Abstract
We examined virus maturation of selected non-enveloped and enveloped ssRNA viruses; retroviruses; bacteriophages and herpes virus. Processes associated with maturation in the RNA viruses range from subtle (noda and picornaviruses) to dramatic (tetraviruses and togaviruses). The elaborate assembly and maturation pathway of HIV is discussed in contrast to the less sophisticated but highly efficient processes associated with togaviruses. Bacteriophage assembly and maturation are discussed in general terms with specific examples chosen for emphasis. Finally the herpes viruses are compared with bacteriophages. The data support divergent evolution of noda, picorna and tetraviruses from a common ancestor and divergent evolution of alpha and flaviviruses from a common ancestor. Likewise, bacteriophages and herpes viruses almost certainly share a common ancestor in their evolution. Comparing all the viruses, we conclude that maturation is a convergent process that is required to solve conflicting requirements in biological dynamics and function.
INTRODUCTION
Virus maturation corresponds to a transition from an initial, non-infectious, assembly product to an infectious virion. This process is observed in virtually all well-studied animal and bacterial viruses. Maturation transitions are a solution to conflicting requirements of particle assembly, particle stability and the energy landscape required for the dynamic processes associated with infection. Initial subunit interactions occur under conditions where the assembling entities have an association energy that favors assembly over disassembly, but that is near equilibrium (normally about 2-4kT). Such moderate interactions are required to permit the associating units to “self-correct” through annealing during assembly. Stronger interactions result in particles trapped in misassembled states not correctable by thermally induced equilibrium dynamics(1). While weak interactions are appropriate for cellular assemblies that associate, transmit appropriate signals and dissociate, virus particles must survive the harsh, extra-cellular environment and require robust stability. The solution to this problem is a staged assembly process in which a procapsid is assembled under conditions required for accurate self-assembly, but the resultant particle carries within it an encoded program for maturation that dictates events to transform the initial assembly product into a robust virion, satisfying the second purpose of maturation. During this process the third reason for maturation is also addressed; that of creating a properly structured energy landscape for additional transitions during entry and genome delivery required for infectivity. Invariably, however, there is still a “safety switch” that must be released to actually confer infectivity. Indeed, creating an infectious virion within an infected cell is not productive from the perspective of a virus. Instead, the late provirion contains all the ingredients required for infectivity but lacks an essential element that confers this activity. Among non-enveloped viruses, activation of infectivity is frequently an autocatalytic cleavage that covalently liberates a lytic, fusion-like, peptide that remains associated with the particle(2). This cleavage is designed to occur in the extracellular environment, thus preventing the release of a membrane active polypeptide within the cell. Likewise, among enveloped viruses a final maturation step, often involving a host protease, activates their fusion peptides in the latter stages of budding through membranes in the secretory pathway(3).
The mechanisms of maturation are diverse when different virus families are compared, indicating convergent evolution toward greater sophistication in this essential process. In this review we chose virus families that have been well studied (non enveloped noda, tetra and bacteriophage from our own laboratory) and that are representative of the breadth of approaches to maturation that have been discovered. Some are medically important and others highly accessible to a variety of biophysical methods. Given the space limitations, the review could not be comprehensive, nor was it possible to reference all of the original literature associated with the different systems described and we apologize in advance to the authors not properly cited. We do hope that the reader will find this review informative and share with us the interest in these accessible, programmed processes that inform us of the larger domain of biological dynamics.
SMALL, SSRNA, NON ENVELOPED VIRUS MATURATION
Noda and tetraviruses are formed by a single subunit type and undergo post assembly maturation cleavage that is required for particle stability and infectivity. Nodaviruses grow in insects, plants, mammals and yeast while tetraviruses have only been grown in Lepidoptera and yeast. Picornaviruses are more complex with different gene products occupying the positions occupied by a single gene product in T=3 (Figure 1) viruses and they also undergo a maturation cleavage required for stability and infectivity. The single subunit viruses are discussed first, then the picornaviruses.
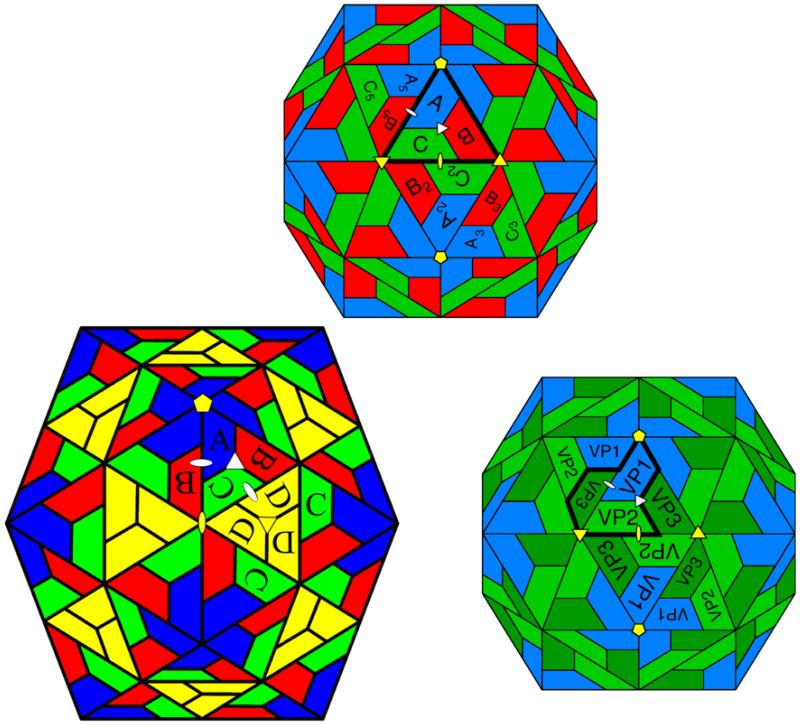
Figure 1.
Virus capsids with T=3, T=4 and picornavirus subunit organizations. Top The T=3 surface lattice presented as a rhombic triicontahedron. Icosahedral symmetry axes are in yellow and quasi symmetry axes are in white. Each trapezoid represents a subunit and A, B and C (identical gene products) occupies an icosahedral asymmetric unit (heavy outline). The three subunits are related by a quasi 3-fold axis (white triangle) and icosahedral asymmetric units are related by an icosahedral 2-fold (yellow ellipse) relating C to C2 etc. and a quasi 2-fold axis (yellow ellipse) relating A to B5 etc. Icosahedral 3-fold axes are coincident with quasi 6-fold axes relating green and red trapezoids. There are 180 subunits in the T=3 particle. Lower left The T=4 quasi-equivalent surface lattice with 4 subunits (labeled A, B, C, and D, corresponding to the same gene product) in the icosahedral asymmetric unit. The triangle containing A, B, and C is closely similar to the T=3 asymmetric unit. It is related to the D,D,D trimer by a quasi-2-fold axis and to the neighboring A,B,C triangle by a different quasi 2-fold axis. Note that the icosahedral 2-fold axis is coincident with the quasi 6-fold axis. There are 240 subunits in the T=4 particle. Lower right The picornavirus capsid. The particle is geometrically identical to the T=3 particle, but now VP1, VP2, and VP3 are different gene products, but all have similar jelly roll folds. The heavy outline shows the “biological” asymmetric unit, with these three trapezoids initially corresponding to a poly-protein prior to proteolytic processing. There are 60 copies of each gene product in the particle.
Nodaviruses
Nodaviruses are among simplest infectious agents. Flock House Virus (FHV) is the most studied nodavirus and the effort has revealed a variety of mechanistic features associated with maturation(4). FHV is a 320Å, non-enveloped, T=3 icosahedral virus that encodes 3 gene products (RNA directed RNA polymerase, inhibitor of cellular RNAi response, and capsid protein) in 2, co-encapsidated, RNA molecules with a total coding capacity of ~5000 ribonucleotides (5). Forty-eight hours post infection, ~20% of the cell mass is virus. The FHV subunit alpha is 407 amino acids and assembles to form non-infectious particles (provirions). An autocatalytic cleavage initiates after assembly that cuts the subunits at residue 363 creating beta (residues 1-363) and gamma (residues 364-407). The gamma peptides remain associated with the particle and the first 25 residues (forming an amphipathic helix) are visible in the X-ray structure of the mature virions(6). The remaining gamma peptide residues are not visible in the electron density. Cleavage is required for infectivity and for the virions to lyse dye-filled, artificial liposomes in vitro (7; 8). Thus the peptide is lytic for membranes and assumed to allow either virions or RNA to enter the cytoplasm from particles in endosomes. There is undetectable change in morphology between provirions and virions. The mechanism for the maturation cleavage is well understood (9). ASN 363 is adjacent to an acidic residue (ASP75) that functions as a base to polarize the ASN side chain. The polarized side chain makes a nucleophilic attack on the main chain carbonyl carbon forming a cyclic imide. This is then hydrated, resulting in the cleavage. Closely similar reactions have been performed in vitro(10) and they are known to participate, in vivo(11), in the degradation of proteins (e.g. cleavage or deamidation at ASN residues(12; 13) change the solubility of crystalline in the eye, causing cataracts). Prior to cleavage the particles are fragile and can be disassembled under mild conditions(14). Following cleavage they are robust. The residues that catalyze the cleavage are all on the same polypeptide chain, yet cleavage does not occur until assembly. The quaternary structure interactions required to place ASP75 adjacent to ASN363 only occur in the assembled particle, controlling the timing of the reaction(15).
