Abstract
Background
Adjuvant hormonal therapy significantly improves long-term survival of breast cancer patients with hormone receptor-positive disease. Despite the proven clinical efficacy of tamoxifen and aromatase inhibitors, many breast cancer survivors either fail to take the correct dosage at the prescribed frequency (adherence) or discontinue therapy (persistence). This systematic review aims to: 1) determine the prevalence of adherence and persistence to adjuvant hormonal therapy among breast cancer survivors in clinical practice, and 2) identify correlates of adherence and persistence.
Methods
We searched Medline, PubMed, PsycINFO, and CINAHL for studies that measured rates and/or correlates of adherence and/or persistence to adjuvant hormonal therapy. Studies were reviewed in a multi-step process: 1) the lead author screened titles and abstracts of all potentially eligible studies; 2) each coauthor reviewed a random 5% sample of abstracts; and 3) two sets of coauthors each reviewed half of all “maybe” abstracts. Any disagreements were discussed until consensus was reached.
Results
Twenty nine studies met inclusion criteria. Prevalence of adherence ranged from 41–72% and discontinuation (i.e., nonpersistence) ranged from 31–73%, measured at the end of 5 years of treatment. Extremes of age (older or younger), increasing out-of-pocket costs, follow-up care with a general practitioner (vs. oncologist), higher CYP2D6 activity, switching from one form of therapy to another, and treatment side effects were negatively associated with adherence and/or persistence. Taking more medications at baseline, referral to an oncologist, and earlier year at diagnosis were positively associated with adherence and/or persistence.
Conclusions/Implications
Adherence and persistence to adjuvant hormonal therapy among breast cancer survivors is suboptimal. Many of the correlates of adherence and persistence studied to date are not modifiable. Our review reveals a critical need for further research on modifiable factors associated with adherence to adjuvant hormonal therapy, and the development of behavioral interventions to improve adherence in this population.
Keywords: adherence, adjuvant hormonal therapy, systematic review, survivorship
1. Background
With a growing number of breast cancer survivors, post-treatment surveillance and risk-reducing maintenance behaviors are important components of survivorship care in clinical practice. Critical to the transition from active treatment to survivorship care is the continued use of adjuvant hormonal therapy because it significantly improves the long-term survival outcomes of breast cancer patients with hormone receptor-positive disease [1–5]. A meta-analysis of randomized trials of tamoxifen use for early breast cancer demonstrates significant 15-year risk reductions in cancer recurrence and mortality [6]. Aromatase inhibitors (e.g., anastrozole, letrozole, exemestane) have been shown to provide additional reductions in breast cancer recurrence in post-menopausal women [2–5]. Thus, five years of hormonal therapy, with either tamoxifen or aromatase inhibitors, is recommended as a preventative measure for recurrence in women with hormone receptor-positive tumors, approximately 75–80% of all breast cancer cases [7–8].
As evidence of improvement in survival from these treatments has mounted, researchers have become interested in adherence to adjuvant hormonal therapy as a possible major contributor to differences in therapeutic effect [9–11]. Despite the proven clinical efficacy of tamoxifen and aromatase inhibitors, evidence from clinical trials suggests 8–28% of patients do not complete treatment as recommended [2, 4–5, 12]. Previous narrative reviews have similarly found that 10–50% of breast cancer survivors in both clinical trial and clinical practice settings either fail to take the correct dosage at the prescribed frequency (i.e., adherence) or discontinue therapy (i.e., persistence), thus increasing their risk for new and recurrent breast cancers [13–18]. These reviews, however, have not focused exclusively on treatment delivered in routine clinical settings, instead relying on data from randomized controlled trials of treatment efficacy, where adherence to recommended treatment is not the primary outcome of interest. Further, most adjuvant treatment is delivered in clinical practice settings, rather than in clinical trials, where support from ancillary personnel, more frequent clinic visits, and patient motivation to participate in clinical trials is likely to positively affect adherence. It is important to better understand treatment adherence in routine clinical settings where patients may not be as closely monitored so that the survival benefits of adjuvant hormonal therapy are realized by patients receiving treatment under “real world” conditions. Identifying a benchmark measure of adherence in clinical practice could provide a basis for further research and intervention development to promote treatment adherence, improve the coordination of survivorship care, and ultimately reduce mortality.
To address the gap in the literature regarding adherence in routine clinical settings (versus clinical trials), we conducted a systematic review of the adjuvant hormonal therapy literature in clinical practice settings, with the specific aims to: 1) determine the prevalence of adherence and persistence to adjuvant hormonal therapy among breast cancer survivors, and 2) identify correlates of adherence and persistence.
2. Methods
Search methods were conducted according to the Preferred Reporting of Systematic Reviews and Meta-Analysis (PRISMA) Statement guidelines [19]. With the assistance of a health sciences librarian, we searched Medline (via Ovid; 1996 to April Week 4 2012, In-Process & Other Non-Indexed Citations May 07, 2012; searched May 8, 2012), PubMed (National Library of Congress; searched May 8, 2012), PsycINFO (via Ovid; 1987 to May Week 2 2012; searched May 11, 2012), and CINAHL (via Ebsco; searched May 11, 2012) for studies that measured prevalence and/or correlates of adherence and/or persistence to adjuvant hormonal therapy among breast cancer survivors in the clinical practice setting from 1998 through 2012. Scopus (via Elsevier; searched May 16, 2012) was used to search the bibliographies of selected studies for additional relevant cited articles. The search was limited to studies after 1998 because of the Early Breast Cancer Trialists’ Collaborative Group’s publication of their review that demonstrated the efficacy of tamoxifen in the prevention of breast cancer recurrence in randomized trials [20]. Search terms included nonadherence or non-adherence or adherence, continuance or persist* or complian* or discontinu*, estrogen antagonists, tamoxifen, and aromatase inhibitors, and were adapted according to the database searched. Search strategies for each database are listed in the appendix.
Inclusion and Exclusion Criteria
Studies were considered eligible for review if they (1) were written in English; (2) were published in a peer-reviewed journal in 1998 through 2012; (3) reported data from a primary study (i.e., not a review, editorial, or commentary); (4) included female breast cancer survivors who were prescribed and initiated adjuvant hormonal therapy after completing primary treatment for breast cancer; and (5) measured or assessed the prevalence and/or correlates of adherence and/or persistence to treatment in clinical practice settings. Adherence was defined as the degree of conformity to provider prescription with respect to timing, dosage, and frequency of day-to-day medication use. Persistence was defined as the duration from initiation to discontinuation of therapy [21]. Adjuvant hormonal therapy included both selective estrogen-receptor modulators (e.g., tamoxifen, raloxifene) and aromatase inhibitors (e.g., anastrozole, letrozole, exemestane). Studies that examined adherence and/or persistence to extended hormonal therapy or exclusively after therapy switches, in predominantly male breast cancer patient populations, in women with ductal carcinoma in situ (DCIS), for the treatment of metastatic disease, or in study populations without a definitive breast cancer diagnosis were excluded.
Studies were screened and reviewed in a collaborative, multi-step process. First, the lead author screened the titles and abstracts of all potentially relevant articles to determine eligibility. Abstracts were coded as “no” or “maybe” for further inclusion. For quality assurance, each coauthor independently reviewed the titles and abstracts of a random 5% sample. Abstracts coded as “no” by all authors were excluded from further review; abstracts coded as “maybe” by any author were discussed. Two pairs of coauthors reviewed half of all “maybe” abstracts. Disagreements occurred in less than 5% of all articles. Any disagreements were discussed until consensus was reached.
Completeness of Reporting
The International Society of Pharmacoeconomics and Outcomes Research (ISPOR) has published a checklist for studies of medication compliance and persistence that use administrative databases [22]. The checklist provides a list of items that should be included in reporting adherence studies, with the ultimate aim of improving the consistency and quality of adherence and persistence analysis. We assessed the completeness of reporting of studies included in our review using selected variables from the checklist, with particular emphasis on outcome variables, methodology, statistical analysis, limitations, and generalizability. We recorded whether studies adhered to these criteria by scoring each item as “YES (Y),” “NO (N),” or “INFERRED (I).”
3. Results
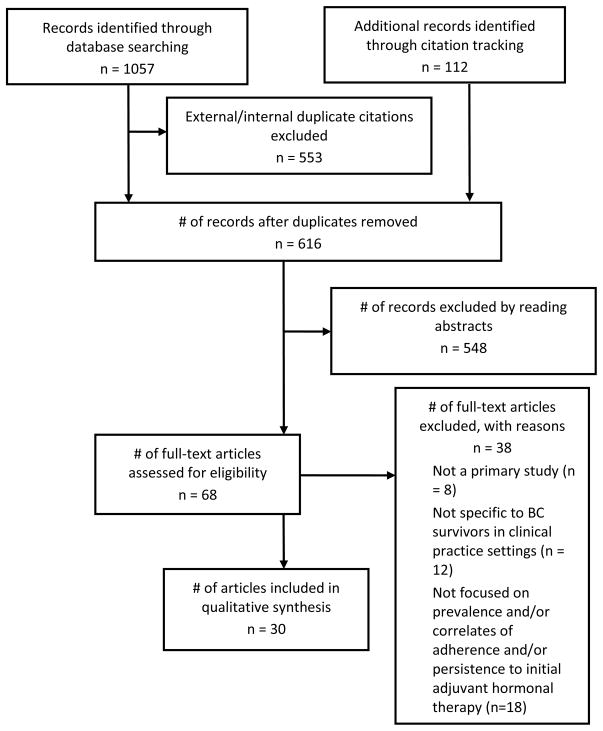
Our search strategy identified 616 potentially eligible citations, of which 30 (29 studies reported in 30 articles, 4.9%) met inclusion criteria (see Figure 1 for PRISMA flow diagram). Although the outcomes were not uniformly defined, studies measured adherence (11) [10, 23–32], persistence (9) [33–41], and both (9) [9, 11, 42–48]. Twenty four [9, 11, 23–29, 31–41, 43–44, 46, 48] studies also examined correlates of persistence and/or adherence.
