Abstract
Adiponectin, an adipokine secreted by adipocytes, exerts beneficial effects on glucose and lipid metabolism and has been found to improve insulin resistance by decreasing triglyceride content in muscle and liver in obese mice. Adiponectin is found in several isoforms and the high-molecular weight (HMW) form has been linked most strongly to the insulin-sensitizing effects. Fat content in skeletal muscle (intramyocellular lipids, IMCL) and liver (intrahepatic lipids, IHL) can be quantified noninvasively using proton magnetic resonance spectroscopy (1H-MRS). The purpose of our study was to assess the relationship between HMW adiponectin and measures of glucose homeostasis, IMCL and IHL, and to determine predictors of adiponectin levels. We studied 66 premenopausal women (mean BMI 31.0 ± 6.6 kg/m2) who underwent 1H-MRS of calf muscles and liver for IMCL and IHL, computed tomography (CT) of the abdomen for abdominal fat depots, dual-energy X-ray absorptiometry (DXA) for fat and lean mass assessments, HMW and total adiponectin, fasting lipid profile and an oral glucose tolerance test (homeostasis model assessment of insulin resistance (HOMAIR), glucose and insulin area under the curve). There were strong inverse associations between HMW adiponectin and measures of insulin resistance, IMCL and IHL, independent of visceral adipose tissue (VAT) and total body fat. IHL was the strongest predictor of adiponectin and adiponectin was a predictor of HOMAIR. Our study showed that in premenopausal obese women HMW adiponectin is inversely associated with IMCL and IHL content. This suggests that adiponectin exerts positive effects on insulin sensitivity in obesity by decreasing intracellular triglyceride content in skeletal muscle and liver; it is also possible that our results reflect effects of insulin on adiponectin.
INTRODUCTION
Adipose tissue is an active endocrine organ, secreting hormones and adipokines, including adiponectin. Adiponectin exerts beneficial effects on glucose and lipid metabolism and plays a protective role against the development of cardiovascular disease, dyslipidemia, insulin resistance, and nonalcoholic steatohepatitis. Consistent with these effects, adiponectin concentrations are low in patients with obesity, insulin resistance and the metabolic syndrome (1–4). Circulating adiponectin is found in different isoforms with distinct biological functions. Most insulin-sensitizing effects of adiponectin have been linked to the high-molecular weight (HMW) form. The HMW form is inversely associated with the risk for diabetes, independent of total adiponectin, and is inversely associated with visceral adipose tissue (VAT) and presence of the metabolic syndrome (5–7). Adiponectin has been found to improve insulin resistance by decreasing triglyceride content in muscle and liver in obese mice (8).
In humans, the role of adiponectin in insulin resistance and its effects on intrahepatic lipids (IHL) and intramyocellular lipids (IMCL) is not well understood. An inverse association between total adiponectin and IMCL has been found in obese adults and adolescents (9–11), whereas no such association was detected in men ranging from normal-weight to obese (12) and subjects with type 2 diabetes (13). Similarly, and inverse association between total adiponectin and IHL was found in subjects with obesity and type 2 diabetes (10,13,14) but not in healthy nondiabetic men and women (15).
No studies have been performed investigating the role of the HMW form of adiponectin and muscle and liver fat accumulation, determined by proton magnetic resonance spectroscopy (1H-MRS). Moreover, no studies have examined the relationship between any form of adiponectin and these measures in nondiabetic premenopausal women ranging from normal-weight to obese.
We hypothesized that in obese premenopausal women HMW adiponectin would be inversely associated with measures of glucose homeostasis, IMCL and IHL, independent of body fat, and that IMCL and IHL would be predictors of HMW adiponectin levels. We therefore evaluated IMCL, IHL, and abdominal fat depots and total body fat using 1H-MRS, computed tomography (CT), and dual-energy X-ray absorptiometry (DXA), in premenopausal women over a wide range of BMIs, to determine whether there is an association between these parameters and adiponectin and insulin resistance (by homeostasis model assessment of insulin resistance (HOMAIR), glucose and insulin area under the curve).
METHODS AND PROCEDURES
The study was approved by Partners Healthcare institutional review board and complied with Health Insurance Portability and Accountability Act guidelines. Written informed consent was obtained from all subjects after the nature of the procedures had been fully explained.
Subjects
The study group was comprised of 66 healthy premenopausal women who were recruited from the community through advertisements. Inclusion criteria were female gender, ages 18 to 45 years, and eumenorrhea. Exclusion criteria included hypothalamic or pituitary disorders, diabetes mellitus or other chronic illnesses, estrogen or glucocorticoid use, weight >280 pounds (due to limitations of the DXA, CT, and magnetic resonance imaging scanner), and contraindications to magnetic resonance imaging such as the presence of a pacemaker or metallic implant. None of the patients had a history of liver disease, and all patients had a normal alanine aminotransferase.