Tetraviruses
Tetraviruses are named for their T=4 quasi-equivalence. They are the only non-enveloped virus group studied with this quasi-symmetry, although the alpha viruses in the enveloped category also have T=4 symmetry. The majority of tetravirus maturation data is derived from studies of Nudaurelia Omega Capensis Virus (NWV) and Helicoverpa armigera Stunt Virus (HaSV). Crystal structures of these viruses were determined at 2.8 (16) and 2.5Å resolution (unpublished) respectively, providing atomic coordinates for interpreting other data. The four subunits in the icosahedral asymmetric unit have closely similar jellyroll folds with an entire Ig domain of ~ 100 residues inserted between strands E and F of the jellyroll. The insect tetraviruses are similar to the T=3 insect nodaviruses(17), undergoing an autocatalytic maturation cleavage with the active sites virtually superimposable and displaying high structural homology in the secondary structures of the jellyrolls(18). The N-terminal portions of the gamma peptides are also closely similar in their positions within the capsid. Unlike the noda viruses the NWV and HaSV gamma peptides associated with the C and D subunits participate in the molecular switching to form a flat contact at the quasi 2-fold axes relating these two subunits (Figure 1).
The expression and spontaneous assembly of tetravirus subunits in a baculovirus system facilitated the study of their maturation (19). Unless otherwise noted, the two viruses share very similar maturation properties. NWV particles purified from the expression system at pH 7.6 are 480 Å. CryoEM analysis revealed subunits distributed with T=4 symmetry and associated as dimers. Lowering the pH to 5 reduced the particle size to 410Å with subunits distributed as trimers with morphology indistinguishable from the authentic virions (Figure 2) (20). As in nodaviruses, maturation cleavage in tetraviruses covalently liberates a polypeptide, an event required for membrane lysis and particle entry. NWV cleavage occurs at pH 5.0 when the particle is in the smaller, mature conformation. It is a slow, non-linear, autocatalytic reaction with 50% of the subunits cleaved in 30 minutes while the remaining subunits require more than 4 hours to cleave(21). If at least10% of the subunits cleave, the particle cannot expand when the pH is raised to 7 or above (22). While near-atomic resolution structural data is not available for provirions, it is likely that the mature conformation is required for positioning the catalytic residues for cleavage, since the cleavage reaction proceeds at the same rate at pH 7 as at pH 5 following cleavage of 10% of the subunits(21). If the subunits are not cleaved the entire maturation conformational change is reversible when the pH is raised to 7.6 (23). The stabilization from the cleavage is due to parts of the C and D gamma peptides being fully positioned into the interface between these subunits, stabilizing these interactions. Prior to cleavage the switch portion of the C and D gamma peptides are dynamic and not properly wedged into the interface, although the morphology of the non-cleaved particle at pH 5 is virtually identical to authentic virions (24).
Figure 2.
Tetravirus maturation. Particles purified from the baculovirus expression system at pH 7.6 are 480Å in diameter and the subunits form dimeric “dumbells” in the T=4 surface lattice(upper left). By carefully controlling the pH the particles can be titrated to different intermediate dimensions and cryoEM structures were determined. As the pH is lowered the subunits form trimers and at pH 5.5 that is the dominant morphology. At that pH the particles are 410Å in diameter. Subunit cleavage at residue 570 initiates at pH 5.5. At 30 minutes roughly half the subunits are cleaved and it takes more than 4 hours for the remainder to cleave.
The formation of the provirion in vivo facilitates the organization of the T=4 capsid and allows subunits to assemble in a near reversible manner. Evidence suggests that the subunits are dimers in solution prior to assembly and these associate to make the spherical provirion. There is an electrostatic attraction between the basic N-terminal region of the capsid protein and the RNA and there is electrostatic repulsion between the subunit interfaces at pH 7 that maintains the expanded state(25). The provirions are readily disassembled in moderate salt concentrations(26). The expanded state of the provirion minimizes the difference between the two classes of quasi 2-fold axes (relating A and B subunits and C and D subunits), with both displaying radial orientations from the particle center. As the pH is lowered, the acidic residues causing subunit repulsion are protonated and the negative charge neutralized allowing the decrease in particle size and obvious faceting with an icosahedral shape. The two classes of 2-fold axes dramatically differentiate with the 2-fold relating C and D subunits becoming “flat” with the axial direction perpendicular to a 3-fold face of the icosahedron and the 2-fold relating A and B subunits remaining bent with the axial direction still roughly radial. This particle is stable and infectious after the autocatalytic cleavage. The timing of maturation is controlled by the viral infection and the induction of apoptosis(27). At that point the pH of the cell is reduced and maturation begins. The stable infectious particle is released from the cell and the infection propagated.
Picornaviruses
Picornaviruses are the most common and diverse small, non-enveloped viruses. The most studied genera being aptho (e.g. foot and mouth disease virus) and entero (e.g. poliovirus and rhinoviruses). Particle assembly has been investigated for decades and involves a complex cascade of polyprotein processing by virally encoded proteases with well-defined pentamers of VP0, VP3, and VP1 as assembly intermediates(28). The provirion of mammalian picornaviruses contains these polypeptides in the icosahedral asymmetric unit, each formed by a virus jellyroll fold and positioned at the C, B, and A positions of the T=3 particle shown in Figure 1. The provirion of poliovirus is fragile, but morphologically similar to the mature virion. Infectivity and stability is gained by a cleavage that liberates a myristoylated, 68 residue, N-terminal portion of VP0 (VP4) from the remainder of VP0 (VP2). The reaction is autocatalytic and occurs between ASN68 of VP4 and SER1 of VP2. It requires the packaged RNA genome and at least one catalytic residue, identified as HIS195 in VP2. Mutation of that residue to THR, ARG, ASP and GLY were lethal(29). Like gamma in noda and tetraviruses, VP4 is dynamic and, although internal in the X-ray structure, is accessible to the outside of the particle and proposed to function as a lytic peptide for virus entry(30). Inhibition of VP4 dynamic behavior by well-studied antiviral agents renders the particles non-infectious(31).
SMALL, SSRNA, ENVELOPED, VIRUS MATURATION
The alpha and flavi togaviruses have significant literature describing their assembly and maturation(32). Viruses in each family considered here have icosahedral symmetry with an inner membrane. Sindbis virus and semliki forest virus are the primary subjects of Alpha virus maturation, while Dengue virus is the most investigated member of the flaviviruses. Alpha viruses have T=4 surface quasi-symmetry with two surface glyco-proteins (E1 and E2), the internal membrane and an inner nucleoprotein core formed by the RNA genome, packaged within a T=4 nucleic acid binding protein shell. Dengue virus has a T=1 surface with 180 copies of a major glyco-protein subunit type (E protein) arranged in a herringbone manner that does not correspond to classical Caspar-Klug quasi-symmetry. The M protein critical for maturation resides in the lipid bilayer and between the bilayer and the E protein. Maturation of flaviviruses depends on processing of the pre M protein (prM) protein that permits formation of the mature particle. The structures of the tick borne encephalitis virus (a flavivirus) subunit(33) and the E1 subunit of semliki forest virus(34) are closely similar, strongly suggesting a common ancestor in their development. In spite of this unifying element, the two virus families have significantly different maturation strategies.
Alphaviruses
The mature alphavirus particles have an internal nucleoprotein particle with T=4 quasi-symmetry. The subunits interacting with the RNA (C-subunits) have a chymotrypsin like fold from residues 106-264, while the N-terminal residues are basic, invisible in the X-ray structure, internal and presumably interact with RNA(35). The protein is an active protease that functions only in cis and releases the E1-prE2 polyprotein from the initial C-E1-prE2 polyprotein. The outer surface of the C-protein mediates interactions with the C-terminal region of glyco-protein E2 that is anchored in the particle membrane. E1, also anchored in the membrane, interacts with E2 on the surface to form the T=4 surface lattice(36; 37). The shape of E2 suggests that it may be related to the known atomic model of E1(38). The characteristic surface spikes are formed primarily by E2 oriented roughly perpendicular to the lipid bilayer, while E1 is oriented roughly parallel to the bilayer except where is splays upward to interact with E2(39). The entire disposition in the mature particle is set for receptor binding via E2 and fusion with the target membrane mediated by E1. Maturation of alpha viruses occurs within the cell during its late stage of egress to the surface. The initial translation product of E2 contains 64 amino acids (called E3) at its N-terminus that prevents the exposure of the fusion peptide associated with E1 as the heterodimer proceeds through the golgi apparatus (40; 41). Later in the secretory pathway a furin-like cellular protease digests E3, maturing the hetero dimer as it is placed in the outer plasma membrane prior to budding(42).
Flaviviruses
Dengue and West Nile virus are among the best structurally characterized of the 70 odd, medically important, viruses in this family. Like alpha viruses they have a capsid protein that interacts with the genomic RNA, but it has a different structure from the capsid protein of alpha viruses and it has not been possible to establish any symmetry of this internal nucleoprotein particle(43). The lipid bilayer anchors the E protein and it has been possible to describe its orientation, topological organization (C-terminal helices are embedded in the membrane) and its bizarre non quasi-equivalent organization of three E proteins in the icosahedral asymmetric unit(44). Density corresponding to the M protein is located in the bilayer and under E. Maturation of Dengue virus has been studied in detail with an excellent quality reconstruction of the immature particles (45) and a high resolution structure of a designed, unprocessed prM-E protein heterodimer (46). Prior to cleavage prM essentially covers the fusion peptide of the E protein, preventing exposure under the variety of conditions within the cell during assembly and egress. The large-scale pH dependent reorganization of the Dengue virus immature particle was shown to be reversible. Indeed, the prM protein reversibly moves from the top of the spike in the provision at pH 7, where it protects the fusion protein from premature release, to the outer portion of the surface at low pH where it allows exposure of the fusion protein. When the pH is lowered, the prM protein can be digested in vitro with a furin-like protease.
