Christelle Dufour, Christian Dorfer, Christian Sainte-Rose, Conor Malluci, Darren Hargrave, David Walker, Dannis Van Vuurden, Emilie De Carli, Eric Bouffet, Frank Van Calenbergh, Didier Frappaz, Paolo Frassanito, James Goodrich, Heidi Baechli, Jacques Grill, Jessica Ternier, Johan Cappelen, John Caird, Josué Pereira, Laurent Riffaud, Marc Baroncini, Marion Walker, Mark Kieran, Memet Ozek, Nada Jabado, Karsten Nysom, Pascale Varlet, John Goodden, Patricia Bertolini, Giorgio Perilongo, Philippe Mercier, Richard Grundy, Rolf Dieter Kortmann, Roger Packer, Stefan Pfister, Shlomi Constantini, Spyros Sgouros, Stefan Holm, Thomas Czech, Thomas Merchant, Tore Stokland, Vita Ridola, and Peter Vandertop.
References
- 1.Little J. Epidemiology of Childhood Cancer, International Agency for Research on Cancer (IARC) Publications, Lyon: France. 1999. pp. 47–51. [Google Scholar]
- 2.Stokland T, Liu J-F, Ironside J, et al. A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702) Neuro-Oncology. 2010;12:1257–1268. doi: 10.1093/neuonc/noq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wisoff J, Boyett J, Berger M, et al. Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: a report of the Children's Cancer Group Trial No. CCG-945. Journal of Neurosurgery. 1998;89:52–59. doi: 10.3171/jns.1998.89.1.0052. [DOI] [PubMed] [Google Scholar]
- 4.Taylor T, Jaspan T, Milano G, et al. Radiological classification of optic pathway gliomas: experience of a modified functional classification system. British Journal of Radiology. 2008;81:761–766. doi: 10.1259/bjr/65246351. [DOI] [PubMed] [Google Scholar]
- 5.Epstein F, Constantini S. Practical decisions in the treatment of pediatric brain stem tumors. Pediatric Neurosurgery. 1996;24:24–34. doi: 10.1159/000121011. [DOI] [PubMed] [Google Scholar]
- 6.Listernick R, Louis D, Packer R, et al. Optic pathway gliomas in children with neurofibromatosis 1: Consensus statement from the NF1 Optic Pathway Glioma Task Force. Annals of Neurology. 1997;41:143–149. doi: 10.1002/ana.410410204. [DOI] [PubMed] [Google Scholar]
- 7.Jones J, Hunter D. Consensus methods for medical and health services research. British Medical Journal. 1995;311:376–380. doi: 10.1136/bmj.311.7001.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy M. Consensus development methods, and their use in clinical guideline development: a review. Health Technology Assessment 2. 1998;3 http://www.hta.ac.uk/fullmono/mon23.pdf . [PubMed] [Google Scholar]
- 9.Wilne S, Koller K, Collier J, et al. The diagnosis of brain tumours in children: a guideline to assist healthcare professionals in the assessment of children who may have a brain tumour. Archives of Disease in Childhood. 2010;95:534–539. doi: 10.1136/adc.2009.162057. [DOI] [PubMed] [Google Scholar]