Abstract
Chemosensory‐based communication is a vital signaling tool in most species, and evidence has recently emerged in support of the notion that humans also use social chemosignals (so‐called pheromones) to communicate. An ongoing controversy does exist, however, concerning the receptor organ through which these chemicals are processed. There is a widespread belief that the vomeronasal organ (VNO) is responsible for processing social chemosignals in humans. Here we demonstrate that functional occlusion of the VNO does not change the percept of, sensitivity toward, or functional neuronal processing of a putative human pheromone. Perithreshold and suprathreshold perception of the endogenous chemical androstadienone (AND) were compared, as were positron emission tomography brain activations evoked by AND when the VNO was either occluded or left open. In addition, we compared sensitivity to AND in subjects with an identifiable VNO to those in whom no VNO could be detected. Thus we could examine the effects of the VNO at several different levels of processing. Occlusion or absence of the VNO did not affect either the perceptual measurements or the functional processing of the putative human pheromone, AND. These results provide strong evidence that the human VNO has no obvious function. Pheromonal communication in humans may be conveyed via the main olfactory system. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: VNO, pheromone, olfaction, smell, human
INTRODUCTION
Chemosensory‐based communication has been demonstrated to be a vital signaling tool in most species studied [McClintock,2000]. Social signals hidden within the individual's body odor, so‐called pheromones, communicate such disparate information as kin recognition, mating compatibility, social status, the presence of danger, and other vital informational cues [Johnston,1998; Lundstrom et al.,2009; Porter et al.,1985; Potts et al.,1991; Stowe et al.,1995]. It was long assumed that pheromones were processed not in the main olfactory system but exclusively in a separate accessory system, with the vomeronasal organ (VNO) as a central receptor organ situated in the nasal cavity [Meredith,1991; Wysocki,1979]. Recent studies, however, indicate that the division between the main and accessory olfactory systems is not as clear as initially believed. The VNO has been demonstrated to mediate both pheromonal and olfactory signals [Kelliher,2007; Sam et al.,2001; Trinh and Storm,2003], with similar evidence existing for the main olfactory system [Boehm et al.,2005; Dorries et al.,1995; Yoon et al.,2005]. Nevertheless, a functional VNO has been demonstrated to be of utmost importance for many animal and insect species with respect to eliciting appropriate behavior to conspecifics [Kimchi et al.,2007].
In humans, an anatomically similar structure can typically be found in the anterior third of the epithelium of the nasal septum [Jacob et al.,2000; Knecht et al.,2001]. For the sake of brevity, we will refer to the structure as the VNO. Earlier studies report percentages of human subjects showing a VNO ranging from 25 to 100%, depending on the technique used to search for it [Gaafar et al.,1998; Johnson et al.,1985; Knecht et al.,2001; Moran et al.,1991; Potiquet,1891; Stensaas et al.,1991; Trotier et al.,2000; Won et al.,2000]. Moreover, repeated observations on 130 subjects revealed changes in VNO visibility over time, from nothing visible to well‐defined pits and vice versa; in a given subject, a VNO can be found unilaterally or bilaterally with no obvious predictors [Trotier et al.,2000].
To date, however, it is not clear whether or not the VNO has a function in humans—and therefore whether it should be classified as an organ at all. The human VNO shows properties that are clearly different from the nearby respiratory epithelium [Jahnke and Merker,1998; Witt et al.,2002]. This does support a possible function of the VNO in humans but appears to be best supported in fetuses [Bhatnagar and Smith,2001; Witt et al.,2002]. Moreover, stimulating the VNO with a steroid compound (androstadienone) evoked an electrical response [Monti‐Bloch and Grosser,1991]. However, there are clear neurochemical and neuroanatomical arguments against a functioning VNO in humans [Meisami et al.,1998; Meredith,2001; Trotier et al.,2000; Witt et al.,2002].
Although in nonhuman animals the VNO to some extent processes common odors, its main function appears to be processing of social chemosignals. Thus, one might postulate that if the VNO in humans is a functional organ, the perception and/or the central processing of chemosignals should be altered when the VNO is occluded. To date, only one study has investigated the VNO's role in the perception of a human endogenous odor. Knecht et al. [2003] measured sensitivity to the odor of androstenone, a steroid found in underarm sweat [Gower and Ruparelia,1993], before and after functionally occluding the VNO by covering its duct with a latex patch. Functional occlusion of the VNO did not change participants' perception of androstenone or that of a nonendogenous control odor. However, androstenone is not generally considered to be a human chemosignal [Lundstrom et al.,2006]. The endogenous odorant that has been singled out as the most likely candidate to be a human chemosignal is the closely related steroid androstadienone (AND). AND is found in axillary secretion [Nixon et al.,1988] and has been reported to influence women's mood [Bensafi et al.,2004; Jacob and McClintock,2000; Lundstrom and Olsson,2005; Lundstrom et al.,2003a], psychophysiological state [Bensafi et al.,2003; Jacob et al.,2001a; Lundstrom and Olsson,2005], regional cerebral blood flow [CBF; Gulyas et al.,2004; Jacob et al.,2001b; Savic et al.,2001,2005], and blood cortisol levels [Wyart et al.,2007]. Furthermore, cerebral processing of AND has been demonstrated to be faster than that of comparable common odorants [Lundstrom et al.,2006]. Because of the demonstrated sex‐specific effects in some of these studies [Jacob and McClintock,2000; Savic et al.,2001], AND has been proposed as a human pheromone [Sobel and Brown,2001]. In a recent report Savic et al. investigated brain activations after stimulation with AND in anosmic men and healthy controls. The patients suffered from severe nasal polyposis, which prevented odor molecules to reach the olfactory cleft, therefore the main olfactory epithelium, and which rendered patients anosmic. However, the VNO, which is located much more distally, was not affected. Savic et al. observed typical activations of the hypothalamus after stimulation with estratetraenol (EST), the female counterpart of AND; in the control group but not in the patient group [Savic et al.,2009]. They interpreted these results as a proof that putative human pheromones are perceived via the main olfactory epithelium.
The notion of the VNO as a functional organ that processes social chemosignals in humans is widespread, although there is little evidence to support it. Here, we report three experiments in which the functional significance of the human VNO is for the first time explored extensively. In a series of well controlled experiments we investigated the effect of occluding the VNO either on women's perception or on their central processing of a putative human pheromone.
Our specific hypotheses were that if the VNO had a function, (a) functional occlusion of the VNO should alter the perception of or sensitivity to the endogenous odorant AND; (b) there should be a difference between subjects with and without a detectable VNO in their olfactory perception of AND; (c) functional occlusion of the VNO should change patterns of brain activation after stimulation with AND.