Figure 1.
PRISMA Flow Diagram: Selection of Studies for Systematic Review
Fifteen [9–11, 23, 28, 31–32, 40, 42–48] studies measured the prevalence of adherence and/or persistence using administrative or prescription claims data linked with medical data, while 8 [25–27, 29, 37–39, 41] reviewed medical records or existing hospital databases/registries, and 6 [24, 30, 33–36] relied on patient self-report. Four [33–35, 39] were prospective studies.
Adherence to adjuvant hormonal therapy was most often defined as a medication possession ratio (MPR) of ≥80% [9–11, 23, 27–29, 31–32, 42–48]. To be considered adherent, prescription users must have evidence of a supply of medication for more than 80% of a given time period (usually measured in 1 year intervals). Persistence was generally defined as continuous use of tamoxifen or aromatase inhibitors, with few gaps in treatment or prescription refills. The minimum treatment gap allowable to remain persistent ranged from 45–180 days [9, 11, 37, 40, 42–44, 47–48]. A small percentage of studies measured persistence as self-reported continuous medication use at each follow-up interview [33–35]. Adherence and persistence were labeled in a variety of ways (e.g., adherence, nonadherence, persistence, nonpersistence). To avoid the confusion of multiple terms to describe the same phenomena, we converted authors’ labels as either prevalence of adherence or prevalence of discontinuation (i.e., nonpersistence).
Prevalence of Adherence and Discontinuation
As shown in Table 1, adherence to and/or discontinuation of adjuvant hormonal therapy varied widely, as did the methodology of each study. The prevalence of adherence ranged from 41–88% among tamoxifen users [9, 23–25, 27, 29–31, 45–46] and 50–91% for aromatase inhibitors [27–28, 30–31, 42, 44–46]. Studies that examined both forms of therapy together reported adherence rates of 46–100% [11, 27, 32, 43, 47]. Similarly, mean or median MPR ranged from 58–93% [9–10, 23, 47], measured at various time intervals. When limited to studies that measured adherence over periods greater than 4 years, prevalence ranged from 41–72% for all forms of therapy [11, 23, 30, 45–47].
Table 1.
Prevalence of Adherence and/or Persistence to Adjuvant Hormonal Therapy among Breast Cancer Survivors in Clinical Practice Settings
| Primary author, year | Study type | Sample size/characteristics | Eligibility criteria | Follow-up period | Hormone Therapy | Adherence defined | Persistence defined | Relevant outcome variable(s)a | Results |
|---|---|---|---|---|---|---|---|---|---|
| Prescription and Medical Claims Database/Registry (k=15) | |||||||||
| Huiart, 2012 [48] | Historical cohortb | 246/mean age 36.9 yrs; majority stage I-II; France residence | Diagnosed with BC; age 18–40 yrs; received at least one TAM rx | 3 yrs | TAM | MPR ≥ 80%c | No more than 90 days between rx refills or in tx gaps | % did not fill rx % discontinued at 1, 2, and 3 yrs % continuous users with MPR ≥ 80% |
6.1% 17.0%/29.7%/39.5% 93.9% |
| Wigertz, 2012 [32] | Historical cohort | 1,741/majority age 50–69 yrs; 73.4% postmenopausal; Sweden residence | Diagnosed with stage I-III BC; ER+ tumor; received at least one rx for oral hormonal therapy | 3 yrs | TAM, AI | MPR 80% and no more than 180 days between rx refills | Not measured | % adherent | 69% |
| Weaver, 2012 [47] | Historical cohort | 857/mean age 67.7 yrs; 56.9% white; 75.9% ER/PR+ tumors | Diagnosed with nonmetastatic, invasive locoregional stage BC; confirmed surgery after dx; filled at least one TAM or AI rx within 12 mos of dx | 5 yrs | TAM, AI | MPR ≥ 80% | No more than 90 days between rx refills or in tx gaps | % adherent at 1, 2, 3, 4, and 5 yrs Mean MPR at 1, 2, 3, 4, and 5 yrs % discontinued tx during first yr |
63%/62%/60%/55%/46% 77%/71%/70%/65%/58% 18% |
| Riley, 2011 [31] | Historical cohort | 9,446/81% white; majority age 70–79 yrs; postmenopausal | Diagnosed with invasive BC; ER/PR+ tumor; treated with surgery; age 65 yrs; entitled to Medicare Part A and B benefits; enrolled in Medicare Part D for at least 12 mos; filled at least one rx for SERM or AI | 19 mos | SERM, AI | MPR 80% | Not measured | % non-LIS recipients adherent to SERM % LIS recipients adherent to SERM % non-LIS recipients adherent to AI % LIS recipients adherent to AI |
79% 76% 70% 80% |
| Neugut, 2011 [44] | Historical cohort | 22,160 (8,110 < 65 yrs; 14,050 ≥ 65 yrs)/mean age 67.4 yrs; postmenopausal; 89.5% white; 74.3% married | Diagnosed with early stage BC; filled at least two 90-day mail order rx for an AI; age ≥50 yrs | 2 yrs | AI | MPR 80% | No more than 45 days elapsed from prior rx without a refill, with no subsequent refills before end of study period | % discontinued (age <65 yrs) % adherent (age < 65 yrs, of those who continued tx) % discontinued (age ≥65 yrs) % adherent (age ≥ 65 yrs, of those who continued tx) |
21.2% 89.7% 24.7% 91.1% |
| Huiart, 2011 [45] | Historical cohort | 13,479d/mean age 62 yrs (TAM), 70.8 yrs (AI); UK residence | Diagnosed with BC; received at least one rx of TAM or AI | 5 yrs (Cohort 1 and 2); 3 yrs (Cohort 3) | TAM, AI | MPR 80% | No more than 90 days between rx refills or in tx gaps | % discontinued all tx/TAM/AI in entire cohort % discontinued tx in first year of therapy among Cohort 1/Cohort 3 % Cohort 1 discontinued tx at 5 yrs % adherent at 1, 2, 3, 4, and 5 yrs in Cohort 1 % adherent at 1, 2, 3, 4, and 5 yrs in Cohort 2 % adherent at 1, 2, and 3 yrs in Cohort 3 % switch therapy in TAM/AI pts |
29.8%/31.0%/18.9% 20.1%/5.2% 50.7% 76.4%/68.5%/65.5%/58.7%/48.2% 84.9%/82.9%/79.9%/78.3%/72.5% 90.5%/87.2%/82.2% 26.2%/14.0 % |
| Nekhlyudov, 2011 [46] | Historical cohort | 2,207/majority age 50–59 yrs, white, non-hispanic | Diagnosed with early-stage BC; continuously enrolled in insurance prgm from at least 12 mos before and after dx | Median follow-up 923 days | TAM, AI | MPR 80% | No more than 60, 90, or 180 days elapsed from prior rx without a refill | % initiated hormone therapy % used TAM alone/AI alone/switched from TAM to AI % discontinued (no more than 60 day tx gaps) at 1, 2, 3, 4, and 5 yrs % discontinued (no more than 180 day tx gaps) at 1, 2, 3, 4, and 5 yrs % restarted tx after 30/60/180 day tx gap % adherent at 1 ,2, 3, 4, and 5 yrs (total) % adherent at 1 ,2, 3, 4, and 5 yrs (TAM only) % adherent at 1 ,2, 3, 4, and 5 yrs (AI only) % adherent at 1 ,2, 3, 4, and 5 yrs (TAM switch to AI) |
58% 54.6%/25.1%/20.3% 21%/30%/38%/47%/73 % 15%/22%/29%/38%/71% 41%/25%/6% 78.4%/75.2%/70.1%/67.1%/61.7% 76.7%/73.9%/66.5%/62.2%/58.8% 81.2%/69.7%/63.6%/72.4%/66.7% 79.7%/80.5%/77.6%/73.2%/65.2% |
| Sedjo, 2011 [28] | Historical cohort | 13,593/mean age 55.5 yrs; postmenopausal; commercially insured | Continuously enrolled in insurance prgm for 2 yrs; dx claime for primary or secondary BC; rx claim for AI | 1 yr | AI | MPR 80% | Not measured | % adherent | 77% |
| Hershman, 2011 [11]f | Historical cohort | 8,769/age <50 to 65 yrs; 11% Asian, 7.2% Hispanic, 5.6% black, 76.2% white | Diagnosed with stage I-III BC; ER/PR + tumors; received 1 rx for hormone therapy within 12 mos of dx | 4.5 yrs | TAM, AI | MPR 80% | No more than 180 days elapsed from prior rx without a refill | % prescribed only AI, TAM, or both % discontinued tx % adherent % non-adherent and/or discontinued over 4.5 yr period |
29%/43%/30% 31.5% 81% of total; 72% of pts who continued therapy 51% |
| van Herk-Sukel, 2010 [40] | Historical cohort | 1,451/age < 35 to 70 yrs; southeastern region of Netherlands | Diagnosed with stage I-IIIa BC; started TAM in first year after dx | 5 yrs | TAM | Not measured | No more than 60 days between rx refills or in tx gaps | % discontinued TAM at 1, 2, 3, 4, and 5 yrs % discontinued any endocrine therapy at 1, 2, 3, 4, and 5 yrs % switched to AI after TAM |
17%/30%/45%/50%/60% 13%/22%/31%/37%/51% 26% |
| Dezentje, 2010 [10] | Historical cohort | 1,962/mean age 59.6 yrs; 99.3% femaleg; 74% ER/PR + tumors; Netherlands | Diagnosed with BC and history of curative surgery; age 18 yrs; prescribed TAM 1–9 mos. after surgery; TAM user 120 days | 7,631 PYs | TAM | MPR 80% or 90% | Not measured | Mean MPR at 1, 3 yrs | 93%/84% |
