Abstract
Adenosine is an extracellular signaling molecule that is generated in response to cell injury where it orchestrates tissue protection and repair. Whereas adenosine is best known for promoting anti-inflammatory activities during acute injury responses, prolonged elevations can enhance destructive tissue remodeling processes associated with chronic disease states. The generation of adenosine and the subsequent activation of the adenosine 2B receptor (A2BR) is an important processes in the regulation of both acute and chronic lung disease. The goal of this study was to examine the contribution of the A2BR in models of bleomycin-induced lung injury that exhibit varying degrees of acute and chronic injury. Intratracheal bleomycin exposure results in substantial acute lung injury followed by progressive fibrosis. In this model, genetic removal of the A2BR resulted in enhanced loss of barrier function and increased pulmonary inflammation, with few differences in indexes of pulmonary fibrosis. These results support an anti-inflammatory role for this receptor in this model of acute lung injury. In contrast, systemic exposure of mice to bleomycin resulted in modest acute lung injury together with progressive pulmonary fibrosis. In this model, the effects of A2BR removal on acute lung injury were negligible; however, there were substantial reductions in pulmonary fibrosis, supporting a profibrotic role for this receptor. A2BR-dependent regulation of IL-6 production was identified as a potential mechanism involved in the diminished pulmonary fibrosis seen in A2BR knockout mice exposed to i.p. bleomycin. These studies highlight the distinct roles of A2BR signaling during acute and chronic stages of lung injury.
Acute lung injuries and chronic lung diseases are collectively the third leading cause of death in the United States (1). Treatment options for these pulmonary disorders are limited due in part to an incomplete understanding of the cellular and molecular pathways involved in the lung’s ability to respond to various forms of injury. Adenosine is an extracellular signaling molecule that is generated in response to cell injury where it orchestrates tissue protection and repair (2). Whereas adenosine is best known for promoting anti-inflammatory activities during acute injury responses (3, 4), prolonged elevations in adenosine can enhance destructive tissue remodeling processes associated with chronic disease states (5, 6). Consistent with this paradigm, adenosine serves as an anti-inflammatory molecule in models of acute lung injury (7-10), whereas elevations in adenosine contribute to disease progression in models of chronic lung disease (11, 12). Thus, adenosine signaling may play an important role in regulating how the lung responds to injury, and targeting this pathway may provide avenues for enhancing the resolution of acute lung injury or halting the progression of chronic lung disease.
Adenosine exerts its activities by engaging cell-surface adenosine receptors. There are four adenosine receptors (A1R, A2AR, A2BR, A3R) that are widely distributed on inflammatory and stromal cells (13, 14). Recent studies have identified the adenosine 2B receptor (A2BR) as an important target in the regulation of both acute and chronic lung disease; however, with opposing activities in these disorders. Agonists for the A2BR are being considered for the treatment of acute lung injury based on findings demonstrating that activation of this receptor can enhance pulmonary barrier function and dampen inflammation in models of acute lung injury (7, 8). In contrast, A2BR antagonists are being considered for the treatment of chronic lung diseases based on results demonstrating that blockade of this receptor can diminish airspace destruction and fibrosis (11). These findings emphasize that understanding the contribution of the A2BR to the regulation of processes associated with acute and chronic lung injury will be essential for guiding the use of A2BR agonists and antagonists for the treatment of these disorders.
Recent studies from our laboratory suggest that A2BR signaling represents an important pathway for balancing the resolution of acute lung injury versus the progression to chronic disease (6). Mice deficient in the purine catabolic enzyme adenosine deaminase (ADA) develop features of chronic lung disease in association with prolonged adenosine elevations (15). The prominent aspects of pulmonary disease seen in this model include macrophage and neutrophil rich inflammation, alveolar airspace destruction, and fibrosis (15, 16). Genetic removal the A2BR from ADA-deficient mice resulted in enhanced acute lung injury characterized by the loss of vascular–alveolar barrier function and enhanced neutrophil accumulation (17). However, treatment of these mice with a selective A2BR antagonist during chronic stages of disease resulted in diminished pulmonary inflammation, airspace destruction, and fibrosis (11). These findings suggest that the A2BR plays a protective role during acute stages of lung injury, whereas detrimental effects of this signaling pathway are manifested at stages where tissue fibrosis is prominent.
These findings support the hypothesis that the A2BR is anti-inflammatory during acute lung injury and profibrotic during chronic disease stages. The goal of the current study was to examine this hypothesis further by exploring the consequences of genetic removal of this receptor in models of bleomycin-induced lung injury. Exposing mice to this toxicant by direct intratracheal (i.t.) administration or systemic exposure resulted in pulmonary fibrosis that was preceded by varying degrees of acute lung injury. Mice exposed to i.t. bleomycin developed substantial acute lung injury followed by fibrosis. In this model, genetic removal of the A2BR resulted in enhanced acute lung injury, with only modest decreases in fibrosis. In contrast, systemic exposure of mice to bleomycin resulted in modest acute lung injury followed by fibrosis, and genetic removal of the A2BR in this model resulted in substantial reductions in pulmonary fibrosis in association with diminished IL-6 production. These studies highlight the distinct roles of A2BR signaling during acute and chronic stages of lung injury.
Materials and Methods
Generation and genotyping of mouse lines
Wide-type C57BL/6 mice (A2BR+/+) were purchased from Harlan Laboratories (Indianapolis, IN). A2BR-deficient mice (A2BR−/−) congenic on a C57BL/6 background were generated and genotyped as described (18). Animal care was in accordance with National Institutes of Health guidelines and the Animal Care Committee at the University of Texas Health Science Center at Houston. All animals were housed under strict containment protocols and maintained in ventilated cages equipped with microisolator lids. Serologies on cage littermates were negative for 12 of the most commonly seen viruses in mice. No evidence of fungal, parasitic, or bacterial infection was found.
Intratracheal bleomycin treatment
Avertin (250 mg/kg, i.p.) was used to anesthetize 10-wk-old female C57BL/6 A2BR+/+ or A2BR−/− mice, and saline alone or 2.5 U/kg bleomycin (Teva Parenteral Medicines, Irvine, CA) diluted in 50 μl sterile saline was instilled intratracheally (19). Endpoints were examined at 7 and 21 d after exposure.
Intraperitoneal bleomycin treatment
Six-week-old male A2BR+/+ or A2BR−/− mice were treated intraperitoneally with 0.035 U/g bleomycin (Teva Parenteral Medicines) diluted in 150 μl sterile PBS or PBS alone (20). Mice were treated twice weekly for 4 wk and were sacrificed on day 10 and 33.
Bronchoalveolar lavage and histology
Avertin was used to anesthetize mice, and airways were lavaged three times with 0.4 ml PBS, and 1 ml bronchoalveolar lavage (BAL) fluid was recovered. Total cell counts were determined using a hemocytometer and cellular differentials determined by cytospinning BAL aliquots onto microscope slides and staining with Diff-Quick (Dade Behring, Deerfield, IL). After lavage, the lungs were inflated with 10% buffered formalin at 25 cm of pressure and fixed at 4°C overnight. Lungs were dehydrated in ethanol gradients and embedded in paraffin, and 5-μm tissue sections were collected on microscope slides and stained with H&E (Shandon-Lipshaw, Pittsburgh, PA) or Masson’s trichrome (EM Science, Gibbstown, NJ) according to manufacturer’s instructions.