Each participant underwent 1H-MRS and CT as detailed below and fasting blood tests, and an oral glucose tolerance test. IMCL and IHL data, body composition, and clinical characteristics have been previously reported in 54 of the 66 subjects (16–18).
Endocrine testing
Subjects underwent the following blood tests: fasting determination of lipid profile (total, high-density lipoprotein, low-density lipoprotein cholesterol, and triglycerides), glucose, and insulin. Adiponectin (total and HMW forms) was measured by enzyme-linked immunosorbent assay (ALPCO Diagnostics, Salem, NH). Intra- and interassay coefficients of variation are less than 8% and 10%, respectively. A standard oral glucose tolerance test with 75 g glucose load was performed, and insulin and glucose area under the curve were calculated using the trapezoidal model. HOMAIR was calculated as (fasting glucose (mmol/l) × fasting insulin (μU/ml))/22.5 (19).
1H-MR spectroscopy of muscle and liver
1H-MRS was performed in 48 subjects using a 3.0 Tesla (Siemens Trio; Siemens Medical Systems, Erlangen, Germany) magnetic resonance imaging system. After an 8 h overnight fast, each subject underwent 1H-MRS of calf muscle and 1H-MRS of the liver. For calf muscle 1H-MRS, a voxel measuring 15 × 15 × 15 mm (3.4 ml) was placed on the axial T1-weighted slice with largest muscle cross-sectional area of the tibialis anterior (TA) and subsequently the soleus (SOL) muscles, avoiding visible interstitial tissue, fat or vessels. Single-voxel 1H-MRS data were acquired using point-resolved spatially localized spectroscopy pulse sequence with repetition time of 3,000 ms, echo-time of 30 ms, 64 acquisitions, 1,024 data points, and receiver bandwidth of 1,000 Hz. Water suppression was used for metabolite acquisition, and unsuppressed water spectra of the same voxel were obtained for each scan with the same parameters as the metabolite acquisition except for the use of eight acquisitions. For liver 1H-MRS a breath-hold True Fast Imaging with Steady Precession sequence was obtained. A voxel measuring 20 × 20 × 20 mm (8 ml) was placed within the right hepatic lobe, avoiding vessels or artifact. Breath-hold single-voxel 1H-MRS data was acquired using point-resolved spatially localized spectroscopy pulse sequence without water suppression with the following parameters: repetition time of 1,500 ms, echo-time of 30 ms, 8 acquisitions, 1,024 data points, and receiver bandwidth of 2,000 Hz.
1H-MR spectroscopic data analysis
Fitting of all 1H-MRS data was performed using LCModel (version 6.1-4A; Stephen Provencher, Oakville, ON, Canada) (20). Data were transferred from the MR scanner to a Linux workstation and metabolite quantification was performed using eddy current correction and water scaling. The fitting algorithm was customized for muscle analysis providing estimates for lipid peaks (0.9, 1.1, 1.3, 1.5, 2.1, and 2.3 p.p.m.), creatine (2.8 and ~3.0 p.p.m.), trimethylamines (3.2 p.p.m.), and putative taurine signal (~3.5 p.p.m.). Data for IMCL (1.3 p.p.m.) methylene protons were used for statistical analyses. For liver 1H-MRS, a customized fitting algorithm for liver analysis provided estimates for all lipid signals combined (0.9, 1.3, and 2.3 p.p.m.). LCModel IMCL and EMCL estimates and liver lipid estimates were automatically scaled to unsuppressed water peak (4.7 p.p.m.) and expressed in lipid to water ratio (%).
CT-body composition
Each subject underwent cross-sectional CT scan of the abdomen at the level of L4 (LightSpeed; General Electric, Milwaukee, WI). Assessment of total, visceral, and subcutaneous abdominal fat compartments was performed by single slice CT. Fat attenuation coefficients were set at −50 to −250 Hounsfield unit as described by Borkan et al. (21). VAT, subcutaneous adipose tissue areas, and total adipose tissue (the sum of subcutaneous and VAT) areas were determined based on offline analysis of tracings obtained utilizing commercial software (VITRAK; Merge/eFilm, Milwaukee, WI; and Alice version 4.3.9; Parexel, Waltham, MA).
Dual-energy X-ray-absorptiometry
Each subject underwent DXA to measure total body fat and lean mass, extremity fat and lean mass, and trunk fat mass (kg) using a Hologic QDR 4500 scanner (Hologic, Waltham, MA).
Statistical analysis
JMP Statistical Database Software (version 5.0.1; SAS Institute, Cary, NC) was used for statistical analyses. Nonparametric Spearman rank correlation coefficients are reported. Correlation coefficients of HMW and total adiponectin were compared by applying Fisher’s z-transformation and then the large sample z-test for independent samples. Multivariate standard least squares regression modeling was performed to control for VAT and total body fat, IMCL, and IHL. Forward stepwise regression modeling was also performed to determine predictors of adiponectin, body composition, and insulin resistance. For stepwise regression analysis, only subjects who had undergone assessment of all parameters entered into the models were included. P < 0.05 was used to denote significance. Data are presented as mean ± s.d.