| Kimmick, 2009 [43] | Historical cohort | 1,491/mean age 67 yrs (range: 29–102); 59% white; low-come; insured via gov’t prgms; 60% ER/PR + tumors | Diagnosed with nonmetastatic, invasive BC; continuously enrolled in Medicaid for 24 mos after dx; ER/PR +/indeterminate tumors | 1 yr | TAM, AI | MPR 80% | No more than 90 days between rx refills or in tx gaps | Rate of rx fill Mean MPR Median MPR % adherent % discontinued tx |
64% overall; 70% among women with ER/PR + tumors 75% (range 8–100%) 86% 60% 20% |
| McCowan, 2008 [9] | Historical cohort | 2,080/mean age 61.4 yrs; 48.6% ER + tumors; majority T1–2 tumors | Diagnosed with BC; resident of Tayside, Scotland during entire study period | Median follow-up 3.16 yrs | TAM | MPR 80% | No more than 180 from first rx to break | % prescribed TAM Median MPR % adherent % discontinued tx % non-adherent and/or discontinued therapy at 1, 2, 3.5, and 5 yrs Median duration of TAM use |
78.5% 93% 81% 33% 10%/19%/32%/51% 2.42 yrs |
| Partridge, 2008 [42] | Historical cohort | 1,498 (Plan A), 1,899 (Plan B), 8,994 (MarketScan)h/postmenopausal; mean age 57.9–64.3 yrs | Diagnosed with early stage BC; initiated AI | 3 yrs | AI | MPR 80% | No more than 4 mos between rx refills or in tx gaps | % short-term analysisi group adherent during first yr % long-term analysis group adherent during first yr % long-term analysis group adherent at 3 yrs % non-adherent pts still undergoing tx during first yr % switched to other endocrine therapy |
72–81% 69–78% 50–68% 23.9% 9.3% (Plan A), 14.2% (Plan B) |
| Partridge, 2003 [23] | Historical cohort | 2,378/mean age 75.4 yrs; 83% white; 63% locally staged disease | Continuously enrolled in state Medicaid prgm during study period; age 18 yrs; fill at least one TAM rx; history of definitive BC surgery | 1–4 yrs | TAM | MPR 80% | Not measured | Mean MPR during first yr of therapy % adherent during first yr % adherent in long-term cohortj at 1, 2, 3, and 4 yrs |
87% 77% 83%/68%/61%/50% |
| Medical Record Review/Hospital Database (k=8) | |||||||||
| Guth, 2011 [41] | Historical cohort | 427/mean age 65.9 yrs; majority stage I-II; Basel, Switzerland | Diagnosed with non-metastatic BC; treated with surgery at author institution; ER/PR + tumors; postmenopausal | Median follow-up 16.5 mos | TAM, AI | Not measured | Length of time from initiation to discontinuation of tx; intentional action to stop tx | % initiated therapy % discontinued tx at 5 yrs % refused to continue further tx after initiation Total number of therapy switches % therapy switches successful/unsuccesful % discontinued due to side effects |
93.7% 62.7% 9.3% 82(20.5%) 65.9%/34.1% 64.9% |
| Thompson, 2011 [29] | Historical cohort | 257k/Caucasian women from Dundee and Manchester, UK | Diagnosed with ER+ BC; prescribed TAM | Not reported | TAM | MPR 80% | Not measured | % adherent | 85.6% |
| Rae, 2009 [39] | Prospective | 280 | Enrolled in NCCTG adjuvant tamoxifen trial; CYP2D6 genotype available for analysis | 4 mos | TAM | Not measured | Withdrawing from tx | % discontinued | 14.6% |
| Schwartzberg, 2009 [38] | Historical cohort | 200/mean age 63 yrs; 87.5% Caucasian, 6.5% African American; 92.5% stage I or II disease | Diagnosed with stage I-IIIa BC; postmenopausal; ER/PR + tumors; received any hormonal therapy during study period | Not reported (study period 1998–2006) | TAM, AI | Not reported | Not reported | % first line AI users switched to another hormone therapy/discontinued tx Mean time to therapy switch among first line AI users % first line TAM users switched to another hormone therapy/discontinued tx Mean time to therapy switch among first line TAM users |
18.9%/2.8% 6.5 mos 58.5%/9.6% 33.5 mos |
| Ziller, 2009 [27] | Hybridl (historical cohort and cross-sectional) | 100/mean age 65 yrs (TAM), 72 yrs (AI); stage 0-IIIC BC; Halburg, Germany | Treated with surgery for BC at author institution; assigned to adjuvant endocrine therapy; tx started 12–24 mos before interview; postmenopausal | Median duration of tx 13.6 mos (TAM), 16.6 mos (AI) | TAM, AI | MPR 80% | Not measured | % adherent by self-report % adherent to TAM/AI by rx records |
100% 80%/69% |
| Ma, 2008 [25] | Historical cohort | 788/70.6% White, 14.1% Black, 12.7% Hispanic | Diagnosed with invasive BC; ER+ tumors; advised to take TAM by surgeon | Not reported | TAM | Not taking TAM as recommend ed or took TAM < 1 yr (if not stopped on advice of physician)m | Not measured | % adherent | 63% |
| Guth, 2008 [26] | Historical cohort | 325/mean age 67.3 yrs (range 47–95); majority stage I-IIA; Basel, Switzerland | Diagnosed with non-metastatic BC; treated with surgery at author institution; ER/PR + tumors; postmenopausal | 5 yrs | TAM, AI | Composite of compliance and persistence: the patient started therapy, but discontinued the planned treatment; intentional action | Not measured | % never initiated therapy % discontinued tx % clinical trial participants discontinued tx % discontinued tx due to choice/death/cancer recurrence/physician recommendation % non-adherent pts discontinued at 1, 2, 3, 4, and 5 yrs |
11.7% 33.4% 33.3% 10.8%/3.8%/15.0%/3.1% 38.7%/19.4%/29.0%/9.7%/3.2% |
| Owusu, 2008 [37] | Historical Cohort | 961/81% white; 66% age 65–74 yrs; | Diagnosed with stage I-IIB BC; age 65 yrs; enrolled in health plan at least 12 mos pre-and post-dx; ER+/indeterminate tumors; primary surgical therapy; received at least one TAM rx | 5 yrs | TAM | Not measured | No more than 60 days between rx refills or in tx gaps | % discontinued TAM at 1, 2, 3, 4, and 5 yrs from initial rx | 15%/24%/33%/40%/49% |
| Self-Report (k=6) | |||||||||
| Dittmer, 2011 [30] | Cross-sectional | 438n/Germany residence | Diagnosed with BC; treated at author’s clinic | 5 yrs | TAM, AI | Completing tx as recommendedo | Not measured | % adherent in Group 1, 2, 3, and 4 | 41.0%/75.3%/61.6%/42.9% |
| Kahn, 2007 [36] | Cross-sectional | 881/85% white; 1/3 age 65 yrs; 92% ER/PR + tumor | Diagnosed with stage I-III BC; registered by ACoS hospital cancer registry; initiated TAM; age 21–80 yrs at dx | Survey administered 4 yrs post-dx | TAM | Not measured | Continuing with tx (no time gaps specified) | % discontinued tx % discontinued within first 3 yrs of tx |
21% 54% (of total pts that discontinued) |
| Lash, 2006 [35] | Prospective | 462/58% age 70–79 yrs; 87% ER+ tumors | Diagnosed with stage I-IIIA BC; age 65 yrs; ER+/indeterminate tumors; initiated TAM | 63 mos | TAM | Not measured | Continuous TAM use via pt self-report at each interview | % discontinued tx | 31% |
| Grunfeld, 2005 [24] | Cross-sectional | 110/mean age 56.3 yrs; 93% white, 7% black | In remission from BC; age 35–65 yrs; prescribed TAM | N/A (mean TAM use 2.75 yrs) | TAM | Taking TAM consistently in last week at point of pt interview | Not measured | % adherent (via self-report) % discontinued due to side effects/for get fulness/doctor recommendation/change of routine/misc. |
88% 46%/18%/18%/9%/9% |
| Fink, 2004 [34] | Prospective | 516/majority age 70 yrs | Diagnosed with stage I-IIIA BC; age 65 yrs; ER+ tumors; prescribed and taking TAM | 27 mos | TAM | Not measured | Continuous TAM use via pt self-report at each interview | % of larger cohort prescribed TAM % discontinued TAM during follow-up |
86.4% (of 597) 17% (21% accounting for loss to follow-up) |
| Demissie, 2001 [33] | Prospective | 303/mean age 67.7yrs | Diagnosed with stage I-II BC; age 55 yrs | 33 mos | TAM | Not measured | Continuous TAM use via pt self-report at each interview | % prescribed TAM % discontinued tx |
65% 15% |
Studies labeled adherence and/or persistence outcomes in a variety of ways (e.g., % adherent, % nonadherent, % persistent, or % nonpersistent). To avoid the confusion of multiple terms to describe the same phenomena, we converted authors’ labels to either prevalence of adherence or prevalence of discontinuation. Nonadherence and persistence rates reported in studies were inverted to arrive at prevalence of adherence and discontinuation, respectively.