RETROVIRUSES
Although different retroviral genera were historically defined based on capsid morphology, the folds observed in equivalent structural proteins among several retroviruses were conserved despite lack of any sequence identity (47). We will provide an overview of the mechanisms governing retroviruses formation in the context of the well-characterized Human Immuno-Deficiency virus 1 that is the focus of many studies due to its clinical relevance.
Following cell entry, the virally encoded reverse transcriptase copies the viral RNA genome into DNA that is then transferred from the cytoplasm to the nucleus where the viral integrase mediates its insertion into the host genome to hijack the cell protein synthesis machinery. Translation of the viral mRNAs results in the production of structural polyprotein precursors (Pr55Gag, Pr160GagPol and gp160) as well as of accessory and regulatory proteins (48). Pr55Gag is co-translationally myristoylated and encompasses four major domains (matrix (MA), capsid (CA), nucleocapsid (NC), and p6) along with two spacer peptides (SP1 and SP2). It encodes all the necessary information for particle assembly though infectious virion production requires specific packaging of dimeric RNA genome and assistance from various cellular factors (47). Virion assembly takes place at lipid rafts in the cytoplasmic membrane that contains the surface (gp120) and transmembrane (gp41) envelope glycoproteins resulting from gp160 processing by a cellular protease (48). Therefore, Pr55Gag anchors to the membrane via its myristoyl moiety and through interactions between a MA basic patch and phosphatidyl-inositol biphosphate whose presence is required for proper membrane targeting. Formation of immature particles is mediated by Pr55Gag oligomerization (involving CA, SP1 and NC domains), which is coupled to interactions with both the membrane and the viral RNA genome, along with incorporation of Pr160GagPol (resulting from a ribosomal frame-shift during Pr55Gag mRNA translation) that encodes for the three viral enzymes (reverse transcriptase, integrase and protease) (47; 48). HIV-1 immature virions are incomplete spherical particles harboring radially arranged Pr55Gag molecules whose CA and SP1 domains form a lattice of cup-shaped hexamers while MA and NC regions do not obey this hexagonal order (Fig. 3A and B) (49). The NC domains point toward the center of the particle where they cluster Pr55Gag molecules together via interactions with the RNA genome and possibly between NC domains themselves.
Figure 3.
HIV maturation. Cartoon depiction of the immature (A) and mature (B) HIV-1 virions architecture. Central slices through cryo-electron tomograms of the immature (A) and mature (B) HIV-1 virions. The different components of the virions are color-coded: envelope glycoprotein (purple), MA domain (dark red), CA N- (dark green) and C-terminal (light green) domains, NC domain (dark blue) and viral RNA (red trace). (E) The conical fuellerene HIV capsid. The CA protein forms both hexamers (green) and pentamers (red).
Thereafter, immature HIV-1 virions bud and exit the cell in acquiring a lipid membrane featuring a lipid raft-like composition (48). The viral protease is activated concomitantly with particle release and processes both Pr55Gag and Pr160GagPol to yield individual constituent proteins. This triggers the major step of the maturation process during which the Gag shell is disassembled (while MA remains membrane-bound) and resulting in reassembly of ~1500 copies of CA to form the conical core, condensation of the NC/RNA complex at the center of the core (which also encloses both the integrase and reverse transcriptase) and stabilization of the dimeric RNA genome (Fig. 3C and D) (47; 48; 50). The mature HIV-1 capsid is a fuellerene cone built from a curved hexagonal assembly, featuring arrays of CA N-terminal domain hexameric rings connected to six surrounding rings by dimerization of the CA C-terminal domains, which harbor a total of 12 pentons (7 and 5 distributed at the wide and narrow ends, respectively) (Fig. 3E) (47; 50-52). Interestingly, a non negligible degree of polymorphism characterizes retroviral capsids and seems to indicate that assembly proceed through preferred, but non-exclusive, pathways in contrast to what is observed in bacteriophages for instance.
BACTERIOPHAGES
Bacterial viruses are elaborate nanomachines designed to infect their hosts with a high efficiency and specificity. The vast majority of them belong to the Caudovirales order and possess a double-stranded DNA (dsDNA) genome enclosed in an icosahedral head to which is attached a tail. They are the most populated biological entity on earth with an estimated number of 1031 tailed phages in the biosphere. The tail serves as a basis to classify Caudovirales into three distinct families: Myoviridae, with a complex-contractile tail; Podoviridae, bearing a short tail; Siphoviridae, characterized by their long non-contractile tail.
Due to packaging constraints, phage genomes are densely packed with genes often clustered in operons featuring limited distance between open-reading frames, which sometimes overlap. Phage operons group genes with related functions and their order within the genetic map corresponds to the different phases of the lifecycle. Structural proteins (i.e. the proteins found in the virion) are encoded by the morphogenetic module that is expressed at the late stage of infection corresponding to a typical time frame of ~30 min.
Assembly and maturation of dsDNA phage capsids is a tightly regulated process, both at the genetic and biochemical levels, exhibiting conserved features in all Caudovirales and in some eukaryotic viruses such as herpesviruses. It starts by the formation of a precursor particle (prohead) that serves as container for genome packaging and is simultaneously matured through successive structural transitions, thanks to a mechano-chemical reorganization program encoded in it, eventually yielding a robust capsid (Figure 4A).
Figure 4.
(A) Bacteriophage HK97 assembly and maturation. Hexamers and pentamers of the MCP (gp5) assemble with a dodecameric portal (gp3) and the protease to form the first icosahedral intermediate termed Prohead-I. The protease is therefore activated and cleaves the scaffolding domain (fused at the MCP N-terminus in HK97) as well as itself to produce the metastable Prohead-II which serves as a container for genome packaging (indicated by a red arrow). Capsid expansion occurs simultaneously with cross-linking within the successive expansion intermediates formed up to the final mature and robust Head-II that is stabilized thanks to the formation of protein catenanes. (B) Structure of the Head-II coat subunit with each domain color-coded (PDB 1OHG). Structure of the 6-fold symmetric Head-II coat subunit hexon external (C) and side (D) views with each monomer colored-coded. The latter view highlights the tangential orientation of the subunits relative to the capsid surface. (E) Structure of the Prohead-II coat subunit (PDB 3E8K). Structure of the skewed Prohead-II coat subunit hexon external (F) and side (G) views. The latter view highlights the radial orientation of the subunits relative to the capsid surface
Building blocks involved in the formation of a dsDNA bacteriophage procapsid
In vivo, three to four virally-encoded components are implicated in the assembly of a functional procapsid: the major coat protein (MCP), the portal, the scaffolding protein and the protease (found only in some phages). Virions belonging to the Podoviridae family also incorporate ejection proteins within their procapsid.
HK97 is the first tailed bacteriophage for which a high-resolution capsid structure was reported revealing a unique (at the time) coat protein fold (53). This L-shaped mixed α/β protein can be divided into an axial (A) domain, located near the icosahedral five-fold and quasi six-fold axes, and a peripheral (P) domain composed of the N-arm and P-loop portions and occupying the region between 3-fold axes in the capsid (Figure 4B). A number of MCP structures have now been reported demonstrating a striking structural conservation among dsDNA phages belonging to the three Caudovirales families as well as in some eukaryotic (herpesviruses) and archeal counterparts (54; 55). It seems likely that the capsid subunit of all these virions derived from a common ancestor preceding the divergence of eukaryotes, bacteria and archea.
The portal is a keystone protein, located at a unique capsid vertex, involved in DNA packaging during assembly and allowing release at the onset of infection. Portal proteins from different phages do not have detectable sequence similarity and show large variation in their subunit molecular mass, from 37 kDa (in φ29) to 83 kDa (in P22). However, the structural data reported for these proteins in all three Caudovirales families and herpesviruses clearly demonstrate the conservation of their dodecameric core architecture as well as of the fold of the constituent monomers (54) suggesting here also an evolutionary connection (Figure 5A and B). The lower portal moiety (termed clip and stem domains) corresponds to the conserved scaffold while the upper region (wing) fluctuates significantly in shape and size among virions. For instance, some phages (mostly P22-like Podoviridae) exhibit a C-terminal helical coiled-coil portal extension forming a barrel within the capsid (Figure 5A and B) (56; 57).
Figure 5.
(A) Left, structure of the phage P22 gp1 portal subunit with each domain color-coded (PDB 3LJ5). Only the first part of the C-terminal barrel domain is shown Right, structure of the biologically relevant dodecameric assembly with the monomers colored from blue (N-terminus) to red (C-terminus). (B) Fitting of the full-length P22 portal X-ray structure (PDB 3LJ5) into the asymmetric virion reconstruction at 7.8 Å resolution (EMDB 5231). While the dodecameric core is well accommodated into the EM density, the C-terminal barrel region deviates significantly from it. Two close-up views highlight the gp1 conformational rearrangements undergone at the end of genome packaging within the barrel region. Spooling of the dsDNA around the portal, promotes a switch from its low-pressure conformation (represented by the gp1 X-ray structure, yellow) to its high-pressure one (adjusted model, purple) and allows signaling to the terminase complex that the headful density is reached within the capsid and to cease translocation. This coiled-coil barrel structure may also serve as a valve retaining DNA within the capsid.