RESULTS
Clinical characteristics of study subjects
Subject characteristics are shown in Table 1. The age of study participants ranged from 19 to 45 years, with a mean of 34.3 ± 7.4 years. Study participants ranged in BMI from 19 to 46 kg/m2, with a mean BMI of 31.0 ± 6.6 kg/m2. Sixteen subjects were categorized as lean (BMI <25 kg/m2), 19 subjects were overweight (BMI =25 kg/m2 and <30 kg/m2), and 31 subjects were obese (BMI =30 kg/m2) based on World Health Organization definitions (22).
Table 1.
Clinical characteristics of all study subjects (mean ± s.d.)
| Variable | Subjects (n = 66) |
|---|---|
| Age (years) | 34.3 ± 7.4 |
| Weight (kg) | 83.8 ± 17.6 |
| BMI (kg/m2) | 31.0 ± 6.6 |
| HMW adiponectin (μg/ml) | 3.1 ± 1.6 |
| Total adiponectin (μg/ml) | 5.3 ± 2.1 |
| Total serum cholesterol (mg/dl) | 174.8 ± 37.6 |
| HDL cholesterol (mg/dl) | 49.9 ± 11.3 |
| LDL cholesterol (mg/dl) | 105.9 ± 32.9 |
| Serum triglycerides (mg/dl) | 95.1 ± 52.2 |
| Glucose-AUC (mg/dl × 120 min) | 14,968 ± 2,598 |
| Insulin-AUC (mg/dl × 120 min) | 4,468 ± 2,818 |
| HOMAIR | 1.5 ± 1.0 |
| CT: TAT (cm2) | 479.3 ± 208.0 |
| CT: SAT (cm2) | 388.5 ± 169.5 |
| CT: VAT (cm2) | 90.8 ± 58.0 |
| DXA: total body fat (kg) | 33.3 ± 12.2 |
| DXA: trunk fat (kg) | 15.8 ± 6.9 |
| IMCL-TA/H2O (%) (n = 48) | 0.45 ± 0.26 |
| IMCL-SOL/H2O (%) (n = 48) | 2.2 ± 1.1 |
| IHL/H2O (%) (n = 48) | 5.6 ± 7.3 |
AUC, area under the curve; CT, computed tomography; DXA, dual-energy X-ray absorptiometry; HDL, high-density lipoprotein; HMW, high-molecular weight; HOMAIR, homeostasis model assessment of insulin resistance; IHL, intrahepatic lipids; IMCL, intramyocellular lipids; LDL, low-density lipoprotein; SAT, subcutaneous adipose tissue; SOL, soleus muscle; TA, tibialis anterior muscle; TAT, total abdominal adipose tissue; VAT, visceral adipose tissue.
Associations of adiponectin with intramyocellular and IHL, body composition, serum lipids, and measures of insulin resistance
There were inverse associations between adiponectin and measures of insulin resistance, body composition, and serum triglycerides (Table 2). No statistically significant difference was detected when comparing correlation coefficients between HMW and total adiponectin (P > 0.05).
Table 2.
Associations of high-molecular weight and total adiponectin with body composition, measures of insulin resistance, and serum triglycerides
| HMW adiponectin
|
Total adiponectin
|
|||
|---|---|---|---|---|
| r | P | r | P | |
| Weight | −0.30 | 0.02 | −0.25 | 0.04 |
| BMI | −0.31 | 0.01 | −0.25 | 0.05 |
| TAT | −0.32 | 0.008 | −0.28 | 0.02 |
| SAT | −0.25 | 0.04 | −0.22 | 0.08 |
| VAT | −0.47 | <0.0001 | −0.42 | 0.0006 |
| Total body fat (DXA) | −0.34 | 0.006 | −0.30 | 0.02 |
| Trunk fat (DXA) | −0.39 | 0.002 | −0.34 | 0.005 |
| IMCL-TA | −0.45 | 0.001 | −0.37 | 0.009 |
| IMCL-SOL | −0.34 | 0.02 | −0.23 | 0.1 |
| IHL | −0.60 | <0.0001 | −0.57 | <0.0001 |
| Glucose AUC | −0.43 | 0.0004 | −0.40 | 0.001 |
| Insulin AUC | −0.52 | <0.0001 | −0.50 | <0.0001 |
| HOMAIR | −0.44 | 0.0004 | −0.48 | <0.0001 |
| Triglycerides | −0.46 | <0.0001 | −0.45 | 0.0002 |
AUC, area under the curve; DXA, dual-energy X-ray absorptiometry; HMW, high-molecular weight; HOMAIR, homeostasis model assessment of insulin resistance; IHL, intrahepatic lipids; IMCL, intramyocellular lipids; SAT, subcutaneous adipose tissue; SOL, soleus muscle; TA, tibialis anterior muscle; TAT, total abdominal adipose tissue; VAT, visceral adipose tissue.