Historical cohort study (also known as a retrospective cohort study) refers to studies that identified a cohort of breast cancer cases prescribed and/or treated with adjuvant hormonal therapy from existing information before the time during which they were at risk for nonadherence or discontinuation, and collected data on adherence and persistence outcomes through medical record or prescription claims databases [63]
Medication possession ratio (MPR) is commonly used to measure adherence to a variety of medications. To be considered adherent, prescription users must have evidence of a supply of medication for more than 80% of a given time period (usually measured in 1 year intervals)
Study population further divided into three cohorts. Cohort 1 (n=416): women under 40 yrs at dx on TAM; Cohort 2 (n=1,435): women over 50 yrs diagnosed before 2000 on TAM; Cohort 3 (n=1,562): women over 50 yrs diagnosed after 2006 on AI
dx and rx claims often assessed through International Classification of Diseases, Version 9 (ICD-9) codes
Findings also reported in [83]
Study population included 14 (0.3%) men. Because we felt such a small percentage would have no effect on overall adherence rates in a large population (n=1,962), this article was still included in the review
Study population was drawn from three large databases: “Plan A” and “Plan B,” two large commercial health plans, and MarketScan, a nationally representative employer-based claims database. MarketScan patient data overlaps with patient data from “Plan A” and “Plan B.” Therefore, outcome variable results are presented as a range, rather than a single percentage.
Analysis split into two groups according to minimum follow-up. Short-term analysis group (n=7,132) had a minimum 12 month follow-up and was considered eligible for analysis of 1-year adherence rates. Long-term analysis group (n=999) had a minimum 36 month follow-up and was eligible for analysis of 3-year adherence rates.
Long-term cohort consisted of 492 patients with a first tamoxifen prescription filled in 1991
Adherence data only available on 257 study participants out of total 618
In addition to medical record review, this study also utilized a patient questionnaire on adherence to investigate how many tablets a patient had really taken
Authors defined as noncompliance rather than adherence
Patients further divided into four subgroups based on physician treatment recommendation: Group 1 (n=205): five yrs TAM; Group 2 (n=93): five yrs AI; Group 3 (n=112): two yrs TAM, three yrs AI; Group 4 (n=28): five yrs TAM, two years GnRH-Analog
Authors defined completing treatment (e.g., chemotherapy, radiation therapy, hormone therapy) as recommended as compliance, not specifically adherence
ABBREVIATIONS: BC = breast cancer; mos = months; yrs = years; AI = aromatase inhibitor (anastrozole, letrozole, exemestane); TAM = tamoxifen; MPR = medication possession ratio; tx = treatment; rx = prescription; dx = diagnosis; pt(s) = patient(s); ER/PR + = estrogen or progesterone receptor positive; SERM = selective estrogen receptor modulator; LIS = low income subsidy; ACoS = American College of Surgeons; prgm = program; PY = person year; NCCTG = North Central Cancer Treatment Group
Among tamoxifen users, the percentage of women who discontinued treatment ranged from 15–20% in the first year of therapy [37, 39–40, 45, 48] to 31–60% at the end of year 5 [9, 37, 40, 45]. In women exclusively taking aromatase inhibitors, discontinuation ranged from 5–25% during the first two years of therapy [42, 44–45]. In studies that examined both tamoxifen and aromatase inhibitors (i.e., there was no distinction between treatment with tamoxifen and treatment with aromatase inhibitors), discontinuation rates ranged from 32–73% at the end of 5 years of treatment [11, 26, 40–41, 46]. Prospective self-report studies describe discontinuation rates of 21%, 15%, and 31% at the end of 27, 33, and 63 month study periods, respectively [33–35]. These studies examined treatment with tamoxifen alone.
Factors Associated with Adherence and Discontinuation
Overall, factors associated with adherence and persistence to adjuvant hormonal therapy among breast cancer survivors have been largely unexamined to date. Similar to the prevalence of adherence and persistence, correlates were studied and framed in a variety of ways (i.e., as either factors associated with nonadherence, adherence, persistence, or nonpersistence). Because of the general agreement between studies in how adherence and persistence were defined (MPR 80% and minimal gaps in treatment, respectively), we summarized available evidence based on the assumption that there was adequate construct validity among studies. For example, a variable positively associated with nonadherence was also considered to be negatively associated with adherence.
As demonstrated in Table 2, many of the factors that have been studied are either not modifiable, were only examined in a single study, or were found to have no significant associations. The only consistent (i.e., measured in two or more studies and no study reported no effect), statistically significant factors associated with hormone therapy use were cytochrome P450 2D6 (CYPD26) activity and switching from one form of therapy to another. Women with higher levels of CYPD26 were less likely to be adherent to and/or continue treatment [29, 39]. Similarly, patients who switched hormonal therapies (e.g., switched to an aromatase inhibitor after 2–3 years of tamoxifen) were less likely to adhere to treatment [28, 32]. All other variables either had mixed findings or no effect. Extremes of age (i.e., older or younger), increasing out-of-pocket costs, follow-up care with a general practitioner (vs. cancer specialist), and treatment side effects were largely negatively associated with adherence [9, 11, 23, 25–26, 28, 31, 33, 35–37, 40–41, 44, 46]. In contrast, taking more medications at baseline, referral to an oncologist, and earlier year at diagnosis were generally positively associated [11, 23, 28, 34–35, 46]. Some studies reported no effect for each of these variables.
Table 2.
Correlates of Adherence and/or Persistence to Adjuvant Hormonal Therapy among Breast Cancer Survivors in Clinical Practice Settings
| Variablea | Adherence | Persistence | No Effect |
|---|---|---|---|
| Sociodemographic Factors | |||
| Age | ** | ||
| Older age (>65–75+ yrs) | − | −− | ** |
| Younger age (<45–50 yrs) | +/−− | − | * |
| Married (vs. other—single, divorced, etc.) | +/− | +/− | * |
| Non-White race/ethnicity | +/− | * | |
| Black | − | ** | |
| Hispanic | + | ** | |
| Asian | + | * | |
| Socioeconomic status | − | *** | |
| Employed† | * | ||
| Higher level of vocational/educational training | ** | ||
| Urban residence/geographic locationb | − | − | ** |
| Hospital size† | * | ||
| Disease/Treatment Characteristics | |||
| Positive lymph nodes | *** | ||
| Stage at diagnosis | *** | ||
| Earlier year began therapy/diagnosed | + | + | ** |
| Larger tumor size | +/− | ** | |
| Tumor grade | * | ||
| Pathology (ductal vs. lobular)† | − | ||
| Regional disease† | + | ||
| Standard primary therapy | * | ||
| Received chemotherapy | +/− | *** | |
| Received radiation therapy | − | + | *** |
| Lumpectomy/breast conserving surgery (vs. mastectomy) | + | − | *** |
| HER2 Status† | * | ||
| Higher CYP2D6 activity | − | − | |
| Menopausal status† | * | ||
| Switching to another form of therapy | − | ||
| Higher co-morbidity index | +/− | +/−− | *** |
| Previous estrogen replacement therapy use† | * | ||
| Nursing home use† | * | ||
| BMI† | * | ||
| Increasing time since curative surgery† | − | ||
| Type of drug program/insurance | ** | ||
| Longer prescription refill intervals† | + | ||
| Mail-order pharmacy use† | + | ||
| More prescriptions at baseline and/or during treatment | +/− | ** | |
| Increasing out-of-pocket costs | − | − | * |
| Treatment side effects | −− | * | |
| Preventive health visit† | + | ||
| Referred to oncologist/increasing number of visits prior to initiating therapy | + | −c | * |
| Follow-up with general practitioner vs. oncologist | − | − | * |
| Increasing number of outpatient visits | * | ||
| Increasing number of inpatient days | * | ||
| Familial Risk† | * | ||
| Using books/magazines in treatment decision making† | * | ||
| Type of HT (TAM vs. AI)/AI claim other than anastrozole | − | * | |
| Behavioral Constructs | |||
| Physician’s decisional balance score† | * | ||
| Negative or neutral decisional balance scores† | − | ||
| Lower perceived necessity† | − | ||
| Reporting less than needed support from healthcare provider during treatment† | − | ||
| Reporting a less than desired role in treatment decision-making process† | − | ||
| Reporting making treatment decisions alone† | − | ||
| Reporting side effects not told about before treatment† | − | ||
| Forgetting to take a dose† | − | ||
| Low social support† | − | ||
| Low material support† | − | ||
| Received psychological support since diagnosis† | * |
Three studies found an ER/PR + tumor to be positively associated with persistence to tamoxifen [27–28, 31]. Although adjuvant hormonal therapy use is only indicated in breast cancer patients with hormone receptor-positive disease, several studies included in this review did not stipulate ER/PR + tumors as an inclusion criterion. Therefore, in statistical analysis hormone receptor-positive status was predictive of treatment adherence as compared to an unknown or receptor-negative status. ER/PR + tumor was not included as a variable in this evidence table.