At first glance, some variability is also present among dsDNA phage scaffolding proteins as their size can fluctuate between 100 (φ29) and 303 residues (P22) and they can be independent proteins (P22 or φ29), N-terminally fused to the MCP (HK97 or T5) or both an isolated unit and C-terminally fused to the viral protease (λ, P2, herpesviruses). Nevertheless, these proteins share common features among phages and herpesviruses, exhibiting elongated architectures with a high α-helical content (58; 59), and structures of the P22 coat protein-binding region and full-length φ29 scaffolding proteins reveal the conservation of a similar helix-loop-helix motif but lying at opposite termini (Fig.6 A) (60; 61).
Figure 6.
(A) Crystal structure of the dimeric coiled-coil φ29 scaffolding protein (PDB 1NOH) colored from blue (N-terminus) to red (C-terminus). (B) Interactions established within P22 procapsids between the C-termini of ten scaffolding proteins and the portal dodecamer. The P22 portal X-ray structure (PDB 3LJ4) has been fitted in the 8.7 Å resolution asymmetric reconstruction of P22 wild-type procapsid (EMDB 1827) and is depicted as a grey surface. The EM density corresponding to the scaffolding proteins is depicted in yellow with the structure of the P22 scaffolding protein C-terminal domain fitted inside and depicted in red (PDB 2GP8). Only three out of the five coat subunit hexamers surrounding the portal vertex are shown for clarity with each monomer colored independently (PDB 2XYY).
Considering the sequence conservation (although weak) between tailed phages and herpesviruses maturational proteases along with the apparent common evolution of the other basic capsid building-blocks, one can put forward the idea that the proteases of all these virions also result from divergent evolution from a single ancestral gene. Hence, the typical fold disclosed by the reported herpesviruses serine protease structures is likely to be observed in at least part of the dsDNA bacteriophages possessing such an enzyme (62-64).
Scaffolding protein-mediated Prohead assembly
HK97 (Siphoviridae) and P22 (Podoviridae) have been the most extensively studied, due to their well characterized genetics as well as their ease of manipulation in vitro, and we will use the data available for these two virions as a connecting thread to describe a generic assembly and maturation pathway. We propose that common mechanisms govern capsid formation in virtually all dsDNA phages (and herpesviruses) based on the high conservation of the gene order organization of the components taking part in this process as well as on their striking 3D-structural similarity.
At a genetically defined time, induction of the morphogenesis genetic module results in expression of the different gene products involved in procapsid assembly. The capsid subunit becomes the most highly expressed protein and is handled in many phage systems by the GroEL/S chaperone machinery. Two classes of phages are distinguished based on the oligomeric state of their coat protein after release from GroEL/S. In the case of HK97 (or T4), the MCP is detected only in its capsomeric state forming a dynamic mixture of hexons and pentons that are incorporated in the procapsid (65; 66). In contrast, P22, φ 29 and HSV-1 MCP remain monomeric at that point (67-69). Concomitantly, expression occurs for the portal protein (assembling as dodecamers), the scaffolding protein, the viral protease and ejection proteins. Scaffolding proteins/domains act as maestros, orchestrating with high fidelity the initial steps of prohead assembly, to initiate the capsid maturation pathway.
In phage P22, it was proposed that a scaffolding protein dimer interacts via its C-terminal domains with two MCP monomers resulting in a local increase of the MCP concentration (“entropic sink”) to overcome the nucleation energy barrier and promote particle assembly (59). However, recent work suggested that the N-termini would also be involved in such contacts (70).
Scaffolding proteins also determine the architecture of the assembly formed by guiding the association of several hundreds of MCP subunits. Their absence in P22 results in a dramatic reduction of the number of particles produced and prolonged infection times lead to the formation of three different particle types: T=7 icosahedral shell that are indistinguishable from the native procapsid (but without scaffolding proteins), T=4 smaller shells and spirals (71). For phages with a prolate head, as is the case of φ29 or T4, the formation of a scaffolding protein network within the procapsid interior has been suggested to govern capsid elongation during morphogenesis (60).
A recruiting role is exerted by scaffolding proteins through interactions with the portal dodecamer guiding its incorporation into assembled virions (Fig. 6B) (71; 72).
Finally, scaffolding proteins appear to be responsible for priming maturation by inducing dramatic structural distortions in the HK97 MCP within capsomers before their assembly into procapsid (63; 73-75). However, the observation of a similar structural distortion in P22 procapsids formed in the absence of scaffolding proteins (demonstrated by the presence of skewed hexamers) suggests the involvement of a different mechanism in some other virions (71; 72).
Although poorly understood, procapsid nucleation probably occurs through formation of an initiation platform comprising the portal dodecamer onto which scaffolding and coat subunits assemble therefore guaranteeing insertion of the portal into an unique capsid vertex (60; 72). Capsid proteins are then successively added to the growing shell either as capsomers (HK97 or T4) or as monomers/dimers interacting with scaffolding protein dimers (P22 or φ29) (60; 72; 76). The ejection proteins are also incorporated before procapsid assembly is completed though the exact timing is not known. This process results in a procapsid with 60 hexamers and 11 pentamers of the MCP, arranged on a T=7 laevo icosahedral surface lattice that is common for isometric bacteriophages (except T5 with its T=13 capsid (77)). The portal dodecamer, several hundred scaffolding proteins and 60-120 copies of the viral protease molecules (where relevant, Figure 4A) are also present (59; 63; 74).
Structural basis for Prohead metastability
The first particle formed during maturation of viruses encoding a protease (HK97, λ, T4, T5), is termed Prohead-I (according to the HK97 nomenclature). This icosahedral assembly is an organizational intermediate relying on weak quaternary structure (inter-capsomer) interactions to allow annealing of potentially misassembled subunits and avoiding kinetic traps (63). Prohead-I in HK97 is a 560 Å transient particle with a round shape and the long dimension of the MCP subunits oriented roughly radial relative to the capsid surface (63; 75). This reversibly associated particle is fragile and exhibits pores in its shell due to the incomplete sealing of the P-loop mediated inter-capsomer interactions at 3-fold contact points (~40% of such contacts are not formed). The scaffolding domains exert a critical role in modulating these P-loop quaternary interactions by constraining the conformation of the adjacent N-arms and preventing in turn further maturation (63). A striking feature of this particle is the skewed hexamers that exhibit 2-fold symmetry, while the pentons obey strict 5-fold symmetry. This skewed architecture results from the MCP secondary and tertiary structure distortions (bending of the spine helix through twisting of the subunit around the P-domain β-sheet) induced by the scaffolding domains at the capsomeric state.
Upon Prohead-I assembly, the viral protease (which is tethered on the scaffolding domains) is activated, probably through a dimerization mechanism (64; 78), digests the scaffolding proteins and auto-digests to produce small polypeptide fragments that diffuse out of the particle through the pores present at the capsomer junctions (63). Processing of the portal protein by the viral protease has been documented in λ and P2 where it removes residues at the N-terminus of each protomer (64). The result of this first maturation step is a metastable particle termed Prohead-II, which contains only coat proteins and a portal dodecamer with the same overall dimensions and shape as Prohead-I (Figure 4A). Beyond ensuring the irreversible nature of this pathway, removal of the scaffolding domains releases the MCP N-arms and thus the associated constraints on P-loop regions allowing formation of strong contacts at all icosahedral and quasi-3 fold axes (63; 75). This strengthens quaternary structure interactions established between capsomers to stabilize the MCP distortions leading to the formation of a metastable particle (Figure 4E-G). Prohead-II is trapped in a local free-energy minimum and can be compared to a compressed spring, primed to transition to a different (lower-energy) conformation in response to small perturbations.
Phages that do not possess a viral protease, such as P22, T7 or φ29, assemble directly a Prohead-II-like particle (ready for genome packaging) that still contains the scaffolding proteins (71; 79-81). Similarly to the HK97 situation, the procapsids of P22 or T7 phages are made of skewed hexamers and 5-fold symmetric pentamers of MCPs (72; 80). This observation along with the striking conservation of the MCP fold among tailed phages strongly suggests the existence of a common mechanism for generation of a metastable prohead based on twisting of the subunit around the P-domain β-sheet. CryoEM reconstructions of the P22 procapsid show the scaffolding proteins C-terminal regions contacting the MCP shell interior with an apparent ratio of 1:1 while the N-terminal moieties are disordered (61; 72). The scaffolding protein C-termini interact with the portal wing domains and probably help the accommodation of the symmetry mismatch between the dodecamer and the pentavalent environment (Fig. 6B) (72). Although less extensive, contacts also exist between the portal clip domains and the surrounding five MCP hexamers.
Genome packaging
Caudovirales phages use nanomotors that convert chemical energy into mechanical work to package their dsDNA genome into preformed procapsids with the packaged genomes at near liquid-crystalline concentration (corresponding to pressures of tens of atmospheres) (82). The packaging machinery comprises three components: (i) the portal dodecamer; (ii) the large terminase and (iii) the small terminase. While the portal is part of the procapsid, the two terminase molecules are transiently associated with it during translocation and are released upon completion of genome packaging. Despite a dramatic sequence divergence between equivalent phage terminase subunits, the structural data reported to date indicate that a conserved mechanism is responsible for dsDNA packaging in tailed phages. The large terminase is formed by a N-terminal ATPase domain (powering packaging) and a C-terminal nuclease domain and functions as a five-membered ring when associated with the T4 or φ29 procapsids though the stoichiometry may vary in other phages (82). The small terminase recognizes DNA and interacts with the large terminase but the situation is unclear regarding its biologically relevant oligomeric state due to the observation of octamers and nonamers in different phages (82).