Immunostaining
Rehydrated slides were quenched with hydrogen peroxide (3%), followed by Ag retrieval for 30 min at 95°C (Dako, Carpinteria, CA). Next, endogenous avidin and biotin blocking was performed with a Biotin Blocking System (Dako). For neutrophil staining, slides were incubated with rat anti-mouse neutrophil Ab (AbD SeroTec, Raleigh, NC; 1:500 dilution) overnight at 4°C. For α smooth muscle actin (α-sma) staining, slides were processed with mouse on mouse blocking reagents and incubated with an α-sma mAb (clone 1A4; Sigma-Aldrich, St. Louis, MO; 1:1000 dilution) at 37°C for 1 h. For IL-6 staining, slides were incubated with rabbit anti-mouse Ab (Abcam, Cambridge, MA; 1:100 dilution) overnight at 4°C. ABC streptavidin reagents and individual appropriate secondary Abs were used followed by development with 3,3′-diaminobenzidine (Sigma-Aldrich) and methyl green counterstaining for neutrophils and vector red alkaline phosphatase and Gill’s hematoxylin for α-sma.
Measurement of vascular permeability
Pulmonary vascular permeability was quantified by i.p. administration of Evans blue dye (0.2 ml of 0.5% in PBS) (17). Four hours after dye administration, mice were perfused with PBS, and lungs were harvested and dye extracted in formamide overnight at 55°C. Dye concentrations were quantified by measuring absorbance at 610 nm with subtraction of reference absorbance at 450 nm. The content of Evans blue dye was determined by generating a standard curve from dye dilutions. Vascular permeability was also monitored by quantifying the levels of serum albumin in lavage fluid using ELISA (ICL, Newberg, OR).
Fibrosis assessment
The Sircol assay (Biocolor, Carrick, U.K.) was used to quantify soluble collagen levels in BAL fluid. Ashcroft scores were determined on H&E-stained lung sections (21). Twenty fields per section were used to determine the Ashcroft score, and four mice per group for controls and six mice per group for bleomycin-treated mice were examined. All analysis was conducted in a blinded manner.
Fibrotic mediator measurements
IL-6 and active TGF-β1 levels were quantified in 50 μl clarified BAL samples using an ELISA kit from BD Biosystems (San Diego, CA) following the manufacturer’s instructions.
Statistics
Results were analyzed using the Mann–Whitney U test for comparison between any two groups and the non-parametric equivalents of ANOVA for multiple comparisons. A p value ≤ 0.05 was considered to be significant.
Results
Differences in anti-inflammatory effects of the A2BR in i.t. and i.p. models of bleomycin-induced lung injury
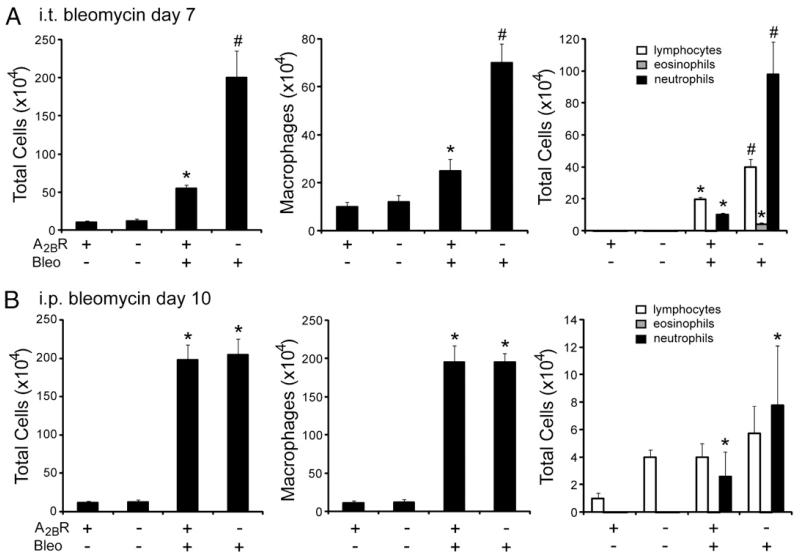
Intratracheal bleomycin exposure in mice results in acute lung injury followed by progressive pulmonary fibrosis (19). The A2BR serves anti-inflammatory roles in several models of acute lung injury (7-9), and we therefore hypothesized that the A2BR plays a similar anti-inflammatory role in the acute injury seen after i.t. bleomycin exposure. To address this, BAL cell counts and differentials were examined at an acute stage (day 7) after a single 2.5 U/kg i.t. bleomycin exposure (Fig. 1A). There were significantly more inflammatory cells recovered from the BAL fluid of A2BR−/− mice compared with that of A2BR+/+ mice after i.t. bleomycin exposure. This included increases in macrophages, lymphocytes, and particularly neutrophils. These results suggest that the A2BR plays an anti-inflammatory role after i.t. bleomycin exposure.
FIGURE 1.
Pulmonary inflammation after bleomycin exposure. BAL fluid was collected, and total cell numbers were determined. BAL cells were cytospun onto microscope slides and stained with Diff-Quick, allowing for determination of cellular differentials. A, BAL cell counts 7 d after i.t. bleomycin exposure. B, BAL cell counts 10 d after i.p. bleomycin exposure. Data are mean cell counts ± SEM. *p ≤ 0.05 versus mice treated with saline or PBS; #p ≤ 0.05 versus A2BR+/+ mice treated with bleomycin; n = 8.
Repeated i.p. injections of bleomycin also result in pulmonary fibrosis (20). To examine the contribution of the A2BR to acute inflammation in this model, BAL fluid cellularity was examined on day 10 (Fig. 1B). There were substantial increases in inflammatory cells, largely macrophages, in the BAL fluid of mice injected with bleomycin; however, there were no differences in the total numbers of inflammatory cells or the cell differentials in the BAL fluid of A2BR+/+ and A2BR−/− mice injected with bleomycin. These findings suggest that the A2BR does not serve an antiinflammatory role in the i.p. model of bleomycin-induced lung fibrosis.