Living in western region associated with higher level of nonpersistence
Hazard ratio only significant when number of oncologist visits in prior year >10
NOTE: +, ≤ three studies and ++, > three studies report positive associations; −, ≤ three studies and −−, > three studies report negative associations;
≤ three studies,
>three studies but six studies, and
>six studies report no effect.
Few studies examined psychosocial or behavioral constructs associated with medication adherence. Kahn [36] found that making treatment decisions alone or having less than desired social support was negatively associated with persistence. Likewise, Huiart [48] reported that women with reported low social and/or material support were more likely to discontinue treatment. Other studies suggested lower perceived need for medication and negative decisional balance scores [24, 34] were negatively associated with adherence and/or persistence. Each of these variables, however, was only examined in one study.
Completeness of Reporting
In studies that utilized prescription or medical claims databases, all reported data sources, used standard methodology (i.e., MPR or gaps method), and provided explicit definitions of adherence and/or persistence (Table 3). Studies were more variable with respect to efforts to address selection bias (e.g., propensity scoring) or potential confounders; efforts to address bias were implied by 13 [9–11, 23, 28, 31–32, 40, 43–46, 48] studies that used adjusted multivariable analysis. None of the studies discussed how MPR values greater than 1 or negative gap values (i.e., study participant had more medication than necessary to adequately cover the time period under review) were handled in statistical analysis, and only a few reported how changes in medications were analyzed. Few [28, 30, 44, 46] studies explicitly addressed external validity.
Table 3.
Completeness of Reporting for Studies of Adherence and/or Persistence Utilizing Prescription Claims Data
| Primary author, year | Adherence/persistence explicitly defined | Verified continuous eligibility for drug benefit | Examined pre-enrollment period | Used standard calculation methods (e.g., MPR or gaps method) | Explained how handled MPR values>1 or negative gap values | Provided appropriate explanation for drug switching | Attempted to control for selection bias | Attempted to control for potential confounders | Discussed limitations | Addressed external validity/generalizability |
|---|---|---|---|---|---|---|---|---|---|---|
| Huiart, 2012 [48] | Y | N/A | N | Y | N | I | N | I | Y | I |
| Wigertz, 2012 [32] | Y | N/A | N | Y | N | N | N | I | Y | N |
| Weaver, 2012 [47] | Y | Y | N | Y | N | Y | I | Y | Y | N |
| Riley, 2011 [31] | Y | Y | N | Y | N | N | N | I | Y | N |
| Neugut, 2011 [44] | Y | N | N | Y | N | N | N | I | Y | Y |
| Huiart, 2011 [45] | Y | N/A | I | Y | N | Y | I | I | Y | I |
| Nekhlyudov, 2011 [46] | Y | Y | Y | Y | N | Y | N | I | Y | Y |
| Sedjo, 2011 [28] | Y | Y | I | Y | N | Y | N | I | Y | Y |
| Hershman, 2011 [11] | Y | N | N | Y | N | N | N | I | Y | N |
| van Herk-Sukel, 2010 [40] | Y | N/A | N | Y | N | Y | N | I | N | N |
| Dezentje, 2010 [10] | Y | N/A | N | Y | N | N | N | I | I | N |
| Kimmick, 2009 [43] | Y | Y | N | Y | N | N | N | I | Y | N |
| McCowan, 2008 [9] | Y | N/A | N | Y | N | N | Y | I | Y | Y |
| Partridge, 2008 [42] | Y | N | Y | Y | N | Y | N | N | Y | I |
| Partridge, 2003 [23] | Y | Y | N | Y | N | N | N | I | Y | N |
ABBREVIATIONS: Y = explicitly reported by study authors; I = inferred by raters, but not explicitly reported by study authors; N = not reported by study authors; N/A = not applicable (i.e., study population was covered by government health insurance and continuously eligible)
4. Discussion
Our review of adherence and persistence to adjuvant hormonal therapy among breast cancer survivors in 29 non-clinical trial settings demonstrates that treatment adherence is suboptimal in these settings, and may be worse than clinical trials that evaluate the efficacy of treatment regimens. When combining the proportion of breast cancer survivors who either discontinued treatment or were nonadherent, more than two-thirds of survivors do not complete 5 years of adjuvant hormonal therapy as recommended [9, 11, 26, 30, 35, 37, 40, 46–47]. In randomized controlled trials, early discontinuation of tamoxifen ranged from 13–28% and 8–24% for aromatase inhibitors [2, 4–5, 12]. Treatment discontinuation in the “real world,” however, ranged from 31–73%, suggesting there may be important differences in how treatment is delivered in clinical practice. Away from the confines of clinical trial protocols, adherence to, and persistence with, adjuvant hormonal therapy may not be as closely monitored, leaving more opportunity for patients to skip doses or stop therapy. Discontinuation or intermittent use of therapy is concerning because high levels of nonadherence may reduce the benefits of treatment observed in clinical trials.
The discrepancy in the ranges of discontinuation rates reported in clinical practice settings (31–73%) compared with clinical trials (8–28%) may be an indication of the differences in provider support and treatment delivery mechanisms. As the Institute of Medicine’s (IOM) report on cancer survivorship reveals, the lack of clear guidelines for caring for patients with a history of cancer creates wide variation in how care is delivered [49]. Once patients complete “active cancer treatment” (i.e., some combination of surgery, radiation, chemotherapy), they may be unclear which provider (cancer specialist or primary care physician) is primarily responsible for cancer follow-up care, further contributing to the wide range of adherence and discontinuation rates of adjuvant therapy found in clinical practice. While a cancer specialist or oncologist may prescribe a particular hormonal therapy agent, breast cancer survivors may not continue care with this provider. Evidence from the 2009 Behavioral Risk Factor Surveillance System (BRFSS) shows that only 20% of all cancer survivors continue to use an oncologist or cancer specialist as their primary provider for cancer follow-up care [50]. With so many breast cancer survivors receiving follow-up care in a primary care setting, increased attention to shared care with an oncologist may be a critical component of post-treatment surveillance and maintenance. Yet, a recent survey comparing the attitudes and practices of oncologists and primary care physicians (PCP) related to the care of cancer survivors found that there was disagreement between oncologists and general practitioners regarding who should be primarily responsible for follow-up care [51]. A majority (57%) of oncologists preferred an oncologist-led model of survivorship care, while only 16% favored a shared model with PCPs. In contrast, 38% of PCPs preferred a shared model of survivorship care. These findings, combined with the IOM’s observation that many PCPs are not familiar with the consequences of cancer or rarely receive guidance from oncologists [49], demonstrates that lack of coordination in the transition to survivorship care may compromise the utility of adjuvant hormonal therapy.
Our review revealed that little is known about the factors associated with continued use of adjuvant hormonal therapy, and few of the correlates studied to date are modifiable. This finding is relatively consistent with a literature review on the predictors of adherence to tamoxifen as both adjuvant therapy and chemoprevention [52], which found that sociodemographic variables such as extremes of age, non-white ethnicity, socioeconomic status, marital status, and education are associated with treatment adherence. Although demographic and treatment-related factors may help identify target populations in which to promote adherence, they do not identify factors that could be used to modify behavior. We found very few studies that examined modifiable factors, and those that did included only a single factor. Lower perceived necessity of treatment, perception of less than optimal role in the treatment decision-making process, low social and/or material support, and lower decisional balance scores were negatively associated with tamoxifen adherence [24, 34, 36, 48]. Preliminary findings from the Breast Cancer Quality of Care (BQUAL) Study measuring factors associated with initiation of hormonal therapy also suggests that patients with negative beliefs about the efficacy of treatment were less likely to initiate treatment. Patients that rated the quality of patient/physician communication more favorably, reported treatment decision making to be easier, and held positive beliefs about treatment efficacy were more likely to initiate treatment [53]. In addition, a recent study of factors associated with tamoxifen interruption reported that patients given the opportunity to ask questions about treatment at diagnosis were less likely to have treatment interruptions [54]. With such limited evidence, a critical need in this area is to further identify modifiable determinants that influence adherence in order to develop behavioral interventions to improve it.