GENERAL MATERIAL AND METHODS
The experiments were conducted following the Declaration of Helsinki and after approval of the local ethics committee. All subjects gave written informed consent after they were informed in detail about the study. Subjects were asked to refrain from drinking anything other than water, eating or smoking 1 h before commencement of testing. They underwent a detailed nasal endoscopy examination prior to the experiments to exclude nasal obstruction, nasal pathology, and anatomical features that would have prevented the functional occlusion of the VNO. During the examination it was determined whether or not a VNO could be detected in either nostril. If a VNO was detected in both nostrils, the nostril with best accessibility to the VNO was selected. If a VNO was detected only unilaterally, that nostril was tested. In the case that no VNO could be detected, a nostril was selected randomly and the subject was considered as not having a VNO.
Occlusion of the VNO
Functional occlusion of the VNO was achieved using the occlusion technique described previously [Knecht et al.,2003]. In short, a latex piece of ∼0.5 cm2 was placed over the VNO in such a manner that the latex piece would fully cover the duct of the VNO (“occluded”). This technique has been shown to effectively block air and chemical access to the VNO [Knecht et al.,2003]. Throughout the different experimental procedures, the position of the latex patch was repeatedly ascertained by nasal endoscopy. Experiments 1 and 2 included subjects in whom no apparent VNO could be detected, and for those subjects the typical position of the VNO on the mucosa was covered. In all experiments, a spot on the lateral nasal wall was covered with a similar latex patch (“VNO open”) as a control measure. The order of which spot (VNO, lateral wall) was covered was randomized and counterbalanced [Knecht et al.,2003]. Measures were obtained monorhinally in all experiments, with the untested nostril occluded by tape (3M, London, ON).
Odorants
Identical odorants were used in all three experiments. AND (androsta‐4,16‐dien‐3‐one, Steraloids, Newport, RI) is the most likely candidate for a human pheromone. AND is a component of human sweat, and its scent is described as sweaty, algae‐like, urinous, and sandalwood‐like [Kraft and Popaj,2004]. As a control, we used polysantol® (CON: 3,3‐dimethyl‐5‐[2,2,3‐trimethyl‐3‐cyclopenten‐1‐yl]‐4‐penten‐2‐ol; Firmenich, Meyrin, Switzerland). Polysantol is an artificial odorant with sandalwood‐like notes, qualitatively perceived as having a scent similar to that of AND, as determined in a pilot experiment. Odorants were diluted in propylene glycol (Sigma Aldrich, Oakville, ON). Starting from pure substances, we mixed stock solutions of 0.79 g L−1 AND and 6.25% CON.
EXPERIMENT 1—SUPRATHRESHOLD ODOR PERCEPTION
In Experiment 1, we investigated the effect of occluding the VNO on ratings of intensity and pleasantness of AND and CON in healthy young women. In addition we tested whether subjects with a VNO and those in whom no VNO could be detected differed with respect to these measures.
Materials and Methods
Subjects
A total of 54 women between 18 and 33 years (mean age 22.9 years) participated. A VNO was detected in 41 subjects (75%), and in the remainder no VNO could be detected. After the endoscopic examination, we occluded either the VNO or the sham location in a randomized counterbalanced fashion.
Procedure
Subjects were asked to rate the intensity and pleasantness of three concentrations of AND and CON on a visual analog scale [Aitken,1969]. We used three concentrations for each odorant (100, 50, and 25% v/v) of the respective stock solutions. The intensity rating scale ranged from not perceivable (0) to very strong (100), and the pleasantness scale from very unpleasant (0) to neutral (50) and very pleasant (100). After the initial ratings, the position of the cover was changed to the other position (VNO or lateral wall) and the measurements were repeated. All testing procedures were performed by a male tester (JF).
Statistical analysis
Statistical analyses were applied with the following paradigm. First, we analyzed the AND ratings of the subjects with and those without a detectable VNO. We computed two separate repeated measures ANOVAs, one with AND intensity ratings and the other with AND pleasantness ratings. We applied a two (VNO: detected, not detected; between subjects) × two (position: VNO occluded, VNO open; within subjects) × three (concentration: high, medium, low; within subjects) design. A significant effect of VNO and/or position and/or an interaction between these two factors would indicate that the VNO is involved in the perception of suprathreshold stimuli and thus suggest its functionality.
Subjects without a detectable VNO were then excluded from further analysis. We next analyzed perceptual ratings to determine a potential impact of the VNO. Two separate repeated measures ANOVAs (one for intensity, the other for pleasantness) were computed, using a two (position: VNO occluded, VNO open; within subjects) × two (odor: AND, CON; within subjects) × three (concentration: high, medium, low; within subjects) design. Again, a significant effect of position and/or an interaction position × odor would indicate that the VNO is involved in the perception of suprathreshold stimuli and thus suggest its functionality.
Finally, we performed planned comparisons based on our a priori hypothesis in which we had predicted an effect of occlusion of the VNO on AND ratings. We performed paired t‐tests on the ratings for the three concentrations of AND between the two positions (VNO occluded, VNO open). A significant difference in any of these tests would indicate involvement of the VNO in the perception of suprathreshold concentrations of AND. Alpha level was set at 0.05 in all analyses.
Results
In the first analysis, both groups of subjects (with VNO and without VNO) were included. With regard to intensity ratings, there was an expected significant effect of concentration (F(2,104) = 5.56, P < 0.01), indicating that stronger AND concentrations rendered higher intensity ratings. However, neither the factor VNO nor position was significant. There was no other significant main effect or interaction. With regard to pleasantness ratings, no significant effects were observed.
In the second analysis, subjects without a detectable VNO were excluded. With regard to intensity ratings, we again observed a significant effect of concentration (F(2,80) = 4.53, P < 0.01), indicating that stronger concentrations evoked higher intensity ratings. In addition, there was a significant effect of odor (F(1,40) = 50, P < 0.01), indicating that CON was perceived as more intense than AND. There was no significant effect of VNO occlusion, and there was no significant interaction between odor and position. With regard to pleasantness ratings, we observed a significant effect of odor (F(1,40) = 4.32, P < 0.05), indicating that CON was perceived as more pleasant than AND. There was no other significant effect. Importantly, neither the factor position nor an interaction between odor and position was found to be significant.
Since our a priori hypothesis had predicted an effect of VNO occlusion, we directly compared ratings for the three concentrations of both odorants under both cover conditions, as a third step. No comparison revealed a significant difference for the ratings.