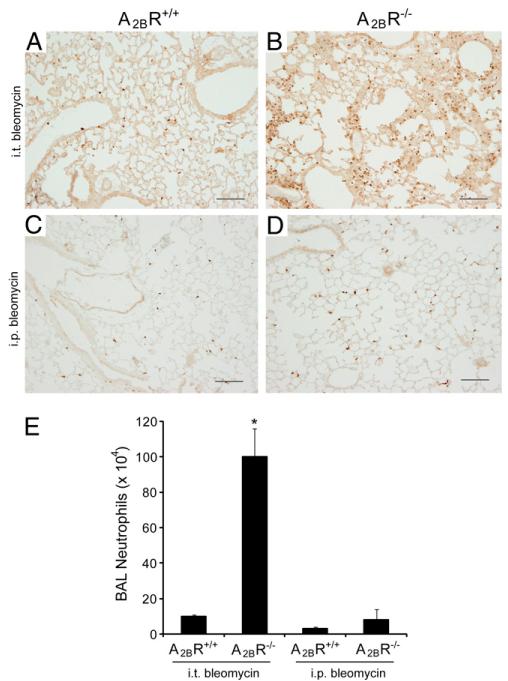
Previous studies suggest that the A2BR plays an important role in regulating neutrophil infiltration during acute lung injury (7, 8, 17). To examine more closely the differences in pulmonary neutrophils in these models of bleomycin-induced lung injury, lung sections from A2BR+/+ and A2BR−/− mice were stained with an anti-neutrophil Ab to localize these cells (Fig. 2). Results demonstrated enhanced numbers of neutrophils in the alveolar airspaces and septae of A2BR−/− mice exposed to i.t. bleomycin (compare Fig. 2A and 2B). Increased neutrophil staining was not seen in the lungs of A2BR−/− mice exposed to i.p. bleomycin (compare Fig. 2C and 2D). These findings are consistent with a selective neutrophil increase in the lavage of A2BR−/− mice exposed to i.t. bleomycin (Fig. 2E). These results suggest that the A2BR serves an anti-inflammatory role in acute inflammatory stages in the i.t. bleomycin model and that these responses are not seen in the i.p. model of pulmonary fibrosis.
FIGURE 2.
Pulmonary neutrophils after bleomycin exposure. A–D, Lung sections from A2BR+/+ (A, C) and A2BR−/− (B, D) mice collected 7 d after i.t. bleomycin exposure (A, B) or 10 d after the first i.p. exposure (C, D). Sections were reacted with an anti-neutrophil Ab to identify tissue neutrophils (brown signal). Sections are representative of six to eight different mice from each genotype. Scale bars, 200 μm. E, Neutrophil counts from BAL fluid collected 7 d after i.t. bleomycin and 10 d after i.p. bleomycin. Data are mean cell counts ± SEM. *p ≤ 0.05 versus mice treated with saline or PBS.
Differences in the contribution of the A2BR to vascular permeability in i.t. and i.p. models of bleomycin-induced pulmonary injury
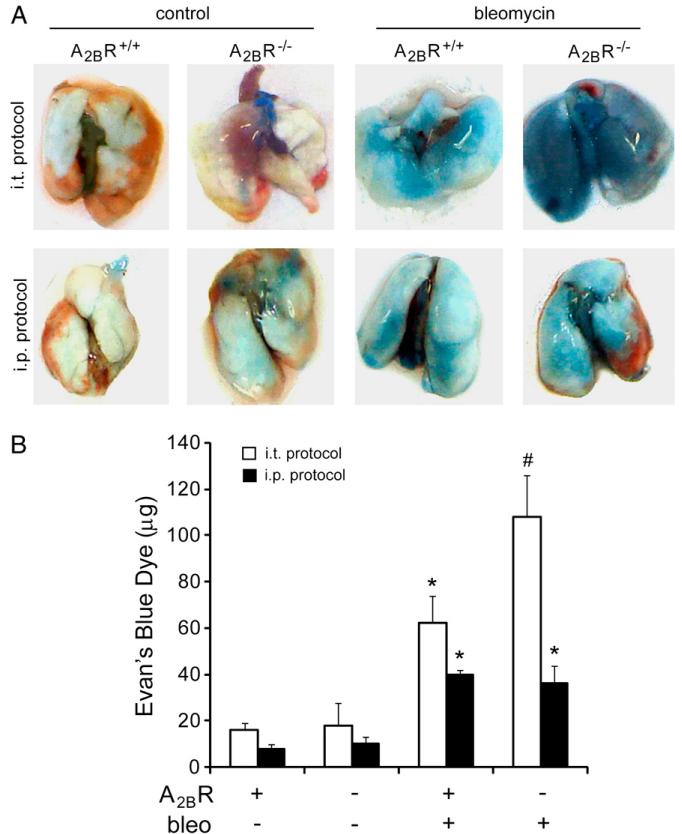
Acute lung injury is commonly associated with the loss of alveolar–vascular barrier function, which can contribute to enhanced neutrophil infiltration (8). Previous studies have shown that the A2BR plays a protective role in the maintenance of pulmonary barrier function in models of acute lung injury (7, 8, 17). We hypothesized that the A2BR plays a similar role in the acute injury seen after i.t. bleomycin exposure and that this effect would be attenuated in the i.p. model of bleomycin exposure. To address this, Evans blue dye extravasations were quantified in the lungs of A2BR+/+ and A2BR−/− mice at acute stages after either i.t. or i.p. bleomycin exposure (Fig. 3). Results revealed that there was enhanced vascular leakage in A2BR−/− mice compared with that in A2BR+/+ mice after i.t. bleomycin exposure (Fig. 3A, 3B). In contrast, there was only mild loss of vascular permeability in A2BR+/+ mice after i.p. bleomycin exposure, and this was not enhanced in A2BR−/− mice (Fig. 3A, 3B). These findings suggest that the A2BR plays an important role in the maintenance of pulmonary barrier function in the i.t. bleomycin model. Furthermore, the loss of pulmonary barrier function and the protective role of the A2BR are less apparent in the i.p. model of bleomycin exposure.
FIGURE 3.
Pulmonary vascular permeability after bleomycin exposure. Evans blue dye (0.2 ml of 0.5% in PBS) was delivered by i.p. injection to mice 7 d after i.t. bleomycin exposure or 10 d after the first i.p. bleomycin exposure. Four hours later, lungs were harvested and Evans blue dye concentrations examined. A, Representative images of lungs. B, Quantification of Evans blue dye levels in lungs. Data are mean dye amount ± SEM. *p ≤ 0.05 versus mice treated with saline or PBS; #p ≤ 0.05 versus A2BR+/+ mice treated with bleomycin; n = 6.