A consistent finding in the literature is that treatment side effects are strongly associated with adherence to adjuvant therapy. Both qualitative and quantitative studies report that side effects (e.g., menopausal symptoms, arthralgia) are cited as barriers to continued treatment [33, 35–36, 41, 55–64]. Finding ways to ameliorate symptoms may be a way to increase long-term adherence. At present, we have limited evidence-based strategies for reducing symptoms associated with long-term, follow-up treatment. Results from studies evaluating alternative clinical strategies (e.g., Vitamin E or black cohosh supplements for hot flashes) are inconclusive [56, 65–67]. There is some limited evidence on how to effectively manage menopausal symptoms in breast cancer patients using various coping and self-management strategies, as well as several self-management interventions in breast cancer survivors that have been shown to improve coping skills and self-regulatory behavior for a variety of survivorship concerns [68–74]. Although these studies are not hormone therapy specific, they suggest that managing menopausal symptoms requires a complex coordination of care, and that survivors’ self-management skills can potentially ease side effect burden. An intervention focused on self-management of side-effects could potentially reduce the prevalence of nonadherence to and discontinuation of treatment. Others have suggested that health systems interventions utilizing oncology and/or advanced practice nurses may provide patients with the necessary support to achieve treatment completion [75–79]. The current lack of such interventions represents a missed opportunity for health promotion.
Our review may be the first to establish a benchmark measure of adherence to adjuvant hormonal therapy among breast cancer survivors in exclusively clinical practice settings; however, it is limited in several ways. The wide variation in reported prevalence ranges from observational studies underscores potential differences in data collection and quality and highlights the need for better summary effect size estimates. Adherence in clinical practice is often measured using a variety of indirect methods (e.g., pill count, self-report, patient diaries), potentially leading to significant variation in end results.
The majority of studies included in our review indirectly measured treatment adherence by analyzing large prescription and medical claims databases. While these data sources may provide researchers with an easy and objective measure of adherence, this methodology has limitations. First, when using large population-based administrative databases, there exists significant potential for data overlap. Many of the studies included in our review used the same or overlapping databases; thus, their estimates may vary based on inclusion criteria and other considerations. Second, prescription refill does not guarantee a patient ingests medication as directed, thus potentially over-estimating the prevalence of adherence. Our assessment of studies using selected items from the ISOPR checklist [22] revealed that the majority of studies either failed to consider or failed to report efforts to reduce potential biases associated with this type of data. Many of the studies also do not report on issues affecting external validity, leaving questions of study generalizbility unanswered. Several of the prescription databases reflect European or predominantly insured, white American populations, thereby excluding a large proportion of breast cancer survivors. This is especially concerning given that minority and low-income women are less likely to be treated with and/or initiate guideline recommended adjuvant systemic therapies [80–81].
In order to effectively move forward with intervention development aimed at promoting treatment adherence and improving the coordination of survivorship care, research must move beyond registry based data sources that contain limited information on patient characteristics so that modifiable factors associated with adherence can be identified. Factors are likely to be from a myriad of influencing sources—from a survivor’s own perception of treatment to system-related factors involved in the coordination of survivorship care. Identifying and targeting such factors may ultimately promote treatment adherence, and thereby reduce breast cancer recurrence and mortality.
Acknowledgments
This study was partially supported by the National Cancer Institute at the National Institutes of Health (K07CA140159 to Dr. Carpentier and R01CA112223 to Dr. Vernon) and the Susan G. Komen Foundation (KG111378 to Ms. Bluethmann).
The authors would like to thank Helena VonVille for her assistance in developing the search strategy.
Appendix
Search Strategy for Ovid Medline
exp patient compliance/or treatment refusal/
(nonadherence or non-adherence or adherence or continuance or persist* or complian* or discontinu*).ti,ab.
1 or 2
exp Tamoxifen/
tamoxifen.ti,ab.
Estrogen Antagonists/
exp Aromatase Inhibitors/
aromatase inhibitor*.ti,ab.
Antineoplastic Agents, Hormonal/
(adjuvant endocrine therap* or adjuvant hormonal therap*).ti,ab.
or/4–10
breast neoplasms/or (breast adj3 (cancer* or neoplasm*)).ti,ab.
3 and 11 and 12
limit 13 to yr= “1998 – 2012”
limit 14 to english language 16. (15 not animals/) or (15 and humans/)
Search Strategy for PubMed
Search patient compliance[mesh] OR treatment refusal[mesh:noexp]
Search nonadherence[tiab] OR non-adherence[tiab] OR adherence[tiab] OR continuance[tiab] OR persist*[tiab] OR complian*[tiab] OR discontinu*[tiab]
Search #1 OR #2
Search Tamoxifen[mesh]
Search tamoxifen[tiab]
Search Estrogen Antagonists[mesh:noexp]
Search Aromatase Inhibitors[mesh]
Search aromatase inhibitor*[tiab]
Search Antineoplastic Agents, Hormonal[mesh:noexp]
Search adjuvant endocrine therap*[tiab] OR adjuvant hormonal therap*[tiab]
Search #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
Search breast neoplasms[mesh:noexp] OR (breast[tiab] AND (cancer*[tiab] OR neoplasm*[tiab]))
Search #3 AND #11 AND #12
Search (#13 NOT animals[mesh:noexp]) OR (#13 AND humans[mesh])
Search #14 Limits: English, Publication Date from 1998 to 2012
Search Strategy for PsycINFO
exp compliance/or disease management/or treatment barriers/or treatment dropouts/or treatment duration/or treatment refusal/
(nonadherence or non-adherence or adherence or continuance or persist* or complian* or discontinu*).ti, ab, id.
1 or 2
antiestrogens/
(tamoxifen or aromatase inhibitor* or adjuvant endocrine therap* or adjuvant hormonal therap*).ti,ab,id.
4 or 5
exp Breast Neoplasms/
(breast adj3 (cancer* or neoplasm*)).ti,ab,id.
7 or 8
3 and 6 and 9
Search Strategy for CINAHL
-
S1
(MH “Breast Neoplasms”) or (breast cancer* or breast neoplasm*)
-
S2
(MH “Estrogen Antagonists+”) or (MH “Antineoplastic Agents, Hormonal+”) or TI (tamoxifen or estrogen antagonist* or adjuvant endocrine therap* OR adjuvant hormonal therap* OR aromatase inhibitor*) or AB (tamoxifen or estrogen antagonist* or adjuvant endocrine therap* OR adjuvant hormonal therap* OR aromatase inhibitor*)
-
S3
((MH “Patient Compliance+”) OR (MH “Treatment Refusal”)) or TI ((nonadherence or non-adherence or adherence or continuance or persist* or complian* or discontinu*)) or AB ((nonadherence or non-adherence or adherence or continuance or persist* or complian* or discontinu*))
-
S4
S1 and S2 and S3
-
S5
S1 and S2 and S3 Limiters - Published Date from: 19980101-20121231; English Language
Footnotes
Disclosures: None.
The authors declare that they have no conflicts of interest.