EXPERIMENT 2—OLFACTORY THRESHOLD
In the second experiment we investigated possible effects of occluding the VNO on detection thresholds of AND and CON. In addition, we tested whether subjects with a detectable VNO and those in whom no VNO could be detected were different with respect to these measures.
Materials and Methods
Subjects
All subjects from Experiment 1 participated; in addition, 20 more subjects were included, resulting in a total of 74 women between 18 and 35 years (mean age 23.1 years). Endoscopically, a VNO could be detected in 57 women (72%), and no VNO could be observed in 17 (28%).
Procedure
We determined olfactory thresholds for AND and CON using an ascending staircase, three alternative forced choice procedure while subjects were blindfolded [Doty,1991]. Threshold was defined as the mean of the last four of seven staircase reversal points [Frasnelli et al.,2002]. Immediately prior to the threshold measurements we occluded either the VNO or a sham location in a randomized counterbalanced fashion under endoscopic control. So, each subject was tested twice, once with a covered VNO, once with an open VNO. Sixteen dilutions were prepared in a geometric series starting from 100% v/v of the previously mentioned AND stock solution and 12.5% of the CON stock solution (dilution ratio 1:2) using propylene glycol as solvent. Odors were presented in 60 mL amber glass bottles containing 10 mL of the stimulus.
As in Experiment 1, scores were assessed under two conditions (VNO occluded vs. open) and all testing procedures were performed by male testers (JF).
Statistical analysis
A total of 31 subjects could not perceive the highest concentration of AND in one or both of the two threshold assessments for this odorant (ceiling effect), and could therefore be considered anosmic for AND. No subject was anosmic to CON. We analyzed the data in three different ways, from conservative statistics with a large number of subjects to more liberal statistics with fewer subjects. First, using a chi‐square test we compared the rate of anosmic subjects in both groups (those showing a VNO and those without a VNO). In a second analysis we included all subjects without ceiling effects in either of the two conditions or in only one of the two (e.g., in VNO occluded or in VNO open) for a given odorant. Subjects showing a ceiling effect were assigned a threshold score of 1. Since this rendered the dataset nonparametric, we then calculated potential effects of VNO occlusion (VNO occluded/open) on detection threshold scores for each of the two odorants using the nonparametric Wilcoxon ranked test. Moreover, we investigated differences in detection threshold between subjects with versus those without a detectable VNO using the nonparametric Mann‐Whitney U test. Third, we included only subjects with a measurable threshold for AND in any measurement and calculated individual t‐tests; once paired to compare between the VNO occlusion conditions and once unpaired to compare between subjects with versus those without a detectable VNO. The alpha level in all tests was set at 0.05.
Results
Of the 57 women in whom we detected a VNO, 23 showed a ceiling effect in both AND threshold assessments (with and without occlusion). Of the 17 women in whom we did not detect a VNO, 8 showed a ceiling effect in the AND threshold assessment when the region where a VNO would normally be found was open, and 7 when that region was occluded. There was no significant difference between women with versus those without a VNO with respect to anosmia rate (chi‐square; P = 0.62). This indicates that the incidence of subjects anosmic to AND is equal in both groups.
In the second analysis, we compared results between 33 subjects with VNO and 11 subjects without a VNO, in whom at least one of two threshold measurements did not show a ceiling effect for AND. No difference between the two groups of subjects could be observed (Mann‐Whitney; P > 0.35). In addition, within the subjects showing a VNO, no difference in threshold measurements when the VNO was occluded and when the VNO was left open could be detected for AND or CON (Wilcoxon: P > 0.21).
Finally, 35 participants did not show a ceiling effect for AND under either testing condition (VNO occluded vs. VNO open); in 27 of them a VNO had been detected, whereas in 8 no VNO could be found. No difference between subjects with and subjects without a VNO could be observed for any of the four threshold measurements (P > 0.12). In addition, in the 27 subjects with a VNO, no difference could be detected when we compared the thresholds obtained when the VNO was occluded or left open (P > 0.7). Data are presented in Figure 1.
Figure 1.
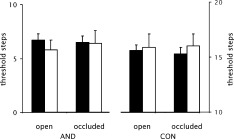
Threshold measurements: mean threshold for AND and CON in subjects with VNO (black bars) and subjects in whom no VNO could be detected (white bars) when the VNO was left open (open) and when the VNO was covered (occluded). Error bars indicate standard error of the mean.
EXPERIMENT 3—CEREBRAL ACTIVATION
Whereas Experiments 1 and 2 focused on behavioral measures, the aim of Experiment 3 was to determine whether functional occlusion of the VNO influences women's neuronal processing of AND. Specifically, we used PET imaging to determine brain activations during stimulation with AND and CON while subjects' VNO was occluded or a sham location in the nasal cavity was covered.
Materials and Methods
Subjects
Twelve women from the previous experiments participated. All subjects showed a VNO and had a measurable AND threshold. All subjects were right handed.
Material
Subjects where stimulated in the scanner with AND and CON. We used concentrations of 100 and 25% v/v of the stock solutions, respectively. As a control, we also presented our subjects with double‐distilled water as an odorless baseline condition. All stimuli were presented in 60 mL amber glass bottles containing 10 mL of the stimulus.
Procedure
On the day before and again just prior to scanning, the presence of a VNO was ascertained with an endoscope. A nostril in which a VNO had been detected was chosen for testing and the other nostril was occluded with a tape (3M, London, ON). We tested six subjects occluding their right nostril and six subjects with a left nostril occlusion. In half of the scans, the VNO was occluded by a small piece of latex (VNO occluded). In the other half we covered a spot on the lateral wall of the nasal cavity for control (VNO open). Within a single scan we presented one of the two odor stimuli (AND or CON) or water (baseline) under one of the two occlusion conditions (VNO occluded or open), for a total of six scan conditions. With the exception of the baseline, each condition was repeated once for a total of 10 PET scans. A condition lasted 60 s with a minimum of 10 min between each.
Subjects were asked to focus their gaze on a cross mark above their heads. Before each scan subjects were told whether they would receive an odor stimulus (i.e., AND or CON) or odorless water (baseline), but they were not told which odorant they would receive. They were instructed and trained to breathe normally through the nose during a scan. Stimuli were presented for 3 s with an interstimulus interval of 5 s, rendering a total of seven stimulations during a scan (see Fig. 2 for an overview of the procedure). During each scan we alternated two identical sets of stimulation bottles to prevent rarefaction of odor concentration in the headspace.
Figure 2.