DNA replication in most tailed phages generate head-to-tail concatemers of the viral genome. The small terminase binds to one of these concatemers and recruits the large terminase that enzymatically cuts the dsDNA. This ternary complex then docks onto the portal dodecamer clip domains, via the large terminase ring, and the DNA is threaded into the procapsid in an ATP-dependent manner via a “pushed and roll” mechanism that doesn’t involve portal rotation (82; 83).
Many phages package DNA until the maximum capacity of the capsid is reached, this strategy is termed “headful” packaging. For instance, P22 incorporates slightly more than exactly one genome into the final mature virion, i.e. 43.5 kbp instead of 41.7 kbp. When the genome is at a defined density within the capsid, the portal triggers packaging termination independently of sequence, acting as a signal transducer by switching between a “low-pressure” to a “high-pressure” conformation (56; 57; 84) (Figure 6C and D). The pressure-sensor domain has been proposed to reside in the upper part of the dodecamer, at the level of the wing domains and the C-terminal barrel extension found in P22-like phages (Figure 5B)(56; 57; 84). As more DNA is introduced and spooled within the prohead, the resulting increase in pressure forces the genome to tighten around the portal. At a critical point, when the capsid has fully expanded (cf. Mechanisms of capsid maturation and stabilization section) and with the genome at a “headful” density, the surrounding ring of DNA exerts such a force on the portal that it changes its conformation, signaling to the terminase complex to cease translocation and to cut the packaged chromosome from the remaining concatemeric DNA. In its new conformation, the portal may also act as a valve allowing retention of the genome inside the capsid (via its C-terminal barrel), and would probably exhibit a reduced affinity of its clip domains for the terminase complex and an increased affinity for the first component of the tail machinery to be assembled, i.e. the first head-completion protein ring (56; 84; 85). Considering the structural conservation among several such proteins in Siphoviridae and Myoviridae (54), a similar affinity switch mechanism is likely involved in many tailed bacteriophages.
Phages of the three Caudovirales families exhibit a dsDNA coaxial spool around the portal vertex axis forming a highly condensed set of hexagonally packed rings filling the capsid with a regular spacing of 24 Å, which appears to be the most space-efficient and energy favorable packing pattern for the cylindrically shaped dsDNA strands (56; 77; 84; 86-89). In some phages, a density within the portal channel was observed and interpreted to be the last end of the packaged genome as if it was poised for injection (84; 87).
Mechanisms of capsid maturation and stabilization
In vivo, DNA packaging triggers prohead maturation corresponding to the transition from local (metastable) stability to global stability and the two processes occur concurrently to produce robust and infectious virions.
The first consequence of genome translocation is the release of scaffolding proteins from the internal particle surface (for virions do not possessing a protease) through pores present in the procapsid (59; 60; 72). It is likely that electrostatic interactions between DNA and the scaffold as well as internal residues of the shell facilitate scaffolding exit. The φ29 scaffolding protein binds non-specifically to DNA, and these interactions may contribute to the synchronization of scaffolding release (60).
Studies of phage HK97 identified numerous maturation intermediates (expansion intermediates, EI) populating the pathway leading from the metastable Prohead-II to the mature Head-II (Figure 4A) (73; 90). The initial transition from Prohead-II to EI-I occurs in a stochastic manner with no populated intermediates, in agreement with the metastable nature of Prohead II (75; 91). EI-I (the maturation ground state) and EI-II are thin-walled, spherical, exhibit a 15% increase of size relative to Prohead-II and bear symmetrical hexamers (92). A striking feature of HK97 capsid maturation is the autocatalytic formation of isopeptide bonds between Lys169, on the E-loop of one subunit, and Asn356, on the P-domain of an adjacent one. EI-II is essentially identical to EI-I but has begun to crosslink (93). Gradual cross-linking occurs simultaneously with expansion up to the balloon state (EI-IV) (Figure 4A) that is even larger (by 9%) and rounder than EI-I and EI-II and possesses all Head-II cross-links except one connecting each penton subunit to a neighboring hexon one (93). Cross-links therefore modulate the propensity of the capsid to undergo its structural reorganization by biasing the thermal motions via a Brownian ratchet mechanism based on the capture of MCP E-loops at the capsid surface (93). The final maturation stage leads to Head-II which is 659 Å wide in its maximum dimension, with pronounced icosahedral facets and flattened (symmetrical) hexons. The shell is only 18Å thick with the MCP oriented tangentially relative to the capsid surface (Figure 4A, C and D)(53). At this stage, 100% of the cross-links are formed generating covalently bonded protein circles that interlock topologically at icosahedral and quasi 3-fold axes to yield molecular chainmail.
The position and quaternary interactions of the P-loops and surrounding β-strands at icosahedral and quasi 3-fold axes are unchanged during expansion demonstrating that it functions as a fixed point of subunit contact in an otherwise highly plastic assembly. Dramatic untwisting and refolding occurs to remove the strain contained in subunit secondary and tertiary structures (Figure 4B and E) (75). Equivalent conformational changes were observed in the P22 MCP between its procapsid and capsid states, providing a structural basis for the exothermic nature of this transition which releases ~21 kcal/mol of capsid (72; 94). We propose that these structural and energetic features are a hallmark of the maturation of all isometric bacteriophages, with “curing” of the hexon skewed asymmetry also observed in bacteriophages λ and T7 (79; 80; 95).
Bacteriophages have evolved different means to stabilize their capsids to withstand the extreme environments encountered during their lifecycle and to deal with the exceptional internal pressures resulting from the density of the packaged genome. Despite closely related molecular architectures and underlying maturation-associated conformational changes in all isometric phages, few of them seem to rely on the HK97 coat subunit cross-linking strategy and several other capsid stabilization schemes have been selected throughout evolution.
For example, phages P-SSP7 and T7 strengthen their capsid during expansion through a reinforcement of the bonding pattern between coat subunits without any major modifications of their structure relative to HK97 (79; 96). P22 and φ29 MCPs are ~150 residues longer than the HK97 MCP and incorporate additional ED or BIG-2-like domains, respectively, which project radially away from the capsid surface (72; 81). These domains are positioned above the E-loop and are involved in capsid stabilization by forming inter-capsomeric contacts (as well as intra-capsomeric contacts in P22) in a region near the chainmail interaction site established in HK97. In phage λ, expansion creates binding sites for the gpD cementing proteins by exposure of regions buried within the procapsid interior (95). The trimeric nature of gpD fastens together six MCP subunits from three different capsomers in a manner and location strikingly reminiscent of the HK97 cross-links. The functional importance of gpD is illustrated by the fact that, in its absence, the internal pressure disrupts the capsid when 90% of the genome is packaged.
At that stage, decoration proteins are added on the surface of the capsid and maturation is finalized by attachment of the head completion proteins to the portal to constitute a platform for tail formation/addition. In Podoviridae, the different components of the tail machine are sequentially assembled onto the capsid and the tail needle protein trimer has been proposed to act as the plug preventing leakage of the highly condensed dsDNA genome (56; 85). In long tailed phages (Siphoviridae and Myoviridae), the tail is separately assembled before being added onto the capsid. The head-completion protein forming the second (most distal ring) of the connector is known to form the plug in SPP1 and considering its structural conservation among both sipho- and myophages, a conserved DNA gatekeeping mechanism is likely to be found in such virions (54).
HERPESVIRUSES ASSEMBLY AND MATURATION
Although they infect a wide range of vertebrate hosts, herpesviruses have retained a similar multilayered organization characterized by a nucleocapsid surrounded by tegument proteins and a lipoproteinaceous envelope. As suggested by the structural conservation among their equivalent capsid components, these viruses share many characteristics with tailed bacteriophages regarding assembly/maturation and they probably arose from a common progenitor (54). However, the HSV-1 MCP is a 150 kDa polypeptide (~5 times the mass of the HK97 MCP) whose structure is formed by a floor domain (folded as Caudovirales MCPs) and an additional mixed α/β protrusion domain accounting for half of the total protein mass. The complex architecture of herpesviruses, consisting of more than 30 virally-encoded proteins, requires a sophisticated production pathway involving several cell compartments (97).
After viral gene expression and DNA replication, the proteins involved in procapsid formation are transported from the cytoplasm to the nucleus where assembly initiation takes place. Procapsids are ~1250 Å, T=16 icosahedral particles containing a dodecameric portal (UL6) at a unique vertex. The shell contains MCP (VP5) hexamers and pentamers as well as triplexes made up of one copy of VP19c and two copies of VP23 present at 3-fold lattice sites. An internal core lines the shell surface interior and is built from a mixture of scaffolding proteins fused (preUL26, 10%) and not fused (preUL26.5, 90%) to the maturational protease as a result of a read-through event. The MCP protrusion domains, extending outwards from the procapsid surface, exhibit a departure from 6-fold symmetry within hexons that is reminiscent of the phage procapsid situation (98). In assembled procapsids, the protease is activated through dimerization and it digests the scaffolding protein terminal peptide disrupting the connection with the outer shell and allowing scaffold release (78; 98). The resulting particles serve as containers for DNA packaging and concomitantly undergo maturation involving symmetrization of the coat protein protrusion domains along with formation of enhanced interactions within hexameric rings and formation of strong lateral contacts between floor domains of neighboring capsomers (98; 99). These conformational changes (occurring without expansion) allow the creation of external binding sites for VP26 (whose role is unknown) at the interface between adjacent protrusion domains within hexamers but not pentamers.