References
- 1.Fisher B, Dignam J, Bryant J, DeCillis A, Wickerham DL, Wolmark N, Costantino J, Redmond C, Fisher ER, Bowman DM, Deschenes L, Dimitrov NV, Margolese RG, Robidoux A, Shibata H, Terz J, Paterson AH, Feldman MI, Farrar W, Evans J, Lickley HL. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996;88:1529–1542. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 2.The Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/s0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 3.The Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/s1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 4.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Eng J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 5.Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lonning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM Intergroup Exemestane Study . A randomized trial of exemestane after two or three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/s0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. [Accessed 23 January 2012];NCCN clinical practice guidelines in oncology: breast cancer. 2012 Version 1.012 http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 8.Burstein HJ, Griggs JJ, Prestrud AA, Temin S. American Society of Clinical Oncology clinical practice guideline update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Oncol Pract. 2010;6(5):243–246. doi: 10.1200/jop.000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCowan C, Shearer J, Donnan PT, Dewar JA, Crilly M, Thompson AM, Fahey TP. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99:1763–1768. doi: 10.1038/sj.bjc.6604758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dezentje VO, van Blijderveen NJC, Gelderblom H, Putter H, van Herk-Sukel MPP, Casparie MK, Egberts ACG, Nortier JWR, Guchelaar H. Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. J Clin Oncol. 2010;28(14):2423–2429. doi: 10.1200/jco.2009.25.0894. [DOI] [PubMed] [Google Scholar]
- 11.Hershman DL, Shao T, Kushi LH, Buono D, Tsai WT, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van de Water W, Bastiaannet E, Hille ETM, Kranenbarg EMM, Putter H, Seynaeve CM, Paridaens R, de Craen AJM, Westendorp RGJ, Liefers G, van de Velde CJH. Age-specific nonpersistence of endocrine therapy in postmenopausal patients diagnosed with hormone receptor-positive breast cancer: a TEAM study analysis. Oncologist. 2012;17:55–63. doi: 10.1634/theoncologist.2011-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncol. 2006;71:1–9. doi: 10.1159/000100444. [DOI] [PubMed] [Google Scholar]
- 14.Hadji P. Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Critic Rev Oncol Hematol. 2010;73:156–166. doi: 10.1016/j.critrevonc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Dogrell SA. Adherence to oral endocrine treatments in women with breast cancer: can it be improved? Breast Cancer Res Treat. 2011;129:299–308. doi: 10.1007/s10549-011-1578-z. [DOI] [PubMed] [Google Scholar]
- 16.Gotay C, Dunn J. Adherence to long-term adjuvant hormonal therapy for breast cancer. Expert Rev Pharmacoeconomics Outcomes Res. 2011;11(6):709–715. doi: 10.1586/erp.11.80. [DOI] [PubMed] [Google Scholar]
- 17.Verma S, Madarnas Y, Sehdev S, Martin G, Bajcar J. Patient adherence to aromatase inhibitor treatment in the adjuvant setting. Curr Oncol. 2011;18(Supplement 1):S3–S9. doi: 10.3747/co.v18i0.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banning M. Adherence to adjuvant therapy in post-menopausal breast cancer patients: a review. Eur J Cancer Care. 2012;21:10–19. doi: 10.1111/j.1365-2354.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG PRSIMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Early Breast Cancer Trialists’ Collaborative Group . Tamoxifen for early breast cancer: an overview of randomized trials. Lancet. 1998;351:1451–1467. doi: 10.1016/s0140-6736(97)11423-4. [DOI] [PubMed] [Google Scholar]
- 21.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 22.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10(1):3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 23.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–606. doi: 10.1200/jco.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 24.Grunfeld EA, Hunter MS, Sikka P, Mittal S. Adherence beliefs among breast cancer patients taking tamoxifen. Patient Educ Couns. 2005;59:97–102. doi: 10.1016/j.pec.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Ma AMT, Barone J, Wallis AE, Wu NJ, Garcia LB, Estabrook A, Rosenbaum-Smith SM, Tartter PI. Noncompliance with adjuvant radiation, chemotherapy, or hormonal therapy in breast cancer patients. Am J Surg. 2008;196:500–504. doi: 10.1016/j.amjsurg.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 26.Guth U, Huang DJ, Schotzau A, Zanetti-Dallenback R, Holzgreve W, Bitzer J, Wight E. Target and reality of adjuvant endocrine therapy in postmenopausal patients with invasive breast cancer. Br J Cancer. 2008;99:428–433. doi: 10.1038/sj.bjc.6604525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziller V, Kalder M, Albert US, Hozhauer W, Ziller M, Wagner U, Hadji P. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol. 2009;20:431–436. doi: 10.1093/annonc/mdn646. [DOI] [PubMed] [Google Scholar]
- 28.Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat. 2011;125:191–200. doi: 10.1007/s10549-010-0952-6. [DOI] [PubMed] [Google Scholar]
- 29.Thompson AM, Johnson A, Quinlan P, Hillman G, Fontecha M, Bray SE, Purdie CA, Jordan LB, Ferraldeschi R, Latif A, Hadfield KD, Clarke RB, Ashcroft L, Evans DG, Howell A, Nikoloff M, Lawrence J, Newman WG. Comprehensive CYP256 genotype and adherence affect outcome in breast cancer patients treated with tamoxifen monotherapy. Breast Cancer Res Treat. 2011;125:279–287. doi: 10.1007/s10549-010-1139-x. [DOI] [PubMed] [Google Scholar]
- 30.Dittmer C, Roeder K, Hoellen F, Salehin D, Thill M, Fischer D. Compliance to adjuvant therapy in breast cancer patients. Eur J Gynecol Oncol. 2011;32(3):280–282. [PubMed] [Google Scholar]
- 31.Riley GF, Warren JL, Harlan LC, Blackwell SA. Endocrine therapy use among elderly hormone receptor-positive breast cancer patients enrolled in Medicare Part D. Medicare Medicaid Res Rev. 2011;1(4):E1–E21. doi: 10.5600/mmrr.001.04.a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wigertz A, Ahlgren J, Holmqvist M, Fornander T, Adolfsson J, Lindman H, Bergkvist L, Lambe M. Adherence and discontinuation of adjuvant hormonal therapy in breast cancer patients: a population-based study. Breast Cancer Res Treat. 2012;133:367–373. doi: 10.1007/s10549-012-1961-4. [DOI] [PubMed] [Google Scholar]
- 33.Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol. 2001;19(2):322–328. doi: 10.1200/JCO.2001.19.2.322. [DOI] [PubMed] [Google Scholar]
- 34.Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2004;22(16):3309–3315. doi: 10.1200/jco.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 35.Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99:215–220. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 36.Kahn KL, Schneider EC, Malin JL, Adams JL, Epstein AM. Patient centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45(5):431–439. doi: 10.1097/01.mlr.0000257193.10760.7f. [DOI] [PubMed] [Google Scholar]
- 37.Owusu C, Buist DSM, Field TS, Lash TL, Thwin SS, Geiger AM, Quinn VP, Frost F, Prout M, Yood MU, Wei F, Silliman RA. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26(4):549–555. doi: 10.1200/jco.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 38.Schwartzberg LS, Cobb P, Senecal F, Henry D, Kulig K, Walker MS, Houts AC, Stepanski EJ. Initial treatment and changes in adjuvant endocrine therapy for early stage breast cancer. Breast. 2009;18:78–83. doi: 10.1016/j.breast.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Rae JM, Sikora MJ, Henry NL, Li L, Kim S, Oesterreich S, Skaar T, Nguyen AT, Desta Z, Storniolo AM, Flockhart DA, Hayes DF, Stearns V. Cytochrome P450 2D6 activity predicts discontinuation of tamoxifen therapy in breast cancer patients. Pharmacogenomics. 2009;9(4):258–264. doi: 10.1038/tpj.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Herk-Sukel MPP, van del Poll-Franse LV, Voogd AC, Nieuwenhuijzen GAP, Coebergh JWW, Herings RMC. Half of breast cancer patients discontinue tamoxifen and any endocrine treatment before the end of the recommended treatment period of 5 years: a population-based analysis. Breast Cancer Res Treat. 2010;122:843–851. doi: 10.1007/s10549-009-0724-3. [DOI] [PubMed] [Google Scholar]
- 41.Guth U, Myrick ME, Schotzau A, Kilic N, Schmid SM. Drug switch because of treatment-related adverse side effects in endocrine adjuvant breast cancer therapy: how often and how often does it work? Breast Cancer Res Treat. 2011;129:799–807. doi: 10.1007/s10549-011-1668-y. [DOI] [PubMed] [Google Scholar]
- 42.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26(4):556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 43.Kimmick G, Anderson R, Camacho F, Bhosle M, Hwang W, Balkrishman R. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27(21):3445–3451. doi: 10.1200/jco.2008.19.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neugut AI, Subar M, Wilde ET, Stratton S, Brouse CH, Hillyer GC, Grann VR, Hershman DL. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29(18):2534–2542. doi: 10.1200/jco.2010.33.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.+ Huiart L, Dell’Aniello S, Suissa S. Use of tamoxifen and aromatase inhibitors in a large population-based cohort of women with breast cancer. Br J Cancer. 2011;104:1558–1563. doi: 10.1038/bjc.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nekhlyudov L, Lingling L, Ross-Degnan D, Wagner AK. Five-year patterns of adjuvant hormonal therapy use, persistence, and adherence among insured women with early-stage breast cancer. Breast Cancer Res Treat. 2011;130:681–689. doi: 10.1007/s10549-011-1703-z. [DOI] [PubMed] [Google Scholar]