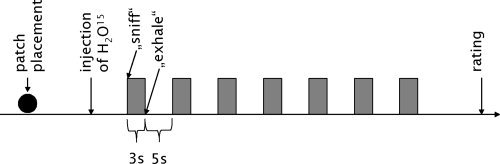
Schematic drawing of the PET procedure: First, under endoscopic control, the patch was placed either on the VNO (VNO occluded) or on the lateral nasal wall (VNO open). Then, after injection of H2O15, subjects were instructed to sniff for 3 s, during which the odor (CON, AND or, in the baseline condition, a blank) was presented by holding a bottle under their nose. Then subjects were instructed to exhale for 5 s, after which the next stimulation cycle began. The whole stimulation period lasted 60 s. After this, subjects rated the odors with regard to intensity, pleasantness, and familiarity.
After each scan, subjects were asked to rate intensity, pleasantness, and familiarity of each odor using an 11‐point visual analog scale (ranging from 0 to 10). Anchor points were “not perceivable,” “very unpleasant”, and “very unfamiliar” for 0, respectively, and “very intense”, “very pleasant”, and “very familiar” for 10, respectively. During the experiment, other than the subject, only men were in the scanner room (JF, JNL, and the PET technician).
PET scanning
We used a Siemens Exact HR+ tomograph (Siemens, Erlangen, Germany) operating in three‐dimensional acquisition mode for measuring the distribution of regional cerebral blood flow (regional CBF). Water labeled with 15O served as the tracer. To provide anatomical details, T1‐weighted structural magnetic resonance imaging scans (160 scans, 1 mm) were obtained with a 1.5T Siemens Sonata Scanner (Siemens, Erlangen, Germany) for each subject.
Data analysis
Preprocessing of the data was done using in‐house programs and following standard conventions [Worsley et al.,1992; Zatorre et al.,1992]. In short, we used a 14‐mm Hanning filter to reconstruct regional CBF images, which were subsequently normalized for differences in global CBF, coregistered with the respective MRI image, and transformed into the Montreal Neurological Institute (MNI) standardized proportional stereotaxic space (ICBM305), based on the Talairach and Tournoux atlas.
Statistical imaging analyses were done using the in‐house program DOT, following automated procedure [Collins et al.,1994]. We established the presence of significant changes in regional CBF initially on the basis of an exploratory search. Here we set a peak's t‐value criterion at >4.45 on a voxel level, corresponding to a corrected P‐value of <0.05 for a whole brain search volume with ∼2,000 resolution elements or resels. For predicted areas we lowered the criterion to t > 3.0 if the activation was significant at P < 0.05 on a cluster level [Worsley et al.,1992,1996].
In addition, for the directed search within a priori selected regions known to be involved in the processing of AND and/or common odors, we defined volumes of interests (VOI). According to earlier reports, we selected the piriform cortex [PIR; Zatorre and Jones‐Gotman,2000], medial orbitofrontal cortex [OFC; Gottfried and Zald,2005], and the hypothalamus [HYP; Savic et al.,2005], separately for left or right hemisphere. We placed the VOI in the right OFC 4‐mm lower (z = −16) than indicated in [Gottfried and Zald,2005] to locate it in the gray matter, based on the averaged anatomical MRI scan. Data on coordinates and size of the VOI are presented in Table I. We extracted normalized regional CBF values using a 5‐mm (7 mm for the mOFC) radius search sphere and calculated the average response within the VOI for individual subjects in each condition (stimulus − baseline). We computed repeated measures ANOVAs with a two (stimulation side: left, right; between subject factor, since half of the subjects were stimulated on the left nostril and half on the right nostril) × two (hemisphere: left, right; within subject factor) × two (odorant: AND, CON; within subject factor) × two (position: VNO occluded, VNO open; within subject factor) design. In addition, we performed planned paired t‐tests between responses when the VNO was occluded or open.
Table I.
Coordinates for VOI analysis
| Structure | X | Y | Z | Size (mm) |
|---|---|---|---|---|
| Left PIR | −21 | 5 | −19 | 5 |
| Right PIR | 21 | 4 | −14 | 5 |
| Left OFC | −24 | 31 | −16 | 7 |
| Right OFC | 24 | 34 | −16 | 7 |
| Left hypothalamus | −6 | 0 | −12 | 5 |
| Right hypothalamus | 6 | 0 | −12 | 5 |
Psychophysical data were analyzed by computing repeated‐measures ANOVAs separately for the respective scales, with odor stimulus as a within‐subject factor.
Results
Behavioral data
There was no significant difference between odor stimuli in ratings of intensity or familiarity. Both odors were rated as neutral; CON was rated as slightly pleasant [5.8 (0.4) of 10 on the VAS] whereas AND was rated as slightly unpleasant [4.3 (0.3) of 10; P = 0.022].
PET data
To determine whether the odors activated cerebral areas known to be involved in olfactory processing, we compared brain activation after stimulation with CON and the odorless baseline, independent of the VNO occlusion, using in the contrast [CON(VNO occ) + CON(VNO open)] − [baseline(VNO occ) + baseline(VNO open)]. This revealed activations in traditional olfactory regions, such as the right PIR and the right OFC (Table II, Fig. 3A). In addition, we observed activations in the right putamen and the right occipital cortex. Furthermore the contrast revealed hypothalamic activation. The inverse contrast revealed significant deactivations after CON stimulation in the left angular gyrus and the right superior frontal gyrus.
Table II.
Significant activation specific to perception of (A) CON and (B) AND independent of occlusion of the VNO: x, y, and z denote coordinates in the right (positive) versus left (negative) direction, anterior (positive) versus posterior (negative) direction, and superior (positive) versus inferior (negative) direction, respectively, expressed as distance in mm from the anterior commissure
| Structure | X | Y | Z | t |
|---|---|---|---|---|
| Aa | ||||
| Right occipital pole | 19 | −92 | −11 | 5.76 |
| Left hypothalamus | −1 | −2 | −20 | 5.15 |
| Right putamen | 24 | −9 | 2 | 4.75 |
| Right posterior OFC | 21 | 29 | −18 | 4.32 |
| Right piriform cortex | 31 | 13 | −20 | 3.11 |
| Right piriform cortex | 21 | 5 | −15 | 3.08 |
| Inverse contrast: | ||||
| Left angular gyrus | −46 | −59 | 51 | −4.98 |
| Right superior frontal gyrus | 28 | 25 | 51 | −4.78 |
| Bb | ||||
| Right occipital pole | 21 | −93 | −6 | 5.37 |
| Left hypothalamus | −3 | 1 | −15 | 4.05 |
| Inverse contrast: | ||||
| Right superior frontal gyrus | 24 | 20 | 57 | −6 |
| Left postcentral gyrus | −13 | −45 | 59 | −4.87 |
| Left superior frontal gyrus | −5 | 27 | 60 | −4.81 |
Contrast: [CON(VNO occ) + CON(VNO open)] – [baseline(VNO occ) + baseline(VNO open)] and inverse contrast.