Pulmonary fibrosis in A2BR−/− mice after i.t. bleomycin exposure
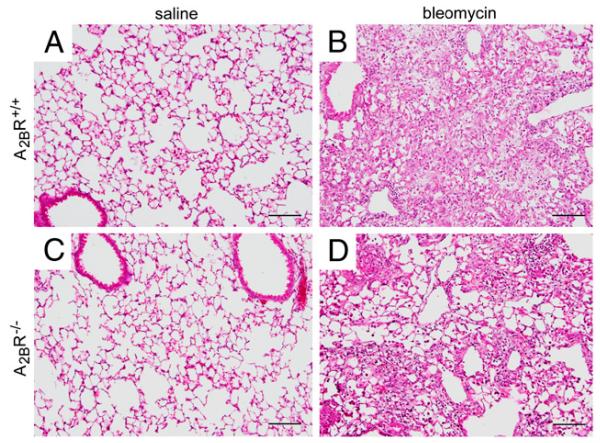
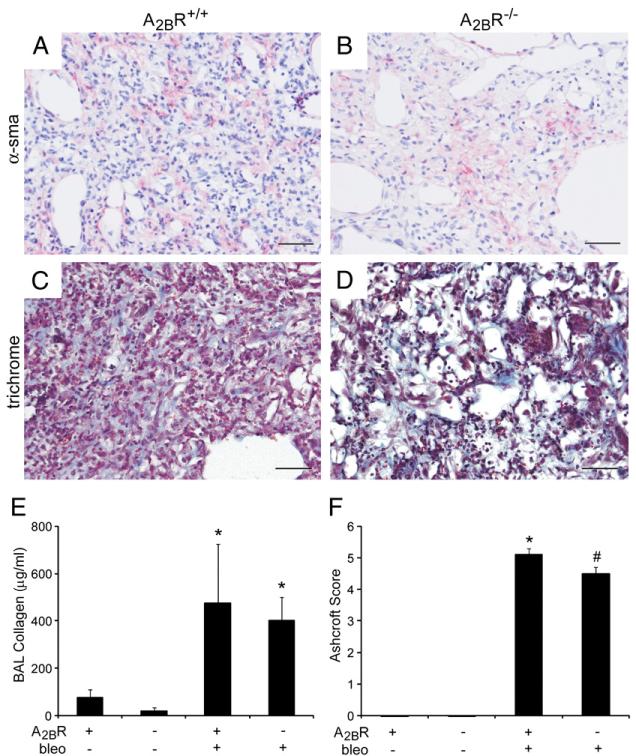
To examine the contribution of the A2BR to fibrosis in the i.t. bleomycin model, A2BR−/− mice were exposed to bleomycin intratracheally, and indicators of fibrosis were assessed 21 d later. Neither A2BR+/+ or A2BR−/− mice exposed to saline showed histological signs of lung injury (Fig. 4A, 4C). However, both A2BR+/+ and A2BR−/− mice exposed to bleomycin exhibited histopathological features of pulmonary fibrosis including congestion and remodeling of the alveolar airspaces (Fig. 4B, 4D). To assess fibrosis in these mice further, the presence of myofibroblasts was examined using α-sma immunohistochemistry (Fig. 5A, 5B), and collagen deposition was examined by staining with Masson’s trichrome (Fig. 5C, 5D). Lungs from both A2BR+/+ and A2BR−/− mice exhibited extensive α-sma and trichrome staining in the alveolar airspaces after the i.t. administration of bleomycin. To quantify the degree of fibrosis, soluble collagen levels were examined in BAL fluid at day 21 (Fig. 5E). Both A2BR+/+ and A2BR−/− mice exhibited increased levels of collagen; however, there was not a significant difference in collagen accumulation between these two genotypes. Ashcroft scoring was conducted to assess the overall degree of fibrosis. Results demonstrated a slight reduction in overall fibrosis in A2BR−/− mice (Fig. 5F). These results suggest that there is little to no difference in the degree of pulmonary fibrosis between A2BR+/+ and A2BR−/− mice exposed to bleomycin by an i.t. route.
FIGURE 4.
Pulmonary histopathology after i.t. bleomycin exposure. Lungs were collected 21 d after i.t. bleomycin or saline exposure and prepared routinely for sectioning and H&E staining. A, Lung section from an A2BR+/+ mouse treated with saline. B, Lung section from an A2BR+/+ mouse treated with bleomycin. C, Lung section from an A2BR−/− mouse treated with saline. D, Lung section from an A2BR−/− mouse treated with bleomycin. Sections are representative of six to eight different mice from each genotype. Scale bars, 200 μm.
FIGURE 5.
Pulmonary fibrosis after i.t. bleomycin exposure. A–D, Lung sections from A2BR+/+ (A, C) and A2BR−/− (B, D) mice collected 21 d after i.t. bleomycin exposure were subjected to α-sma immunostaining to identify myofibroblasts (pink signal in A and B) and stained with Masson’s trichrome to identify collagen (blue signal in C and D). Sections are representative of six to eight different mice from each genotype. Scale bars, 100 μm. E, Soluble collagen protein levels in BAL fluid were quantified using the Sircol assay. Results are presented as mean collagen levels ± SEM. F, Ashcroft scoring was conducted to determine the extent of fibrosis. *p ≤ 0.05 versus ADA+ mice treated with saline; #p ≤ 0.05 versus A2BR+/+ mice treated with bleomycin. n = 6 (saline treated), n = 8 (bleomycin treated).
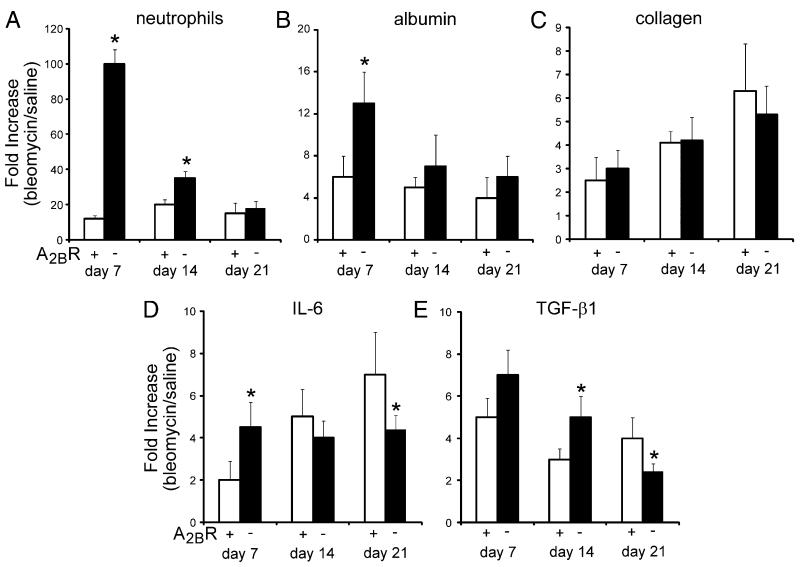
Development of pulmonary fibrosis in the i.t. bleomycin model is closely linked to inflammatory changes; however, we did not see an enhancement in pulmonary fibrosis in A2BR−/− mice despite increased inflammation on day 7. To investigate this further, we examined inflammation, vascular permeability, fibrosis, and fibrotic mediator production at various time points in the progression of this model. As shown earlier, there was enhanced neutrophilia and vascular permeability in A2BR−/− mice at day 7 after i.t. bleomycin exposure (Fig 6A, 6B); however, differences in inflammation and vascular leak between genotypes diminished at day 14 and 21. Collagen levels increased with time in the model; however, there were not significant differences between A2BR+/+ and A2BR−/− mice (Fig. 6C). Investigation of the key profibrotic mediators IL-6 and TGF-β1 revealed significant elevations in A2BR−/− mice during acute stages of the model; however, levels were diminished during chronic stages (Fig. 6D, 6E). These results suggest that IL-6 and TGF-β1 are differentially regulated during acute and chronic stages of i.t. bleomycin exposure, a feature that might account for the lack of increased fibrosis in the lungs of A2BR−/− mice despite enhanced inflammation during acute stages.