DNA-filled capsids initially acquire tegument proteins in the nucleus before they gain an envelope via budding at the inner nuclear membrane during the course of primary envelopment. This occurs is conserved in the three herpesviruses subfamilies investigated (97; 100). There is then a loss of the envelope through fusion of the virus envelope with the outer nuclear membrane releasing non-enveloped capsids in the cytoplasm. Most of the tegument proteins are added to the capsid at this stage, which involves remodeling of existing components, before secondary envelopment occurs at vesicles of the trans-Golgi network. Finally, particles mature by recruiting a glycoprotein-containing lipidic bilayer during egress from the cell through the secretory pathway.
CONCLUSIONS
We surveyed maturation in a variety of well-studied virus families. The non-enveloped animal viruses discussed exhibit common structural and maturation properties that make a compelling argument for them sharing a common ancestor. Nodaviruses are exceptionally efficient killing machines with minimal genetic and structural sophistication, yet their success suggests that they are optimally evolved for the niche that they occupy. No morphological intermediates have been detected during maturation with only the autocatalytic cleavage defining the transition from provirion to virion. The cleaved gamma peptides occupy three non-equivalent positions in the icosahedral asymmetric unit and, based on their positions relative to the surface, we suggest that the cleaved peptides at the pentamers are active in entry. Tetraviruses add another level of sophistication by assembling 240 copies of one gene product. Tetravirus maturation occurs to facilitate assembly, stabilize the particle and activate the autocatalytic cleavage. Here, morphological intermediates can be isolated with maturation requiring pH 5.5 (occurring at the last stage of infection with cellular apoptosis). The resulting gamma peptides are multi-functional; with peptides from the C and D subunits functioning as molecular switches while the A subunit gamma is likely to be the lytic peptide for entry. Picornaviruses share the architecture of the T=3 particles, but each quasi-equivalent position in the icosahedral asymmetric unit is occupied by a separate gene product that retains the jelly roll fold observed in noda and tetra viruses. There is no detectable sequence similarity between VP1, VP2, and VP3, but the folds are closely similar. Like nodaviruses, there is no intermediate morphology observed during maturation and the transition from provirion to virion occurs with the cleavage of VP4 and VP2. Picornaviruses cleavage occurs at the N-terminal region, while noda and tetravirus cleavage occurs at the C-terminal region. The N-terminus of VP4 is myristoylated, facilitating its entry into membranes. The cleaved peptides in noda, tetra and picornaviruses can all access the surface even though they are internal in the X-ray structures.
The alpha and flaviviruses are medically important and share common maturation features, but the mature particles have different architectures and morphologies. Both viruses have an internal ssRNA, nucleoprotein core surrounded by a membrane with outer, icosahedrally distributed, glycoproteins. The two types of provirions have similar appearance with obvious protrusions visible in cryoEM images, but displaying different quasi-symmetries. They both undergo pH dependent morphological changes that facilitate access to the cleavage sites in the glycoproteins that exposes the fusion peptide required for infection. The mature particles however are quite different in the two virus families. Alphaviruses have two glycoproteins that form protrusions arranged with T=4 quasi-symmetry, while flaviviruses have three copies of one type of glycoprotein in the icosahedral asymmetric unit and they display no quasi-equivalent interactions. The flavi glycoprotein is structurally related to the alphaviruses E1 glycoprotein and there is good evidence that alphaviruses E2 also shares the same fold. Receptor binding and fusion are carried out by E2 and E1, respectively, in Alpha viruses, while both functions are performed by the single glycoprotein in flaviviruses.
Retrovirus maturation has been studied extensively. We provided a brief description for comparison with alpha and flaviviruses. The mechanistic understanding of maturation in HIV has lead to the protease inhibitor antivirals that have had enormous success. It is a striking example of the crucial role that proteolysis plays in maturation and the good fortune of having a virally encoded protease that could be uniquely targeted by inhibitors. Alpha and flaviviruses depend on host proteases for their maturation and cannot be controlled by the strategy that has been so successful in HIV.
Double stranded DNA bacteriophages play a central role in the study of viral maturation. They have been studied for nearly 60 years and their accessibility to physical methods has generated paradigms for maturation that are recapitulated in the other systems studied. There has been a renewal of interest in these viruses in the last decade, as detailed structures have provided mechanistic understanding of processes that were carefully analyzed decades ago. While there are clear variations among the different bacteriophage genera, the similarities are striking. The role of scaffold proteins or scaffold domains in creating meta-stable particles, poised for exothermic maturation, appears to be wide spread and the surge in studies of the packaging motors and their structures is also revealing common themes. The fact that these themes are found in members of the herpesviruses is a strong indication that phages and herpesviruses share a common ancestor. When high-resolution structures of the herpesviruses packaging motors will be determined, these would constitute wonderful targets for antiviral agents directed against this virus group.
Finally, virus maturation provides opportunities to explore mechanisms of biological dynamics in exquisite detail with modular systems that can be investigated by single particle analysis, crystallography, electron microscopy and a myriad of biochemical and biophysical methods. Whether dynamic aspects of cell biology uses any of the strategies seen in viruses remains to be seen, but we certainly know what to look for.
Summary Points.
Virus maturation is the programmed structural and biochemical change that occurs between formation of the initial particle and development of the infectious virion.
Virus maturation occurs in the large majority of animal viruses studied and in all bacteriophages.
The final stage of animal virus maturation frequently involves proteolysis (or autocatalytic cleavage) of the capsid proteins to expose a fusion peptide (enveloped viruses) or a lytic peptide to translocate either the particle or the viral genome across a cellular membrane (non enveloped virus).
Maturation of HIV has been an important target for antiviral agents with viral protease inhibitors stopping the formation of an infectious particle.
Bacteriophage maturation is more complex than in most animal viruses requiring the formation of an empty capsid, the use of packaging enzymes to fill the capsid and defined transitions between different capsid morphologies and finally the addition of tails to the particles.
Herpesvirus has a morphogenesis and maturation very similar to bacteriophages.
While bacteriophages have similar assembly and maturation programs, there is a wide variety of animal virus maturation.
ACKNOWLEDGEMENTS
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review. Work in the lab of JEJ was supported by grants from the National Institutes of Health R37 GM034220, R01 GM054076 and R01 AI040101. DV is supported by a FP7 Marie-Curie International Outgoing Fellowship (273427).
Acronyms
- C protein
Capsid protein in retroviruses
- E
Receptor binding and fusion protein in flaviviruses
- E1
Fusion glycoprotein in alphaviruses
- E2
Receptor binding protein in alphaviruses
- Ma protein
Matrix protein in retroviruses
- MCP
major capsid protein
- NC protein
Nucleocapsid protein in retroviruses
- VLP
virus-like-particle
Mini glossary
- Autocatalytic cleavage
The spontaneous cleavage of a polypeptide that does not depend on an exogenous protease. This usually occurs at an aspargine residue and the efficiency depends on the details of the “active site” that is usually formed when assembly or maturation occurs. Noda, tetra and picorna viruses all use this mechanism for maturation.
- Portal
Then entry and exit location for dsDNA in bacteriophages and herpesviruses. In the particle it is formed by 12 subunits and it is positioned at a pentavalent (site normally occupied by a pentamer) in the capsid.
- Procapsid
Any of a number of different maturation intermediates on the pathway to provirion formation. Bacteriophage, such as HK97 have many defined intermediates in the maturation pathway, each corresponding to a different procapsid.
- Provirion
The penultimate particle prior to formation of an infectious virion. Typically a proteolytic event in the provirion subunits will render the provirion infectious. This may be an autocatalytic cleavage (e.g. noda, tetra and picornaviruses) or cleavage by an exogenous, cellular protease such as furin (e.g. alpha and flaviviruses).
- Quasi-equivalence
Symmetry that exists in a local environment and illustrated in Figure 1. It also describes associations that are similar but not equivalent. Pentamers and hexamers are quasi-equivalent since changing an angle from to 60 degrees (hexamer) to 72 degrees (pentamer) can allow the same inter subunit contacts if there is curvature added to the pentamer.
- Scaffold protein; scaffold domain
Either an independent protein or a domain of a capsid protein that guides the assembly of bacteriophage subunits into a properly formed shell. They are scaffolds because they are removed after assembly is complete. When it is a domain, it is removed by proteolysis and when it is an independent protein it leaves during dsDNA packaging. In the case of P22 the same proteins are recycled in multiple rounds of assembly.
- Viral jelly roll
A tertiary structure found in many viral subunits. It is an 8-stranded beta sandwich with strands BIDG on one side and CHEF on the other where the letters indicate the location of the strand in the primary structure.
- Virus like particles
Particles formed when viral subunits are expressed in a heterologous expression system such as the baculovirus system. The particles undergo assembly and maturation in a manner indistinguishable from authentic virions, but they do not contain the viral genome, so they are not infectious.
References
- 1.Katen S, Zlotnick A. In: Methods in Enzymology: Biothermodynamics, part A. Johnson ML, Holt JM, Ackers GK, editors. Vol. 455. Academic Press; New York: 2009. pp. 395–417. Number of 395-417. An excellent quantitative analysis of the range of association energies appropriate for biological self-assembly processes.