Contrast: [AND(VNO occ) + AND(VNO open)] – [baseline(VNO occ) + baseline(VNO open)] and inverse contrast.
Figure 3.
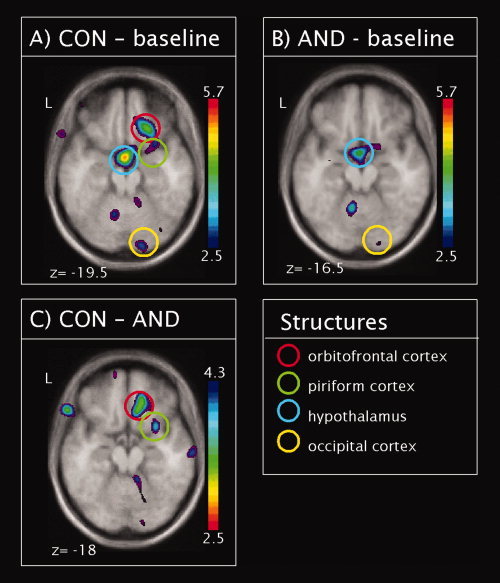
Global contrasts: Statistical parametric maps (t‐statistics as represented by the color scale; note that color scale is inversed in C) superimposed on group averaged anatomical MRI showing group regional CBF response to the processing of (A) the control odor (contrast CON–baseline), (B) androstadienone (contrast AND–baseline). Figure C represents the contrast between regional CBF response to CON and AND (contrast CON–AND). Significant regions are highlighted by colored circles. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The same contrast was computed for AND. Again, we observed activation of the right occipital pole and the right hypothalamus (Table II, Fig. 3B). The inverse contrast revealed significant deactivations after AND stimulation in the bilateral superior frontal gyrus and the left postcentral gyrus.
The two odorants thus did not evoke the same central activation patterns. When we compared the two odorants directly: [CON(VNO occ) + CON(VNO open)] − [AND(VNO occ) + AND(VNO open)], we could observe that CON activated the right OFC and PIR, and thus classical olfactory regions. The inverse contrast, however, did not reveal any activations (Table III, Fig. 3C).
Table III.
Significant differences in the activation patterns between CON and AND: x, y, and z denote coordinates in the right (positive) versus left (negative) direction, anterior (positive) versus posterior (negative) direction, and superior (positive) versus inferior (negative) direction, respectively, expressed as distance in mm from the anterior commissure
| Structure | X | Y | Z | t |
|---|---|---|---|---|
| Right medial OFC | 16 | 36 | −14 | 4.42 |
| Right PIR | 29 | 12 | −20 | 3.83 |
Contrast: [CON(VNO occ) + CON(VNO open)] – [AND(VNO occ) + AND(VNO open)] (please note: the inverse contrast did not reveal any significant differences).
We then analyzed the contribution of the VNO to the AND‐evoked brain activation, resulting in the contrast AND(VNO open) − AND(VNO occ). No significant activation was observed. The inverse contrast, however revealed significant activations in the right inferior temporal gyrus ([x, y, z] = 55, −44, −20) and an area below the gyrus rectus, but outside the brain (3, 8, −23). When we performed the same analysis for the control odor, resulting in the contrast CON(VNO open) − CON(VNO occ), we observed activation of the right postcentral gyrus (11, −40, 55; and 13, −40, 60). Again the inverse contrast revealed significant activations of the right inferior temporal gyrus (54, −42, −20) and the same area below the gyrus rectus, but outside the brain (−3, −1, −23). Since we observed the same peak below the brain (0, 6, −21) when comparing the two baselines [baseline(VNO occ) − baseline(VNO open)], we hypothesize this peak to be an artifact resulting from an increased swelling of the nasal mucosa as a consequence of the placement of the latex patch on the nasal septum. We controlled for this by performing the contrast [AND(VNO occ) − AND(VNO open)] − [CON(VNO occ) − CON(VNO open)]. This contrast reveals the effect of the occlusion of the VNO on AND‐evoked brain activations by eliminating nonspecific effects due to odor perception or placement of the latex patch. We did not observe any significant peaks in this contrast.
In a second step we performed VOI analysis in regions known to be involved in the perception of AND and/or common odors. We detected no significant main effects in the three regions of interest. Specifically, there was no effect of occluding the VNO. Neither did we observe an interaction with this factor.
We additionally performed planned paired t‐tests comparing the response in the VOI to both odors, when the VNO was open or occluded. Again, we did not find any significant difference. Thus, altogether we found no effect of occlusion of the VNO on brain activations.
DISCUSSION
In this series of experiments we provide systematic support for the notion that the human VNO does not have any function in perception and higher processing of AND or that of a common odorant with similar perceptual characteristics to AND. In three experiments we found no differences in the outcome when subjects perceived the odors with the VNO occluded or not.
Specifically, in Experiment 1, we showed that occluding the VNO did not change subjective perception of suprathreshold concentrations of AND and CON in 54 young women. In addition, there were no differences in intensity and pleasantness ratings between subjects in whom a VNO could be detected and those in whom no VNO was detectable by means of nasal endoscopy. We could, however, show that stronger concentrations of the odorants were perceived as stronger; this confirms the validity of our paradigm.
In Experiment 2 we showed that occlusion of the VNO had no effect on odor detection thresholds of AND and CON in 74 subjects. We analyzed our data by manipulating the experimental conditions in three different ways. Covering the VNO did not change threshold in our subjects; furthermore we did not observe any difference between subjects in whom a VNO could be found and those in whom no VNO could be detected. Our data are in keeping with the findings of an earlier study in which the VNO of 19 subjects of both sexes between 16 and 78 years of age was occluded using the same technique and different odorants [Knecht et al.,2003]. Subjects' odor detection thresholds for androstenone and phenylethyl alcohol did not change; in addition they had the same average threshold as 13 subjects in whom no VNO could be detected. We show similar results for another endogenous odor in a larger and more homogenous sample of young women. Taken together, these studies suggest that the VNO in humans is not involved in the perception of perithreshold concentrations of endogenous odors (AND and androstenone) or of common odors.