FIGURE 6.
Developmental analysis of pulmonary inflammation, permeability, and fibrosis after i.t. bleomycin exposure. BAL fluid was collected from A2BR+/+ (open bar) and A2BR−/− (solid bar) mice at days 7, 14, and 21 after i.t. bleomycin exposure. A, Total BAL neutrophil counts. B, Vascular permeability as determined by levels of albumin in the BAL fluid. C, Soluble collagen levels determined by Sircol analysis. D, IL-6 protein levels in BAL fluid. E, Active TGF-β1 levels in BAL fluid. Values are presented as mean fold increases in bleomycin-exposed mice compared with saline-exposed mice ± SEM. n = 4 for each. *p ≤ 0.05 comparing adjacent genotype.
Reductions in pulmonary fibrosis in A2BR−/− mice after i.p. bleomycin exposure
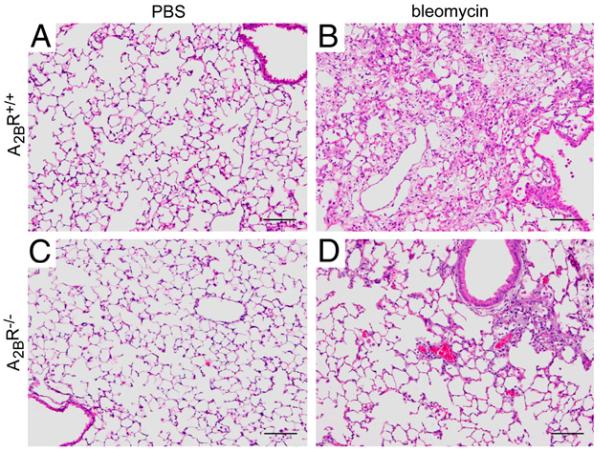
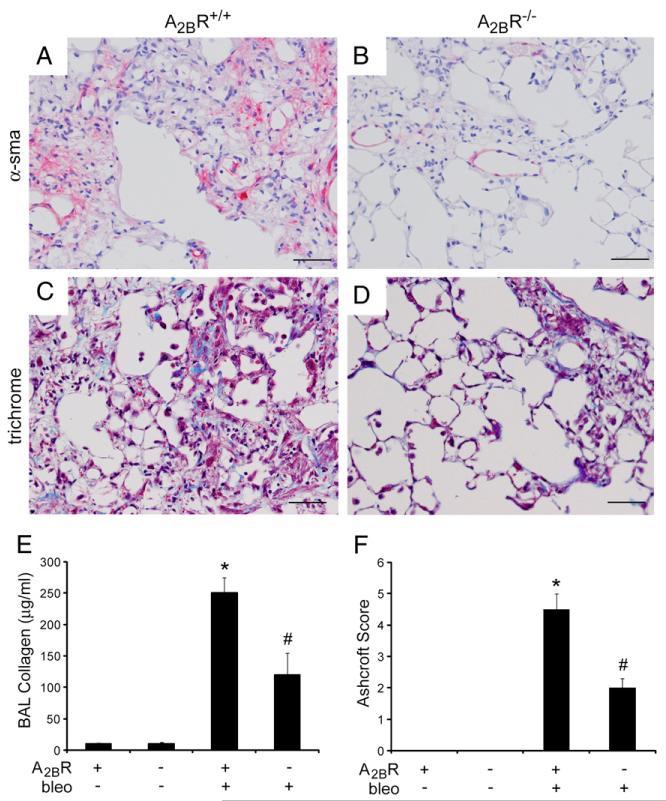
To assess the role of the A2BR in fibrosis in the i.p. model of bleomycin injury, mice were injected intraperitoneally with bleomycin twice a week for 4 wk, and pulmonary fibrosis was assessed a week after the last bleomycin injection (day 33). Mice exposed to PBS were used as controls and exhibited normal pulmonary histopathology (Fig. 7A, 7C). A2BR+/+ mice treated intraperitoneally with bleomycin exhibited histopathological features of pulmonary fibrosis (Fig. 7B), and A2BR−/− mice demonstrated diminished histopathological evidence of pulmonary fibrosis (Fig. 7D). Consistent with this, A2BR+/+ mice treated intraperitoneally with bleomycin exhibited pronounced α-sma (Fig. 8A) and Masson’s trichrome (Fig. 8C) staining, and these metrics of pulmonary fibrosis were reduced in A2BR−/− mice (Fig. 8B, 8D). Quantification of collagen levels (Fig. 8E) and Ashcroft scores (Fig. 8F) confirmed that there was significantly less pulmonary fibrosis in A2BR−/− mice exposed to bleomycin by an i.p. route. These findings suggest that the A2BR plays a profibrotic role in the i.p. model of bleomycin-induced pulmonary fibrosis.
FIGURE 7.
Pulmonary histopathology after i.p. bleomycin exposure. Lungs were collected 33 d after the first bleomycin exposure and prepared routinely for sectioning and H&E staining. A, Lung section from an A2BR+/+ mouse treated with PBS. B, Lung section from an A2BR+/+ mouse treated with bleomycin. C, Lung section from an A2BR−/− mouse treated with PBS. D, Lung section from an A2BR−/− mouse treated with bleomycin. Sections are representative of six to eight different mice from each genotype. Scale bars, 200 μm.
FIGURE 8.
Pulmonary fibrosis after i.p. bleomycin exposure. A–D, Lung sections from A2BR+/+ (A, C) and A2BR−/− (B, D) mice collected 33 d after i.p. bleomycin exposure were subjected to α-sma immunostaining to identify myofibroblasts (pink signal in A and B) and stained with Masson’s trichrome to identify collagen (blue signal in C and D). Sections are representative of six to eight different mice from each genotype. Scale bars, 100 μm. E, Soluble collagen protein levels in BAL fluid were quantified using the Sircol assay. Results are presented as mean collagen levels ± SEM. F, Ashcroft scoring was conducted to determine the extent of fibrosis. *p ≤ 0.05 versus ADA+ mice treated with PBS; #p ≤ 0.05 versus A2BR+/+ mice treated with bleomycin. n = 6 (saline treated), n = 8 (bleomycin treated).