- 2.Banerjee M, Johnson JE. Current protein & peptide science. 2008;9:16–27. doi: 10.2174/138920308783565732. A review comparing lytic peptides in non-enveloped viruses with fusion peptides in enveloped viruses. The conclusion is that non enveloped viruses have converged on similar strategies, but different mechanisms, to deliver their genomes to the cytoplasm
- 3.White JM, Delos SE, Brecher M, Schornberg K. Crit Rev Biochem Mol Biol. 2008;43:189–219. doi: 10.1080/10409230802058320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odegard A, Banerjee M, Johnson JE. Curr Top Microbiol Immunol. 2010;2100:1–22. doi: 10.1007/82_2010_35. [DOI] [PubMed] [Google Scholar]
- 5.Schneemann A, Reddy V, Johnson J. In: Adv Virus Res. Maramorosch K, Murphy F, Shatkin A, editors. Vol. 50. Academic Press; New York: 1998. pp. 381–46. Number of 381-46. [DOI] [PubMed] [Google Scholar]
- 6.Fisher A, Johnson J. Nature. 1993;361:176–9. doi: 10.1038/361176a0. [DOI] [PubMed] [Google Scholar]
- 7.Odegard AL, Kwan MH, Walukiewicz HE, Banerjee M, Schneemann A, Johnson JE. J Virol. 2009;83:8628–37. doi: 10.1128/JVI.00873-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneemann A, Zhong W, Gallagher TM, Rueckert RR. J Virol. 1992;66:6728–34. doi: 10.1128/jvi.66.11.6728-6734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy V, Schneemann A, Johnson J. In: Handbook of Proteolytic Enzymes. Barret A, Rawlings N, Woessner J, editors. Elsevier Ltd.; Amsterdam: 2004. pp. 197–201. Number of 197-201. [Google Scholar]
- 10.Stephenson RC, Clarke S. J Biol Chem. 1989;264:6164–70. [PubMed] [Google Scholar]
- 11.Robinson NE, Robinson AB. Mech Ageing Dev. 2004;125:259–67. doi: 10.1016/j.mad.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Voorter CE, de Haard-Hoekman WA, van den Oetelaar PJ, Bloemendal H, de Jong WW. J Biol Chem. 1988;263:19020–3. [PubMed] [Google Scholar]
- 13.Hains PG, Truscott RJ. Invest Ophthalmol Vis Sci. 2010;51:3107–14. doi: 10.1167/iovs.09-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneemann A, Gallagher TM, Rueckert RR. J Virol. 1994;68:4547–56. doi: 10.1128/jvi.68.7.4547-4556.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang L, Johnson K, Ball L, Lin T, Yeager M, Johnson J. Nat Struct Biol. 2001;8:77–83. doi: 10.1038/83089. [DOI] [PubMed] [Google Scholar]
- 16.Helgstrand C, Munshi S, Johnson JE, Liljas L. Virology. 2004;318:192–203. doi: 10.1016/j.virol.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JE, Munshi S, Liljas L, Agrawal D, Olson NH, et al. Archives of virology. Supplementum. 1994;9:497–512. doi: 10.1007/978-3-7091-9326-6_48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munshi S, Liljas L, Cavarelli J, Bomu W, McKinney B, et al. J Mol Biol. 1996;261:1–10. doi: 10.1006/jmbi.1996.0437. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal DK, Johnson JE. Virology. 1995;207:89–97. doi: 10.1006/viro.1995.1054. [DOI] [PubMed] [Google Scholar]
- 20.Canady MA, Tihova M, Hanzlik TN, Johnson JE, Yeager M. J Mol Biol. 2000;299:573–84. doi: 10.1006/jmbi.2000.3723. [DOI] [PubMed] [Google Scholar]
- 21.Matsui T, Lander G, Johnson JE. J Virol. 2009;83:1126–34. doi: 10.1128/JVI.01859-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canady MA, Tsuruta H, Johnson JE. J Mol Biol. 2001;311:803–14. doi: 10.1006/jmbi.2001.4896. [DOI] [PubMed] [Google Scholar]
- 23.Taylor DJ, Krishna NK, Canady MA, Schneemann A, Johnson JE. J Virol. 2002;76:9972–80. doi: 10.1128/JVI.76.19.9972-9980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang J, Lee KK, Bothner B, Baker TS, Yeager M, Johnson JE. J Mol Biol. 2009;392:803–12. doi: 10.1016/j.jmb.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsui T, Tsuruta H, Johnson JE. Biophys J. 2010;98:1337–43. doi: 10.1016/j.bpj.2009.12.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor DJ, Johnson JE. Protein Sci. 2005;14:401–8. doi: 10.1110/ps.041054605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomasicchio M, Venter PA, Gordon KH, Hanzlik TN, Dorrington RA. J Gen Virol. 2007;88:1576–82. doi: 10.1099/vir.0.82250-0. [DOI] [PubMed] [Google Scholar]
- 28.Tuthill TJ, Groppelli E, Hogle JM, Rowlands DJ. Current topics in microbiology and immunology. 2010;343:43–89. doi: 10.1007/82_2010_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hindiyeh M, Li QH, Basavappa R, Hogle JM, Chow M. J Virol. 1999;73:9072–9. doi: 10.1128/jvi.73.11.9072-9079.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reisdorph N, Thomas JJ, Katpally U, Chase E, Harris K, et al. Virology. 2003;314:34–44. doi: 10.1016/s0042-6822(03)00452-5. [DOI] [PubMed] [Google Scholar]
- 31.Lewis JK, Bothner B, Smith TJ, Siuzdak G. Proc Natl Acad Sci U S A. 1998;95:6774–8. doi: 10.1073/pnas.95.12.6774. An excellent study that shows the dynamic character of VP4 in human rhino virus and its frequent exposure outside of the virus. The paper demonstrates that picornavirus antiviral agents (Win compounds) inhibit the particle dynamics and, thus, virus infection.
- 32.Kuhn RJ, Rossmann MG. Adv Virus Res. 2005;64:263–84. doi: 10.1016/S0065-3527(05)64008-0. [DOI] [PubMed] [Google Scholar]
- 33.Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. Nature. 1995;375:291–8. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 34.Bressanelli S, Stiasny K, Allison SL, Stura EA, Duquerroy S, et al. Embo J. 2004;23:728–38. doi: 10.1038/sj.emboj.7600064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi HK, Tong L, Minor W, Dumas P, Boege U, et al. Nature. 1991;354:37–43. doi: 10.1038/354037a0. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Mukhopadhyay S, Pletnev SV, Baker TS, Kuhn RJ, Rossmann MG. J Virol. 2002;76:11645–58. doi: 10.1128/JVI.76.22.11645-11658.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mancini EJ, Clarke M, Gowen BE, Rutten T, Fuller SD. Molecular cell. 2000;5:255–66. doi: 10.1016/s1097-2765(00)80421-9. [DOI] [PubMed] [Google Scholar]
- 38.Lescar J, Roussel A, Wien MW, Navaza J, Fuller SD, et al. Cell. 2001;105:137–48. doi: 10.1016/s0092-8674(01)00303-8. The structure determination of the E1 protein of sindbis virus demonstrating the close similarity between the flavivirus glycoprotein and the sindbis virus fusion protein.
- 39.Pletnev SV, Zhang W, Mukhopadhyay S, Fisher BR, Hernandez R, et al. Cell. 2001;105:127–36. doi: 10.1016/s0092-8674(01)00302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferlenghi I, Gowen B, de Haas F, Mancini EJ, Garoff H, et al. J Mol Biol. 1998;283:71–81. doi: 10.1006/jmbi.1998.2066. [DOI] [PubMed] [Google Scholar]
- 41.Paredes AM, Heidner H, Thuman-Commike P, Prasad BV, Johnston RE, Chiu W. J Virol. 1998;72:1534–41. doi: 10.1128/jvi.72.2.1534-1541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Presley JF, Polo JM, Johnston RE, Brown DT. J Virol. 1991;65:1905–9. doi: 10.1128/jvi.65.4.1905-1909.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W, Chipman PR, Corver J, Johnson PR, Zhang Y, et al. Nat Struct Biol. 2003;10:907–12. doi: 10.1038/nsb990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, et al. Cell. 2002;108:717–25. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu IM, Zhang W, Holdaway HA, Li L, Kostyuchenko VA, et al. Science. 2008;319:1834–7. doi: 10.1126/science.1153264. A definitive demonstration of the reversibility of the pH dependent conformational change during flavivirus maturation if cleavage is prevented.
- 46.Li L, Lok SM, Yu IM, Zhang Y, Kuhn RJ, et al. Science. 2008;319:1830–4. doi: 10.1126/science.1153263. The crystal structure of a designed precursor of the Dengue virus E protein with the M protein covalently attached. The M protein clearly covers the fusion protein to prevent premature activity.
- 47.Ganser-Pornillos BK, Yeager M, Sundquist WI. Curr Opin Struct Biol. 2008;18:203–17. doi: 10.1016/j.sbi.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balasubramaniam M, Freed EO. Physiology (Bethesda) 2011;26:236–51. doi: 10.1152/physiol.00051.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright ER, Schooler JB, Ding HJ, Kieffer C, Fillmore C, et al. Embo J. 2007;26:2218–26. doi: 10.1038/sj.emboj.7601664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Briggs JA, Wilk T, Welker R, Krausslich HG, Fuller SD. Embo J. 2003;22:1707–15. doi: 10.1093/emboj/cdg143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganser-Pornillos BK, Cheng A, Yeager M. Cell. 2007;131:70–9. doi: 10.1016/j.cell.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 52.Pornillos O, Ganser-Pornillos BK, Yeager M. Nature. 2011;469:424–7. doi: 10.1038/nature09640. A detailed and quantitative model of the HIV fullerene, inner nucleocapsid based on crystal structures of the hexamer and pentamer units that form it.