Among our 74 subjects, we could determine a reliable threshold in all measures in only 35. It is known that the sensitivity toward AND varies greatly within the normal population [Keller et al.,2007; Lundstrom et al.,2003b]. In addition, we assessed olfactory thresholds in only one nostril. Monorhinally obtained odor thresholds are known to be significantly higher than birhinal ones [Frasnelli et al.,2002], indicating that subjects are more sensitive when they can rely on both nostrils. Therefore, it is likely that not all subjects demonstrating a ceiling effect are anosmic to AND since they might have shown a better performance in a birhinal measurement.
In Experiment 3, we used PET to measure changes in brain blood flow while manipulating access to the VNO. Comparisons of both odorants (AND, CON) versus baseline revealed similar results as in earlier studies. Specifically, we observed hypothalamic activation when our subjects smelled AND, in agreement with earlier reports [Savic et al.,2001,2005,2009]. Interestingly, CON, an artificial odorant with (presumably) no biological effects, but like AND described as having sandalwood‐like notes [Kraft and Popaj,2004], also activated the hypothalamic area. As a consequence, hypothalamic activation in women following exposure to AND (and perceptually similar odorants) is probably based on the association of these odors to male body odor, as suggested by Savic et al. [2005], rather than a hard‐wired biological effect.
Interestingly, although both odors, AND and CON, were rated as having the same intensity, AND did not evoke any changes in blood flow in olfactory areas, whereas CON activated known olfactory areas such as the right posterior orbitofrontal and piriform cortex. A predominance of the right hemisphere in olfactory processing has been described earlier [Zatorre et al.,1992]. At first sight, it may seem surprising that AND, although perceivable, did not activate olfactory regions. However, this is in line with earlier reports. Savic et al. [2001,2005,2009] have repeatedly shown that stimulation with AND does not lead to activation of olfactory regions as common odors do. In addition, Lundstrom et al. [2008] showed that body odors, although evoking a clear olfactory percept, did not activate olfactory regions. In the latter study the authors found that body odors activate, among other regions, the right posterior occipital gyrus, in line with our results for both odors. This occipital activation was not due to visualization, as revealed by additional control analysis [Lundstrom et al.,2008]. There is thus converging evidence from imaging and electrophysiological studies [Lundstrom et al.,2006] that AND is processed differently from common odors. Taken together, the overlap between the results of our study and those of earlier reports prove the validity of our approach.
When we occluded the VNO we did not observe any changes in the brain activation patterns evoked by AND. This supplies further support for the notion that the VNO is not involved in the processing and perception of endogenous odors such as AND and androstenone. In a recent study investigating anosmic men, Savic et al. came to the same conclusion [Savic et al.,2009]. In their study the authors investigated brain activations after stimulation with EST, the female counterpart of AND, which in healthy men activated the hypothalamus. However, in the anosmic group the authors did not observe such activation. These patients suffered from a severe nasal polyposis, which blocked access to the olfactory cleft and rendered them anosmic; the VNO, however, which is located much more anteriorly in the nasal cavity, was unaffected by the polyposis. The authors therefore concluded that the hypothalamic activation in their healthy subjects was caused by stimulation of the olfactory epithelium rather than the VNO. Our data confirm this notion and extend it to women. Taken together, there is now convincing evidence that the VNO has no function in central olfactory processing or pheromonal actions of AND and EST. Although the Grueneberg ganglion, an anatomical structure located at the anterior portion of the nostril, was recently demonstrated to act as a pheromonal receptor organ for fear odors in rodents [Brechbuhl et al.,2008] and may therefore be a candidate for having vomeronasal functions in humans; tentative evidence indicates that in humans the functions of the VNO might have migrated to the main olfactory system. So, a vomeronasal receptor gene that in rodents is expressed in the VNO is in humans expressed in the olfactory mucosa [Rodriguez et al.,2000].
It may be that the human VNO has a function in fetuses, where nerve connections exist between the brain and elongated microvillar cells in the VNO [Bhatnagar and Smith,2001; Witt et al.,2002]. However, there is now converging evidence that the VNO in human adults has no apparent function. On a neurochemical level, it has been shown that the VNO lacks typical markers [Trotier et al.,2000; Witt et al.,2002]. Neuroanatomically, no neuronal connections are found between the VNO and the brain after week 32 in gestation [Meisami et al.,1998; Trotier et al.,2000; Witt et al.,2002]. In addition, no accessory bulb or an anatomically equivalent formation that is required for functional VNO signaling in other animals has been demonstrated in humans [Meisami et al.,1998; Meredith,2001]. In genetic studies, it has been shown that the gene TRPC2, which is essential for VNO functions in rodents, is a pseudogene in humans and other primates [Liman and Innan,2003]. Furthermore, the V2R genes that are expressed in mammalian VNOs are completely degenerated in primates [Young and Trask,2007]. Finally, we have demonstrated that the human VNO has no apparent function in olfactory perception of a putative pheromone.
CONCLUSION
We did not find any support for our hypothesis on a role of the VNO in the processing of endogenous odorants. Specifically, functional occlusion of the VNO did not alter the perception of or sensitivity to AND; there was no difference between subjects with and without a detectable VNO in AND perception; and functional occlusion of the VNO did not change patterns of brain activation after stimulation with AND. Thus, our study shows that the human VNO has no function in perception and processing of the most likely human pheromone and a control odor. Chemosignals in humans are probably processed via the main olfactory system.
Acknowledgements
The authors thank Giulia DeProphetis and Monica Hernandez for the help in preparing the odor solutions, and Andreas Frasnelli for his assistance in testing one of the PET subjects.