A2BR-dependent expression of IL-6 after bleomycin exposure
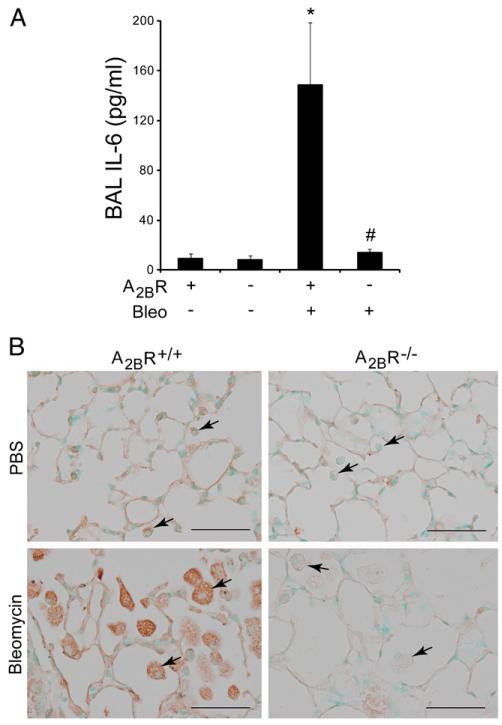
IL-6 is a pluripotent cytokine that has profibrotic activities and is associated with pulmonary fibrosis in animal models and humans (22-24). In addition, we and others have shown that adenosine can promote the expression of IL-6 through the A2BR (11, 25, 26). IL-6 expression was monitored to determine if the regulation of this profibrotic mediator represents a mechanism by which A2BR engagement regulates fibrosis in the i.p. model. IL-6 levels were elevated more than 10-fold in the BAL fluid of A2BR+/+ mice exposed to i.p. bleomycin (Fig. 9A). In contrast, elevations in IL-6 were not detected in BAL fluid from the lungs of A2BR−/− mice exposed to i.p. bleomycin (Fig. 9A). Immunolocalization experiments demonstrated that IL-6 production was abundant in macrophages in the airspaces of A2BR+/+ mice exposed to i.p. bleomycin (Fig. 9B). Furthermore, IL-6 expression in these cells was diminished in A2BR−/− mice exposed to i.p. bleomycin (Fig. 9B). These findings suggest that the A2BR is essential for the production of IL-6 from alveolar macrophages in this model.
FIGURE 9.
IL-6 levels after i.p. bleomycin exposure. A, IL-6 levels were measured in BAL fluid collected from A2BR+/+ or A2BR−/− mice on day 33 after i.p. PBS or bleomycin exposure using ELISA. Data are presented as mean pg/ml + SEM. *p ≤ 0.05 versus ADA+ mice treated with PBS; #p ≤ 0.05 versus A2BR+/+ mice treated with bleomycin. n = 6 (saline treated), n = 8 (bleomycin treated). B, Immunohistochemistry techniques were used to localize IL-6 expression in alveolar macrophages (arrows) in lung sections from A2BR+/+ or A2BR−/− mice on day 33 after i.p. PBS or bleomycin exposure. Scale bars, 100 μm.
Discussion
In the current study, we demonstrate that the genetic removal of the A2BR leads to enhanced acute lung injury in a model of i.t. bleomycin exposure supporting an anti-inflammatory role for this receptor by promoting barrier function in the lung. In contrast, when bleomycin was administered via an i.p. route where acute lung injury was limited, removal of the A2BR resulted in diminished pulmonary fibrosis, suggesting a profibrotic role for this receptor in chronic stages of disease. These findings support the hypothesis that adenosine signaling through the A2BR plays distinct roles during acute and chronic stages of lung injury.
Generation of adenosine after cellular damage is an important component of the injury response where it plays active roles in processes that protect cells and tissues, limit inflammation, and promote wound healing (4-6). Engagement of all four adenosine receptors is involved in some capacity in these anti-inflammatory and tissue-protective activities. The A2BR has a relatively low affinity for adenosine (2) and thus may serve regulatory roles in pathological environments where adenosine is elevated (2, 12). This is supported by observations that the A2BR is markedly upregulated after various injurious stimuli including those seen during acute (7, 8) or chronic (27-29) lung injury. Mechanisms by which the A2BR is upregulated include transcriptional activation by Hif-1α (30) and regulation by micro RNAs (31). The A2BR serves anti-inflammatory and tissue protective roles in many acute injury models including cardioprotection by ischemic preconditioning (32) and protection from renal ischemic injury (33), sepsis (34), and acute lung injury (7-9). Exposure of mice to hypoxia (7), ventilator-induced lung injury (8), or endotoxin (9) leads to acute lung injury in association with adenosine production and A2BR upregulation. The A2BR plays a protective role in these models by promoting vascular–alveolar barrier function and limiting inflammation, including neutrophilia. This was demonstrated by the enhancement of acute lung injury in mice lacking the A2BR and by the protection of mice from injury after treatment with an A2BR agonist. These findings suggest that A2BR agonist may be useful in the treatment of acute lung injuries.
Findings in the current study demonstrate for the first time, to our knowledge, that the A2BR serves an anti-inflammatory and tissue-protective role in the classical i.t. model of bleomycin-induced lung injury. This model is characterized by acute lung injury followed by progressive but resolvable pulmonary fibrosis (19, 35, 36). A2BR knockout mice given i.t. bleomycin demonstrated enhanced loss of pulmonary barrier function and excessive infiltration of neutrophils into the lung. The acute damage to the pulmonary epithelium after direct instillation of bleomycin to the airways likely contributes to the loss of barrier function in this model, and our findings suggest that activation of the A2BR plays important roles in promoting barrier function after this direct chemical injury. The exact mechanisms by which A2BR promotes barrier function in the lung is not clear; however, recent studies demonstrate that this receptor can be rapidly induced in airway epithelial cells and endothelial cells by various inflammatory mediators produced after acute lung injury (9), suggesting that upregulation of this pathway is important for enhanced barrier protection during acute inflammation. It is also possible that antiinflammatory effects may be due to the direct engagement of the A2BR on neutrophils (37); however, additional studies are needed to demonstrate functionality of this receptor on this cell type.
Notably, A2BR knockout mice go on to develop pulmonary fibrosis in the i.t. model to a degree that is similar to or slightly less than that seen in wild-type mice. These results suggest that the A2BR is not necessary for the development of pulmonary fibrosis in this model. Similar findings were seen in mice lacking the ecto enzyme CD73, which is responsible for generating adenosine after bleomycin-induced lung injury (38). CD73 mice exposed to i.t. bleomycin did not exhibit elevations in pulmonary adenosine levels and consequently developed enhanced acute lung injury, a finding that is likely mediated by the A2BR based on the current study. However, CD73 knockout mice went on to develop enhanced fibrosis in conjunction with enhanced inflammation, a common feature of the i.t. bleomycin model (23, 39). Notably, A2BR−/− mice did not develop enhanced fibrosis despite enhanced inflammation suggesting this receptor plays an important role in regulating key mediators that amplify the fibrotic response. Indeed, time course evaluation of the production of IL-6 and TGF-β1 demonstrated that the levels of these fibrotic mediators are elevated during acute stages in the lungs of A2BR−/− mice but are significantly reduced during chronic stages where fibrosis ensues. Thus, the lack of fibrotic enhancement in the presence of increased inflammation is likely due to reduced levels of these important profibrotic molecules. These findings are consistent with those demonstrating that antagonism of the A2BR can reduce pulmonary fibrosis in adenosine-dependent lung injury in conjunction with the regulation of IL-6 and TGF-β1 during chronic stages of fibrosis (11). These findings also suggest that the A2BR may impact processes that are more closely associated with fibrosis such as direct responses of fibroblasts (40), the recruitment of fibrocytes (41), or enhancement of remodeling responses such as epithelial to mesenchymal transition (42) or endothelial to mesenchymal transition (43).