- 47.Weaver KE, Camacho F, Hwang W, Anderson R, Kimmick G. Adherence to adjuvant hormonal therapy and its relationship to breast cancer recurrence and survival among low-income women. Am J Clin Oncol. 2012 doi: 10.1097/coc.0b013e3182436ec1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huiart L, Bouknik A, Rey D, Tarpin C, Cluze C, Bendiane MK, Viens P, Giorgi R. Early discontinuation of tamoxifen intake in younger women with breast cancer: is it time to rethink the way it is prescribed? Eur J Cancer. 2012 doi: 10.1016/j.ejca.2012.03.004. in press. [DOI] [PubMed] [Google Scholar]
- 49.Hewitt ME, Greenfield S, Stovall E, editors. From cancer patient to cancer survivor: lost in transition. Washington, D.C: 2005. [Google Scholar]
- 50.Centers for Disease Control and Prevention. Surveillance or demographic characteristics and health behaviors among adult cancer survivors—behavioral risk factor surveillance system, United States, 2009. [Accessed 19 Jan 2012];MMWR: Morbidity and Mortality Weekly Report. 2012 61:1. http://www.cdc.gov/mmwr. [PubMed] [Google Scholar]
- 51.Potosky AL, Han PKJ, Rowland J, Klabunde CN, Smith T, Aziz N, Earle C, Ayanian JZ, Ganz PA, Stefanek M. Difference between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med. 2011;26(12):1403–1410. doi: 10.1007/s11606-011-1808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin JH, Zhang SM, Manson JE. Predicting adherence to tamoxifen for breast cancer adjuvant therapy and prevention. Cancer Prev Res. 2011;4:1360–1365. doi: 10.1158/1940-6207.capr-11-0380. [DOI] [PubMed] [Google Scholar]
- 53.Neugut AI, Hillyer GC, Kushi LH, Lamerato L, Leoce N, Nathanson SD, Ambrosone CB, Bovbjerg DH, Mandelblatt JS, Magai C, Tsai W, Jacobson JS, Hershman DL. Non-initiation of adjuvant hormonal therapy in women with hormone receptor-positive breast cancer: The Breast Cancer Quality of Care Study (BQUAL) Breast Cancer Res Treat. 2012 doi: 10.1007/s10549-012-2066-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cluze C, Rey D, Huiart L, BenDiane MK, Bouhnik AD, Berenger C, Carrieri MP, Giorgi R. Adjuvant endocrine therapy with tamoxifen in young women with breast cancer: determinants of interruptions vary over time. Ann Oncol. 2012;23:882–890. doi: 10.1093/annonc/mdr330. [DOI] [PubMed] [Google Scholar]
- 55.Garreau JR, DeLaMelena T, Walts D, Karamlou K, Johnson N. Side effects of aromatase inhibitors versus tamoxifen: the patients’ perspective. Am J Surg. 2006;192:496–498. doi: 10.1016/j.amjsurg.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat. 2008;107:167–180. doi: 10.1007/s10549-007-9548-1. [DOI] [PubMed] [Google Scholar]
- 57.Fellowes D, Fallowfield LJ, Saunder CM, Haughton J. Tolerability of hormone therapies for breast cancer: how informative are documented symptom profiles in medical notes for ‘well-tolerated’ treatments? Breast Cancer Res Treat. 2001;66:73–81. doi: 10.1023/a:1010684903199. [DOI] [PubMed] [Google Scholar]
- 58.Salgado BA, Zivian MT. Aromatase inhibitors: side effects reported by 622 women. Breast Cancer Res Treat. 2006;100:S1618. [Google Scholar]
- 59.Henry NL, Giles JT, Ang D, Mohan M, Dadabhoy D, Robarge J, Hayden J, Lemler S, Shahverdi K, Powers P, Li L, Flockhart D, Stearns V, Hayes DF, Storniolo AM, Clauw DJ. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat. 2008;111:365–372. doi: 10.1007/s10549-007-9774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Presant CA, Bosserman L, Young T, Vakil M, Horns R, Upadhyaya G, Ebrahimi B, Yeon C, Howard F. Aromatase inhibitor-associated arthralgia and/or bone pain: frequency and characterization in non-clinical trial patients. Clin Breast Cancer. 2007;7(10):775–778. doi: 10.3816/CBC.2007.n.038. [DOI] [PubMed] [Google Scholar]
- 61.Pellegrini I, Sarradon-Eck A, Soussan PB, Lacour AC, Largillier R, Tallet A, Tarpin C, Julian-Reynier C. Women’s perception and experience of adjuvant tamoxifen therapy account for their adherence: breast cancer patients’ point of view. Pyscho-Oncology. 2010;19:472–479. doi: 10.1002/pon.1593. [DOI] [PubMed] [Google Scholar]
- 62.Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, Hayden J, Tarpinian K, Yakim E, Flockhart DA, Stearns V, Hayes DF, Storniolo AM. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30(9):936–942. doi: 10.1200/jco.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gallicchio L, MacDonald R, Wood B, Rushovich E, Helzlsouer KJ. Menopausal-type symptoms among breast cancer patients on aromatase inhibitor therapy. Climacteric. 2011 doi: 10.3109/13697137.2011.620658. in press. [DOI] [PubMed] [Google Scholar]
- 64.Boonstra A, van Zadelhoff J, Timmer-Bonte A, Ottevanger PB, Beurskens CH, van Laarhoven HW. Arthralgia during aromatase inhibitor treatment in early breast cancer patients: prevalence, impact, and recognition by healthcare providers. Cancer Nurs. 2012 doi: 10.1097/ncc.0b013e31824a7e18. in press. [DOI] [PubMed] [Google Scholar]
- 65.Nedrow A, Miller J, Walker M, Nygren P, Huffman LH, Nelson HD. Complementary and alternative therapies for the management of menopause-related symptoms: a systematic evidence review. Arch Intern Med. 2006;166:1453–1465. doi: 10.1001/archinte.166.14.1453. [DOI] [PubMed] [Google Scholar]
- 66.Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, Nicolaidis C, Walker M, Humphrey L. Nonhomronal therapies for menopausal hot f35es: systematic review and meta-analysis. J Am Med Assoc. 2006;295(17):2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 67.Hickey M, Saunders CM, Stuckey BGA. Management of menopausal symptoms in patients with breast cancer: an evidence-based approach. Lancet Oncol. 2005;6:687–695. doi: 10.1016/S1470-2045(05)70316-8. [DOI] [PubMed] [Google Scholar]
- 68.Cimprich B, Janz NA, Northouse L, Wren PA, Given B, Given CW. Taking charge: a self-management program for women following breast cancer treatment. Psycho-Oncology. 2005;14:704–717. doi: 10.1002/pon.891. [DOI] [PubMed] [Google Scholar]
- 69.Ganz PA, Kwan L, Stanton AL, Krupnick JL, Rowland JH, Meyerowitz BE, Bower JE, Belin TR. Quality of life at the end of primary treatment of breast cancer: first results from the moving beyond cancer randomized trial. J Natl Cancer Inst. 2004;96(5):376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- 70.Ganz PA, Greendale GA, Petersen L, Zibecchi L, Kahn B, Belin TR. Managing menopausal symptoms in breast cancer survivors: results of a randomized controlled trial. J Natl Cancer Inst. 2000;92(13):1054–1064. doi: 10.1093/jnci/92.13.1054. [DOI] [PubMed] [Google Scholar]
- 71.Hunter MS, Conventry S, Hamed H, Fentiman I, Grunfeld EA. Evaluation of a group cognitive behavioral intervention for women suffering from menopausal symptoms following breast cancer treatment. Psycho-Oncology. 2009;18:560–563. doi: 10.1002/pon.1414. [DOI] [PubMed] [Google Scholar]
- 72.Meneses KD, McNees P, Loerzel VW, Su X, Zhang Y, Hassey LA. Transition from treatment to survivorship: effects of a psychoeducational intervention on quality of life in breast cancer survivors. Oncol Nurs Forum. 2007;34(5):1007–1016. doi: 10.1188/07.onf.1007-1016. [DOI] [PubMed] [Google Scholar]
- 73.Mishel MH, Germino BB, Gil KM, Belyea M, Laney IC, Stewart J, Porter L, Clayton M. Benefits from an uncertainty management intervention for African-American and Caucasian older long-term breast cancer survivors. Psycho-Oncology. 2005;14:962–972. doi: 10.1002/pon.909. [DOI] [PubMed] [Google Scholar]
- 74.Mann E, Smith MJ, Hellier J, Balabanovic JA, Hamed H, Grunfeld EA, Hunter MS. Cognitive behavioral treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomised controlled trial. Lancet Oncol. 2012;13:309–318. doi: 10.1186/1471-2407-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mortimer JE. Managing the toxicities of aromatase inhibitors. Curr Opin Obstet Gynecol. 2010;22(1):56–60. doi: 10.1097/gco.0b013e328334e44e. [DOI] [PubMed] [Google Scholar]
- 76.Wengstrom Y. Effectively nursing patients receiving aromatase inhibitor therapy. Breast. 2008;17(3):227–238. doi: 10.1016/j.breast.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 77.Miaskowski C, Shockney L, Chlebowski RT. Adherence to endocrine therapy for breast cancer: a nursing perspective. Clin J Oncol Nurs. 2008;12(2):213–221. doi: 10.1188/08.cjon.213-221. [DOI] [PubMed] [Google Scholar]
- 78.Kelly A, Agius CR. Improving adherence to endocrine therapies: the role of advanced practice nurses. Oncol. 2006;20(10):50–54. [PubMed] [Google Scholar]
- 79.Albert US, Zemlin C, Hadji P, Ziller V, Kuhler B, Frank-Hahn B, Wagner U, Kalder M. The impact of breast care nurses on patients’ satisfaction, understanding of the disease, and adherence to adjuvant endocrine therapy. Breast Care. 2011;6(3):221–226. doi: 10.1159/000329006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu X, Lund MJ, Kimmick GC, Richardson LC, Sabatino SA, Chen VW, Fleming ST, Morris CR, Huang B, Trentham-Dietz A, Lipscomb J. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30(2):142–150. doi: 10.1200/jco.2011.36.8399. [DOI] [PubMed] [Google Scholar]
- 81.Livaudais JC, Hershman DL, Habel L, Kushi L, Gomez SL, Li CI, Neugut AI, Fehrenbacher L, Thompson B, Coronado GD. Racial/ethnic differences in initiation of adjuvant hormonal therapy among women with hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2012;131:607–617. doi: 10.1007/s10549-011-1762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rothman KJ. Epidemiology: an introduction. New York: 2002. [Google Scholar]
- 83.Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai W, Fehrenbacher L, Gomez SL, Miles S, Neugut AI. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–4128. doi: 10.1200/jco.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]