REFERENCES
- Aitken RC ( 1969): Measurements of feelings using visual analogue scale. Proc R Soc Med 62: 989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensafi M,Brown WM,Tsutsui T,Mainland JD,Johnson BN,Bremner EA,Young N,Mauss I,Ray B,Gross J,Richords J,Stappen I,Levenson RW,Sobel N. ( 2003): Sex‐steroid derived compounds induce sex‐specific effects on autonomic nervous system function in humans. Behav Neurosci 117: 1125–1134. [DOI] [PubMed] [Google Scholar]
- Bensafi M,Brown WM,Khan R,Levenson B,Sobel N ( 2004): Sniffing human sex‐steroid derived compounds modulates mood, memory and autonomic nervous system function in specific behavioral contexts. Behav Brain Res 152: 11–22. [DOI] [PubMed] [Google Scholar]
- Bhatnagar KP,Smith TD ( 2001): The human vomeronasal organ. III. Postnatal development from infancy to the ninth decade. J Anat 199( Part 3): 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm U,Zou Z,Buck LB ( 2005): Feedback loops link odor and pheromone signaling with reproduction. Cell 123: 683–695. [DOI] [PubMed] [Google Scholar]
- Brechbuhl J,Klaey M,Broillet MC ( 2008): Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science 321: 1092–1095. [DOI] [PubMed] [Google Scholar]
- Collins DL,Neelin P,Peters TM,Evans AC ( 1994): Automatic 3D inter‐subject registration of MR volumetric data in standardized Talairach Space. J Comput Assist Tomo 18: 192–205. [PubMed] [Google Scholar]
- Dorries KM,Adkins‐Regan E,Halpern BP ( 1995): Olfactory sensitivity to the pheromone, androstenone, is sexually dimorphic in the pig. Physiol Behav 57: 255–259. [DOI] [PubMed] [Google Scholar]
- Doty RL ( 1991): Olfactory System In: Getchell TV, Doty RL, Bartoshuk LM, Snow JBJ, editors. Smell and Taste in Health and Disease. New York: Raven Press; pp 175–203. [Google Scholar]
- Frasnelli J,Livermore A,Soiffer A,Hummel T ( 2002): Comparison of lateralized and binasal olfactory thresholds. Rhinology 40: 129–134. [PubMed] [Google Scholar]
- Gaafar HA,Tantawy AA,Melis AA,Hennawy DM,Shehata HM ( 1998): The vomeronasal (Jacobson's) organ in adult humans: Frequency of occurrence and enzymatic study. Acta Otolaryngol (Stockh) 118: 409–412. [DOI] [PubMed] [Google Scholar]
- Gottfried JA,Zald DH ( 2005): On the scent of human olfactory orbitofrontal cortex: Meta‐analysis and comparison to non‐human primates. Brain Res Rev 50: 287–304. [DOI] [PubMed] [Google Scholar]
- Gower DB,Ruparelia BA ( 1993): Olfaction in humans with special reference to odorous 16‐androstenes: Their occurrence, perception and possible social, psychological and sexual impact. J Endocrinol 137: 167–187. [DOI] [PubMed] [Google Scholar]
- Gulyas B,Keri S,O'Sullivan BT,Decety J,Roland PE ( 2004): The putative pheromone androstadienone activates cortical fields in the human brain related to social cognition. Neurochem Int 44: 595–600. [DOI] [PubMed] [Google Scholar]
- Jacob S,McClintock MK ( 2000): Psychological state and mood effects of steroidal chemosignals in women and men. Horm Behav 37: 57–78. [DOI] [PubMed] [Google Scholar]
- Jacob S,Zelano B,Gungor A,Abbott D,Naclerio R,McClintock MK ( 2000): Location and gross morphology of the nasopalatine duct in human adults. Arch Otolaryngol Head Neck Surg 126: 741–748. [DOI] [PubMed] [Google Scholar]
- Jacob S,Hayreh DJ,McClintock MK ( 2001a): Context‐dependent effects of steroid chemosignals on human physiology and mood. Physiol Behav 74: 15–27. [DOI] [PubMed] [Google Scholar]
- Jacob S,Kinnunen LH,Metz J,Cooper M,McClintock MK ( 2001b): Sustained human chemosignal unconsciously alters brain function. Neuroreport 12: 2391–2394. [DOI] [PubMed] [Google Scholar]
- Jahnke V,Merker H‐J ( 1998): Elektronenmikroskopische Untersuchungen des menschlichen vomeronasalen Organs. HNO 46: 502–506. [DOI] [PubMed] [Google Scholar]
- Johnson A,Josephson R,Hawke M ( 1985): Clinical and histological evidence for the presence of the vomeronasal (Jacobson's) organ in adult humans. J Otolaryngol 14: 71–79. [PubMed] [Google Scholar]
- Johnston RE ( 1998): Pheromones, the vomeronasal system, and communication. From hormonal responses to individual recognition. Ann N Y Acad Sci 855: 333–348. [DOI] [PubMed] [Google Scholar]
- Keller A,Zhuang H,Chi Q,Vosshall LB,Matsunami H ( 2007): Genetic variation in a human odorant receptor alters odour perception. Nature 449: 468–472. [DOI] [PubMed] [Google Scholar]
- Kelliher KR ( 2007): The combined role of the main olfactory and vomeronasal systems in social communication in mammals. Horm Behav 52: 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi T,Xu J,Dulac C ( 2007): A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448: 1009–1014. [DOI] [PubMed] [Google Scholar]
- Knecht M,Kuhnau D,Huttenbrink KB,Witt M,Hummel T ( 2001): Frequency and localization of the putative vomeronasal organ in humans in relation to age and gender. Laryngoscope 111: 448–452. [DOI] [PubMed] [Google Scholar]
- Knecht M,Lundstrom JN,Witt M,Huttenbrink KB,Heilmann S,Hummel T ( 2003): Assessment of olfactory function and androstenone odor thresholds in humans with or without functional occlusion of the vomeronasal duct. Behav Neurosci 117: 1135–1141. [DOI] [PubMed] [Google Scholar]
- Kraft P,Popaj K ( 2004): Total synthesis and olfactory evaluation of 5,10‐dimethyl‐des‐A‐18‐nor‐androstan‐13‐ol: a potential human pheromone? Eur J Organ Chem 2004: 4995–5002. [Google Scholar]
- Liman ER,Innan H ( 2003): Relaxed selective pressure on an essential component of pheromone transduction in primate evolution. Proc Natl Acad Sci USA 100: 3328–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom JN,Olsson MJ ( 2005): Subthreshold amounts of social odorant affect mood, but not behavior, in heterosexual women when tested by a male, but not a female, experimenter. Biol Psychol 70: 197–204. [DOI] [PubMed] [Google Scholar]
- Lundstrom JN,Goncalves M,Esteves F,Olsson MJ ( 2003a): Psychological effects of subthreshold exposure to the putative human pheromone 4,16‐androstadien‐3‐one. Horm Behav 44: 395–401. [DOI] [PubMed] [Google Scholar]