These possibilities are validated further by our findings in the i.p. model of bleomycin-induced pulmonary fibrosis. We found that wild-type mice exposed to repeated i.p. injections of bleomycin did not develop acute lung injury to the degree seen in the i.t. bleomycin model. Consequently, an enhancement of the loss of barrier function and increased pulmonary inflammation were not seen in A2BR knockout mice exposed to i.p. bleomycin. However, A2BR knockout mice exhibited significantly less pulmonary fibrosis than that of wild-type mice, including diminished quantities of myofibroblasts and collagen deposition in the airspaces and reduced levels of soluble collagen. This supports the conclusion above that the enhanced pulmonary inflammation in the i.t. model resulting from the lack of the A2BR leads to promotion of A2BR-independent fibrotic stimuli. Furthermore, our finding suggests that the A2BR serves profibrotic effects in the absence of over-riding consequences of acute lung injury.
Further evidence to support this claim comes from a study demonstrating antifibrotic properties of an A2BR antagonist in the i.t. bleomycin model (11). Notably, in this study, the A2BR an-tagonist was administered during the fibrotic phase of the model and was associated with decreased pulmonary inflammation and decreased numbers of myofibroblasts and collagen content. These results suggest that the profibrotic effects of the A2BR may be directly associated with myofibroblast activity. In support of this, the A2BR has been shown to be the most abundant adenosine receptor expressed on human pulmonary fibroblasts (40). Furthermore, A2BR expression is increased in pulmonary fibroblasts after hypoxia and contributes to elevations in α-sma levels in vitro through the increased production of the profibrotic cytokine IL-6 (40). In addition to direct affects on fibroblasts and myofibroblasts, the A2BR may have indirect influences on fibrosis. Profibrotic mediators such as IL-6, TGF-β1, and osteopontin are elevated in the lungs of humans and animal models with pulmonary fibrosis (22, 44-47). Studies in ADA-deficient mice have demonstrated adenosine-dependent elevations in these profibrotic mediators in macrophages that accumulate in the airways during chronic stages of disease (11). Exposure of these mice to an A2BR antagonist during these stages resulted in decreased expression of these profibrotic mediators in association with decreases in pulmonary fibrosis. In support of these findings, we demonstrate in the current study that the A2BR is essential for the production of IL-6 from alveolar macrophages in the airspaces of mice exposed to i.p. bleomycin. We propose that the diminished levels of IL-6 production from macrophages in the airspaces may play a role in the diminished fibrosis seen in this model. Thus, A2BR-mediated production of profibrotic molecules from macrophages may contribute to fibrotic progression in disease. The mechanisms by which A2BR activation promotes IL-6 production in macrophages is not fully understood but likely involves the upregulation of A2BR mRNA levels in activated reparative macrophages found during chronic stages of disease. Potential mechanisms under investigation include induction of the A2BR by hypoxia and regulation by micro RNAs.
Consistent with these findings in mice, recent studies have shown that the expression of the A2BR is increased in surgical biopsy specimens from the lungs of patients with a particularly severe form of interstitial lung disease known as idiopathic pulmonary fibrosis (IPF) (48, 49). Moreover, expression levels of the A2BR increase with the severity of fibrosis in IPF patients (48, 49). Immunolocalization studies place the A2BR on airway epithelial cells (48), fibroblasts, and alternatively activated macrophages in the lungs of IPF patients (49). Moreover, ex vivo analysis of alternatively activated macrophages from IPF patients demonstrates that activation of the A2BR directly promotes IL-6 production from these cells (49), further supporting the notion that A2BR-dependent production of fibrotic mediators from macrophages may represent a mechanism of disease amplification in pulmonary disorders where fibrosis is present.
In conclusion, our studies highlight that the A2BR serves distinct functions during acute and chronic stages of lung disease. The exact mechanisms involved are not known; however, the A2BR is expressed on key cell types involved in the regulation of pulmonary barrier function [endothelial cells (27) and airway epithelial cells (9, 50)] and on cells that impact the progression of fibrosis [macrophages (11) and myofibroblasts (40)]. Continued efforts to define the contribution of the A2BR to key cellular processes involved in lung disease will be critical for the advancement of A2BR-specific therapies for acute or chronic lung diseases. In this regard, the preclinical data in animal models are striking regarding either the use of A2BR agonist in acute lung injury (36) or A2BR antagonist during chronic stages of disease (11). However, it will be critical to understand the expression and activity of the A2BR in patients with acute lung injury and chronic lung disease to ensure proper treatments (6).
Acknowledgments
We thank Dr. Clay Marsh for sharing the protocol for i.p. bleomycin exposure developed in the Marsh laboratory.
This work was supported in part by National Institutes of Health Grant HL70952 (to M.R.B.).
Abbreviations used in this article
- A2BR
adenosine 2B receptor
- ADA
adenosine deaminase
- BAL
bronchoalveolar lavage
- IPF
idiopathic pulmonary fibrosis
- i.t.