- 53.Wikoff WR, Liljas L, Duda RL, Tsuruta H, Hendrix RW, Johnson JE. Science. 2000;289:2129–33. doi: 10.1126/science.289.5487.2129. The first near atomic resolution of a dsDNA bacteriophage capsid showing what is now known as the HK97 fold for the first time.
- 54.Veesler D, Cambillau C. Microbiology and Molecular Biology Reviews. 2011:75. doi: 10.1128/MMBR.00014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heinemann J, Maaty WS, Gauss GH, Akkaladevi N, Brumfield SK, et al. Virology. 2011 doi: 10.1016/j.virol.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 56.Tang J, Lander GC, Olia A, Li R, Casjens S, et al. Structure. 2011;19:496–502. doi: 10.1016/j.str.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olia AS, Prevelige PE, Jr., Johnson JE, Cingolani G. Nat Struct Mol Biol. 2011;18:597–603. doi: 10.1038/nsmb.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conway JF, Duda RL, Cheng N, Hendrix RW, Steven AC. J Mol Biol. 1995;253:86–99. doi: 10.1006/jmbi.1995.0538. [DOI] [PubMed] [Google Scholar]
- 59.Fane BA, Prevelige PE., Jr. Adv Protein Chem. 2003;64:259–99. doi: 10.1016/s0065-3233(03)01007-6. [DOI] [PubMed] [Google Scholar]
- 60.Morais MC, Kanamaru S, Badasso MO, Koti JS, Owen BA, et al. Nat Struct Biol. 2003;10:572–6. doi: 10.1038/nsb939. [DOI] [PubMed] [Google Scholar]
- 61.Sun Y, Parker MH, Weigele P, Casjens S, Prevelige PE, Jr., Krishna NR. J Mol Biol. 2000;297:1195–202. doi: 10.1006/jmbi.2000.3620. [DOI] [PubMed] [Google Scholar]
- 62.Cheng H, Shen N, Pei J, Grishin NV. Protein Sci. 2004;13:2260–9. doi: 10.1110/ps.04726004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang RK, Khayat R, Lee KK, Gertsman I, Duda RL, et al. J Mol Biol. 2011;408:541–54. doi: 10.1016/j.jmb.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Medina E, Wieczorek D, Medina EM, Yang Q, Feiss M, Catalano CE. J Mol Biol. 2010;401:813–30. doi: 10.1016/j.jmb.2010.06.060. [DOI] [PubMed] [Google Scholar]
- 65.Xie Z, Hendrix RW. J Mol Biol. 1995;253:74–85. doi: 10.1006/jmbi.1995.0537. [DOI] [PubMed] [Google Scholar]
- 66.Fokine A, Leiman PG, Shneider MM, Ahvazi B, Boeshans KM, et al. Proc Natl Acad Sci U S A. 2005;102:7163–8. doi: 10.1073/pnas.0502164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fuller MT, King J. Biophys J. 1980;32:381–401. doi: 10.1016/S0006-3495(80)84963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prevelige PE, Jr., Thomas D, King J. Biophys J. 1993;64:824–35. doi: 10.1016/S0006-3495(93)81443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fu CY, Morais MC, Battisti AJ, Rossmann MG, Prevelige PE., Jr. J Mol Biol. 2007;366:1161–73. doi: 10.1016/j.jmb.2006.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Padilla-Meier GP, Teschke CM. J Mol Biol. 2011;410:226–40. doi: 10.1016/j.jmb.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thuman-Commike PA, Greene B, Malinski JA, King J, Chiu W. Biophys J. 1998;74:559–68. doi: 10.1016/S0006-3495(98)77814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen DH, Baker ML, Hryc CF, DiMaio F, Jakana J, et al. Proc Natl Acad Sci U S A. 2011;108:1355–60. doi: 10.1073/pnas.1015739108. The near atomic resolution cryoEM reconstruction of the authentic prohead of P22 allowing the detailed analysis of the portal interface with the capsid.
- 73.Gertsman I, Fu CY, Huang R, Komives EA, Johnson JE. Mol Cell Proteomics. 2010;9:1752–63. doi: 10.1074/mcp.M000039-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conway JF, Cheng N, Ross PD, Hendrix RW, Duda RL, Steven AC. J Struct Biol. 2007;158:224–32. doi: 10.1016/j.jsb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gertsman I, Gan L, Guttman M, Lee K, Speir JA, et al. Nature. 2009;458:646–50. doi: 10.1038/nature07686. The first near atomic resolution crystal structure of a maturation intermediate for a dsDNA bacteriophage. The structure revealed remarkable distortions in the subunit tertiary structure interpreted as the mechanism for storing energy and making the HK97 prohead II particle metastable.
- 76.Fuller DN, Raymer DM, Rickgauer JP, Robertson RM, Catalano CE, et al. J Mol Biol. 2007;373:1113–22. doi: 10.1016/j.jmb.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huet A, Conway JF, Letellier L, Boulanger P. J Virol. 2010;84:9350–8. doi: 10.1128/JVI.00942-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmidt U, Darke PL. J Biol Chem. 1997;272:7732–5. doi: 10.1074/jbc.272.12.7732. [DOI] [PubMed] [Google Scholar]
- 79.Ionel A, Velazquez-Muriel JA, Luque D, Cuervo A, Caston JR, et al. J Biol Chem. 2011;286:234–42. doi: 10.1074/jbc.M110.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agirrezabala X, Velazquez-Muriel JA, Gomez-Puertas P, Scheres SH, Carazo JM, Carrascosa JL. Structure. 2007;15:461–72. doi: 10.1016/j.str.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 81.Morais MC, Choi KH, Koti JS, Chipman PR, Anderson DL, Rossmann MG. Mol Cell. 2005;18:149–59. doi: 10.1016/j.molcel.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 82.Casjens SR. Nat Rev Microbiol. 2011;9:647–57. doi: 10.1038/nrmicro2632. [DOI] [PubMed] [Google Scholar]
- 83.Moffitt JR, Chemla YR, Aathavan K, Grimes S, Jardine PJ, et al. Nature. 2009;457:446–50. doi: 10.1038/nature07637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lander GC, Tang L, Casjens SR, Gilcrease EB, Prevelige P, et al. Science. 2006;312:1791–5. doi: 10.1126/science.1127981. [DOI] [PubMed] [Google Scholar]
- 85.Lander GC, Khayat R, Li R, Prevelige PE, Potter CS, et al. Structure. 2009;17:789–99. doi: 10.1016/j.str.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fokine A, Kostyuchenko VA, Efimov AV, Kurochkina LP, Sykilinda NN, et al. J Mol Biol. 2005;352:117–24. doi: 10.1016/j.jmb.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 87.Tang J, Olson N, Jardine PJ, Grimes S, Anderson DL, Baker TS. Structure. 2008;16:935–43. doi: 10.1016/j.str.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Effantin G, Boulanger P, Neumann E, Letellier L, Conway JF. J Mol Biol. 2006;361:993–1002. doi: 10.1016/j.jmb.2006.06.081. [DOI] [PubMed] [Google Scholar]
- 89.Cerritelli ME, Cheng N, Rosenberg AH, McPherson CE, Booy FP, Steven AC. Cell. 1997;91:271–80. doi: 10.1016/s0092-8674(00)80409-2. [DOI] [PubMed] [Google Scholar]
- 90.Gan L, Speir JA, Conway JF, Lander G, Cheng N, et al. Structure. 2006;14:1655–65. doi: 10.1016/j.str.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 91.Lee KK, Gan L, Tsuruta H, Hendrix RW, Duda RL, Johnson JE. J Mol Biol. 2004;340:419–33. doi: 10.1016/j.jmb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 92.Gertsman I, Komives EA, Johnson JE. J Mol Biol. 2010;397:560–74. doi: 10.1016/j.jmb.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee KK, Gan L, Tsuruta H, Moyer C, Conway JF, et al. Structure. 2008;16:1491–502. doi: 10.1016/j.str.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Galisteo ML, King J. Biophys J. 1993;65:227–35. doi: 10.1016/S0006-3495(93)81073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lander GC, Evilevitch A, Jeembaeva M, Potter CS, Carragher B, Johnson JE. Structure. 2008;16:1399–406. doi: 10.1016/j.str.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu X, Zhang Q, Murata K, Baker ML, Sullivan MB, et al. Nat Struct Mol Biol. 2010;17:830–6. doi: 10.1038/nsmb.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mettenleiter TC. Vet Microbiol. 2006;113:163–9. doi: 10.1016/j.vetmic.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 98.Steven AC, Heymann JB, Cheng N, Trus BL, Conway JF. Curr Opin Struct Biol. 2005;15:227–36. doi: 10.1016/j.sbi.2005.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heymann JB, Cheng N, Newcomb WW, Trus BL, Brown JC, Steven AC. Nat Struct Biol. 2003;10:334–41. doi: 10.1038/nsb922. [DOI] [PubMed] [Google Scholar]
- 100.Guo H, Shen S, Wang L, Deng H. Protein Cell. 2010;1:987–98. doi: 10.1007/s13238-010-0120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]