- Lundstrom JN,Hummel T,Olsson MJ ( 2003b): Individual differences in sensitivity to the odor of 4,16‐androstadien‐3‐one. Chem Senses 28: 643–650. [DOI] [PubMed] [Google Scholar]
- Lundstrom JN,Olsson MJ,Schaal B,Hummel T ( 2006): A putative social chemosignal elicits faster cortical responses than perceptually similar odorants. Neuroimage 30: 1340–1346. [DOI] [PubMed] [Google Scholar]
- Lundstrom JN,Boyle JA,Zatorre RJ,Jones‐Gotman M ( 2008): Functional neuronal processing of body odors differs from that of similar common odors. Cereb Cortex 18: 1466–1474. [DOI] [PubMed] [Google Scholar]
- Lundstrom JN,Boyle JA,Zatorre RJ,Jones‐Gotman M ( 2009): The neuronal substrates of human olfactory based kin recognition. Hum Brain Mapp 30: 2571–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock MK ( 2000): Human pheromones: Primers, releasers, signalers, or modulators? In: Wallen K, Schneider JE, editors. Reproduction in Context: Social and Environmental Influences on Reproductive Physiology and Behavior. Cambridge: MIT Press; pp 355–420. [Google Scholar]
- Meisami E,Mikhail L,Baim D,Bhatnagar KP ( 1998): Human olfactory bulb: Aging of glomeruli and mitral cells and a search for the accessory olfactory bulb. Ann N Y Acad Sci 855: 708–715. [DOI] [PubMed] [Google Scholar]
- Meredith M ( 1991): Sensory processing in the main and accessory olfactory systems: Comparisons and contrasts. J Steroid Biochem Mol Biol 39: 601–614. [DOI] [PubMed] [Google Scholar]
- Meredith M ( 2001): Human vomeronasal organ function: A critical review of best and worst cases. Chem Senses 26: 433–445. [DOI] [PubMed] [Google Scholar]
- Monti‐Bloch L,Grosser BI ( 1991): Effect of putative pheromones on the electrical activity of the human vomeronasal organ and olfactory epithelium. J Steroid Biochem Molec Biol 39: 573–582. [DOI] [PubMed] [Google Scholar]
- Moran DT,Jafek BW,Rowley JCD ( 1991): The vomeronasal (Jacobson's) organ in man: Ultrastructure and frequency of occurrence. J Steroid Biochem Mol Biol 39: 545–552. [DOI] [PubMed] [Google Scholar]
- Nixon A,Mallet AI,Gower DB ( 1988): Simultaneous quantification of five odorous steroids (16‐androstenes) in the axillary hair of men. J Steroid Biochem 29: 505–510. [DOI] [PubMed] [Google Scholar]
- Porter RH,Cernoch JM,Balogh RD ( 1985): Odor signatures and kin recognition. Physiol Behav 34: 445–448. [DOI] [PubMed] [Google Scholar]
- Potiquet M ( 1891): Le canal de Jacobson. Rev Laryngol Paris 2: 737–753. [Google Scholar]
- Potts WK,Manning CJ,Wakeland EK ( 1991): Mating patterns in seminatural populations of mice influenced by MHC genotype. Nature 352: 619–621. [DOI] [PubMed] [Google Scholar]
- Rodriguez I,Greer CA,Mok MY,Mombaerts P ( 2000): A putative pheromone receptor gene expressed in human olfactory mucosa. Nat Genet 26: 18–19. [DOI] [PubMed] [Google Scholar]
- Sam M,Vora S,Malnic B,Ma W,Novotny MV,Buck LB ( 2001): Neuropharmacology. Odorants may arouse instinctive behaviours. Nature 412: 142. [DOI] [PubMed] [Google Scholar]
- Savic I,Berglund H,Gulyas B,Roland P ( 2001): Smelling of odorous sex hormone‐like compounds causes sex‐differentiated hypothalamic activations in humans. Neuron 31: 661–668. [DOI] [PubMed] [Google Scholar]
- Savic I,Berglund H,Lindstrom P ( 2005): Brain response to putative pheromones in homosexual men. Proc Natl Acad Sci USA 102: 7356–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I,Heden‐Blomqvist E,Berglund H ( 2009): Pheromone signal transduction in humans: What can be learned from olfactory loss. Hum Brain Mapp 30: 3057–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel N,Brown WM ( 2001): The scented brain: pheromonal responses in humans. Neuron 31: 512–514. [DOI] [PubMed] [Google Scholar]
- Stensaas LJ,Lavker RM,Monti‐Bloch L,Grosser BI,Berliner DL ( 1991): Ultrastructure of the human vomeronasal organ. J Steroid Biochem Molec Biol 39: 553–560. [DOI] [PubMed] [Google Scholar]
- Stowe MK,Turlings TC,Loughrin JH,Lewis WJ,Tumlinson JH ( 1995): The chemistry of eavesdropping, alarm, and deceit. Proc Natl Acad Sci USA 92: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh K,Storm DR ( 2003): Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat Neurosci 6: 519–525. [DOI] [PubMed] [Google Scholar]
- Trotier D,Eloit C,Wassef M,Talmain G,Bensimon JL,Døving KB,Ferrand J ( 2000): The vomeronasal cavity in adult humans. Chem Senses 25: 369–380. [DOI] [PubMed] [Google Scholar]
- Witt M,Georgiewa B,Knecht M,Hummel T ( 2002): On the chemosensory nature of the vomeronasal epithelium in adult humans. Histochem Cell Biol 117: 493–509. [DOI] [PubMed] [Google Scholar]
- Won J,Mair EA,Bolger WE,Conran RM ( 2000): The vomeronasal organ: An objective anatomic analysis of its prevalence. Ear Nose Throat J 79: 600–605. [PubMed] [Google Scholar]
- Worsley KJ,Evans AC,Marrett S,Neelin P ( 1992): A three‐dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12: 900–918. [DOI] [PubMed] [Google Scholar]
- Worsley KJ,Marrett S,Neelin P,Vandal AC,Friston KJ,Evans AC ( 1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
- Wyart C,Webster WW,Chen JH,Wilson SR,McClary A,Khan RM,Sobel N ( 2007): Smelling a single component of male sweat alters levels of cortisol in women. J Neurosci 27: 1261–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki CJ ( 1979): Neurobehavioral evidence for the involvement of the vomeronasal system in mammalian reproduction. Neurosci Biobehav Rev 3: 301–341. [DOI] [PubMed] [Google Scholar]
- Yoon H,Enquist LW,Dulac C ( 2005): Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell 123: 669–682. [DOI] [PubMed] [Google Scholar]
- Young JM,Trask BJ ( 2007): V2R gene families degenerated in primates, dog and cow, but expanded in opossum. Trends Genet 23: 212–215. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ,Jones‐Gotman M ( 2000): Functional imaging of the chemical senses In: Toga AW, Mazziotta JC, editors. Brain Mapping: The Applications. San Diego: Academic Press; pp 403–424. [Google Scholar]
- Zatorre RJ,Jones‐Gotman M,Evans AC,Meyer E ( 1992): Functional localization and lateralization of human olfactory cortex. Nature 360: 339–340. [DOI] [PubMed] [Google Scholar]