intratracheal
- α-sma
α smooth muscle actin
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Ward PA, Hunninghake GW. Lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 1998;157:S123–S129. doi: 10.1164/ajrccm.157.4.nhlbi-10. [DOI] [PubMed] [Google Scholar]
- 2.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 3.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu. Rev. Pharmacol. Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 4.Haskó G, Pacher P. A2A receptors in inflammation and injury: lessons learned from transgenic animals. J. Leukoc. Biol. 2008;83:447–455. doi: 10.1189/jlb.0607359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan ES, Cronstein BN. Adenosine in fibrosis. Mod. Rheumatol. 2010;20:114–122. doi: 10.1007/s10165-009-0251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Y, Schneider DJ, Blackburn MR. Adenosine signaling and the regulation of chronic lung disease. Pharmacol. Ther. 2009;123:105–116. doi: 10.1016/j.pharmthera.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J. Clin. Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J. Immunol. 2010;184:5271–5279. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 2009;24:298–306. doi: 10.1152/physiol.00022.2009. [DOI] [PubMed] [Google Scholar]
- 11.Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J. Clin. Invest. 2006;116:2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackburn MR. Too much of a good thing: adenosine overload in adenosine-deaminase-deficient mice. Trends Pharmacol. Sci. 2003;24:66–70. doi: 10.1016/S0165-6147(02)00045-7. [DOI] [PubMed] [Google Scholar]
- 13.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackburn MR, Volmer JB, Thrasher JL, Zhong H, Crosby JR, Lee JJ, Kellems RE. Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction. J. Exp. Med. 2000;192:159–170. doi: 10.1084/jem.192.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chunn JL, Molina JG, Mi T, Xia Y, Kellems RE, Blackburn MR. Adenosine-dependent pulmonary fibrosis in adenosine deaminasedeficient mice. J. Immunol. 2005;175:1937–1946. doi: 10.4049/jimmunol.175.3.1937. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y, Mohsenin A, Morschl E, Young HW, Molina JG, Ma W, Sun CX, Martinez-Valdez H, Blackburn MR. Enhanced airway inflammation and remodeling in adenosine deaminase-deficient mice lacking the A2B adenosine receptor. J. Immunol. 2009;182:8037–8046. doi: 10.4049/jimmunol.0900515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Csóka B, Németh ZH, Virág L, Gergely P, Leibovich SJ, Pacher P, Sun CX, Blackburn MR, Vizi ES, Deitch EA, Haskó G. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood. 2007;110:2685–2695. doi: 10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morschl E, Molina JG, Volmer JB, Mohsenin A, Pero RS, Hong JS, Kheradmand F, Lee JJ, Blackburn MR. A3 adenosine receptor signaling influences pulmonary inflammation and fibrosis. Am. J. Respir. Cell Mol. Biol. 2008;39:697–705. doi: 10.1165/rcmb.2007-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baran CP, Opalek JM, McMaken S, Newland CA, O'Brien JM, Hunter MG, Bringardner BD, Monick MM, Brigstock DR, Stromberg PC, et al. Important roles for macrophage colony-stimulating factor, CC chemokine ligand 2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2007;176:78–89. doi: 10.1164/rccm.200609-1279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 1988;41:467–470. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight DA, Ernst M, Anderson GP, Moodley YP, Mutsaers SE. The role of gp130/IL-6 cytokines in the development of pulmonary fibrosis: critical determinants of disease susceptibility and progression? Pharmacol. Ther. 2003;99:327–338. doi: 10.1016/s0163-7258(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 23.Saito F, Tasaka S, Inoue K, Miyamoto K, Nakano Y, Ogawa Y, Yamada W, Shiraishi Y, Hasegawa N, Fujishima S, et al. Role of interleukin-6 in bleomycin-induced lung inflammatory changes in mice. Am. J. Respir. Cell Mol. Biol. 2008;38:566–571. doi: 10.1165/rcmb.2007-0299OC. [DOI] [PubMed] [Google Scholar]
- 24.Moodley YP, Scaffidi AK, Misso NL, Keerthisingam C, McAnulty RJ, Laurent GJ, Mutsaers SE, Thompson PJ, Knight DA. Fibroblasts isolated from normal lungs and those with idiopathic pulmonary fibrosis differ in interleukin-6/gp130-mediated cell signaling and proliferation. Am. J. Pathol. 2003;163:345–354. doi: 10.1016/S0002-9440(10)63658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J. Pharmacol. Exp. Ther. 2008;324:694–700. doi: 10.1124/jpet.107.131540. [DOI] [PubMed] [Google Scholar]
- 26.Sitaraman SV, Merlin D, Wang L, Wong M, Gewirtz AT, Si-Tahar M, Madara JL. Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J. Clin. Invest. 2001;107:861–869. doi: 10.1172/JCI11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackburn MR, Lee CG, Young HWJ, Zhu Z, Chunn JL, Kang MJ, Banerjee SK, Elias JA. Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway. J. Clin. Invest. 2003;112:332–344. doi: 10.1172/JCI16815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma B, Blackburn MR, Lee CG, Homer RJ, Liu W, Flavell RA, Boyden L, Lifton RP, Sun CX, Young HW, Elias JA. Adenosine metabolism and murine strain-specific IL-4-induced inflammation, emphysema, and fibrosis. J. Clin. Invest. 2006;116:1274–1283. doi: 10.1172/JCI26372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun CX, Young HW, Molina JG, Volmer JB, Schnermann J, Blackburn MR. A protective role for the A1 adenosine receptor in adenosine-dependent pulmonary injury. J. Clin. Invest. 2005;115:35–43. doi: 10.1172/JCI22656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 31.Kolachala VL, Wang L, Obertone TS, Prasad M, Yan Y, Dalmasso G, Gewirtz AT, Merlin D, Sitaraman SV. Adenosine 2B receptor expression is post-transcriptionally regulated by microRNA. J. Biol. Chem. 2010;285:18184–18190. doi: 10.1074/jbc.M109.066555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eckle T, Krahn T, Grenz A, Köhler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-59-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 33.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Csóka B, Németh ZH, Rosenberger P, Eltzschig HK, Spolarics Z, Pacher P, Selmeczy Z, Koscsó B, Himer L, Vizi ES, et al. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J. Immunol. 2010;185:542–550. doi: 10.4049/jimmunol.0901295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izbicki G, Segel MJ, Christensen TG, Conner MW, Breuer R. Time course of bleomycin-induced lung fibrosis. Int. J. Exp. Pathol. 2002;83:111–119. doi: 10.1046/j.1365-2613.2002.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aherne CM, Kewley EM, Eltzschig HK. The resurgence of A2B adenosine receptor signaling. Biochim. Biophys. Acta. 2010 doi: 10.1016/j.bbamem.2010.05.016. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat. Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 38.Volmer JB, Thompson LF, Blackburn MR. Ecto-5′-nucleotidase (CD73)-mediated adenosine production is tissue protective in a model of bleomycin-induced lung injury. J. Immunol. 2006;176:4449–4458. doi: 10.4049/jimmunol.176.7.4449. [DOI] [PubMed] [Google Scholar]
- 39.Hay J, Shahzeidi S, Laurent G. Mechanisms of bleomycin-induced lung damage. Arch. Toxicol. 1991;65:81–94. doi: 10.1007/BF02034932. [DOI] [PubMed] [Google Scholar]
- 40.Zhong H, Belardinelli L, Maa T, Zeng D. Synergy between A2B adenosine receptors and hypoxia in activating human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2005;32:2–8. doi: 10.1165/rcmb.2004-0103OC. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J. Clin. Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl. Acad. Sci. USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Kawabe T, Shimokata K, Hasegawa Y. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2010;43:161–172. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thannickal VJ, Toews GB, White ES, Lynch JP, III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu. Rev. Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 45.Sime PJ, O’Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin. Immunol. 2001;99:308–319. doi: 10.1006/clim.2001.5008. [DOI] [PubMed] [Google Scholar]
- 46.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J. Clin. Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C, Yousem S, Herrera I, Ruiz V, Selman M, Kaminski N. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2:e251. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selman M, Carrillo G, Estrada A, Mejia M, Becerril C, Cisneros J, Gaxiola M, Pérez-Padilla R, Navarro C, Richards T, et al. Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PLoS ONE. 2007;2:e482. doi: 10.1371/journal.pone.0000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Y, Murthy JN, Zeng D, Belardinelli L, Blackburn MR. Alterations in adenosine metabolism and signaling in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. PLoS ONE. 2010;5:e9224. doi: 10.1371/journal.pone.0009224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cagnina RE, Ramos SI, Marshall MA, Wang G, Frazier CR, Linden J. Adenosine A2B receptors are highly expressed on murine type II alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:L467–L474. doi: 10.1152/ajplung.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]