There were strong inverse associations between HMW adiponectin and IMCL-TA (r = −0.45, P = 0.001) (Figure 1), IMCL-SOL (r = −0.34, P = 0.02), and IHL (r = −0.60, P < 0.0001) (Figure 2). After controlling for VAT, there remained a significant inverse association between HMW adiponectin and IMCL-TA (P = 0.02) and IHL (P = 0.009). The associations also remained significant after controlling for total body fat by DXA (P = 0.02 and P = 0.001, respectively). However, there was no significant association between HMW adiponectin and IMCL-SOL after controlling for VAT (P = 0.5) or total body fat by DXA (P = 0.2). There was no significant association between adiponectin and total lean mass or extremity lean mass (P = 0.2 and P = 0.3, respectively).
Figure 1.
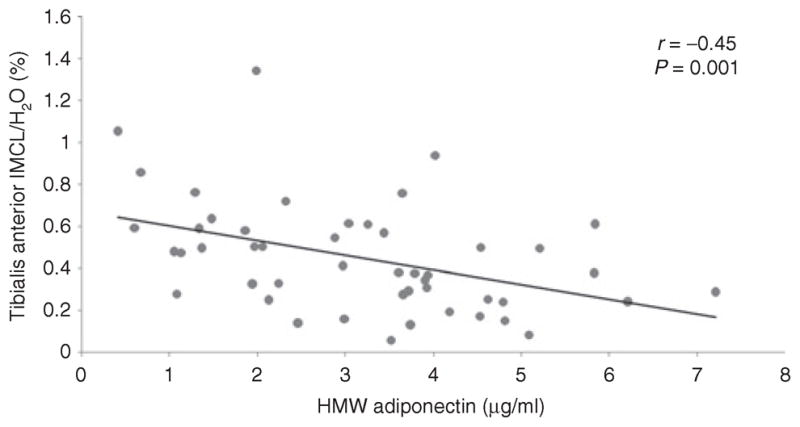
Regression analysis of high-molecular weight (HMW) adiponectin on tibialis anterior intramyocellular lipids (IMCL). There is an inverse association between HMW adiponectin and tibialis anterior IMCL.
Figure 2.
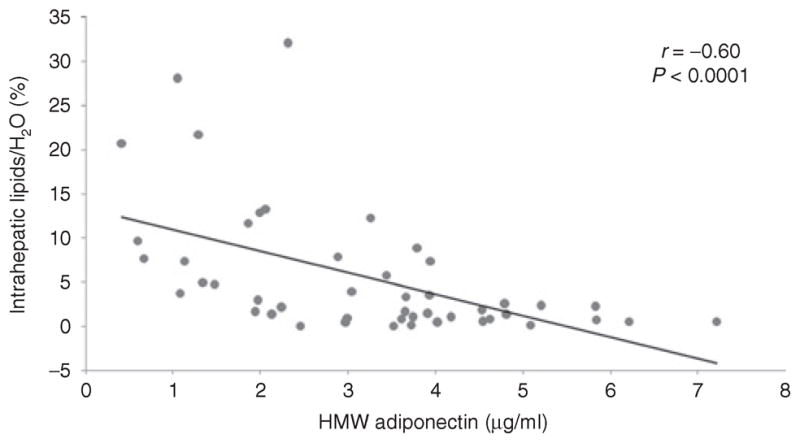
Regression analysis of high-molecular weight (HMW) adiponectin on intrahepatic lipids (IHL). There is an inverse association between HMW adiponectin and IHL.
There was an inverse association between HMW adiponectin and measures of insulin resistance (r = −0.43 to −0.52, P = 0.0004 to <0.0001), which was independent of VAT and total body fat (P = 0.03 to P = 0.006). However, after controlling for IMCL-TA and IHL the association between HMW adiponectin and HOMAIR lost significance (P = 0.06 and 0.07, respectively), suggesting that the insulin sensitizing effects of adiponectin are mediated in part by IMCL and IHL.
There was an inverse association between HMW adiponectin and abdominal fat depots (r = −0.34 to −0.47, P = 0.006 to <0.0001), with the strongest association between HMW adiponectin and VAT (r = −0.47, P < 0.0001) (Figure 3). There was an inverse association between HMW adiponectin and upper extremity fat mass as determined by DXA (r = −0.37, P = 0.002) while there was no significant association between adiponectin and total, and lower extremity fat mass as determined by DXA (P = 0.3).
Figure 3.
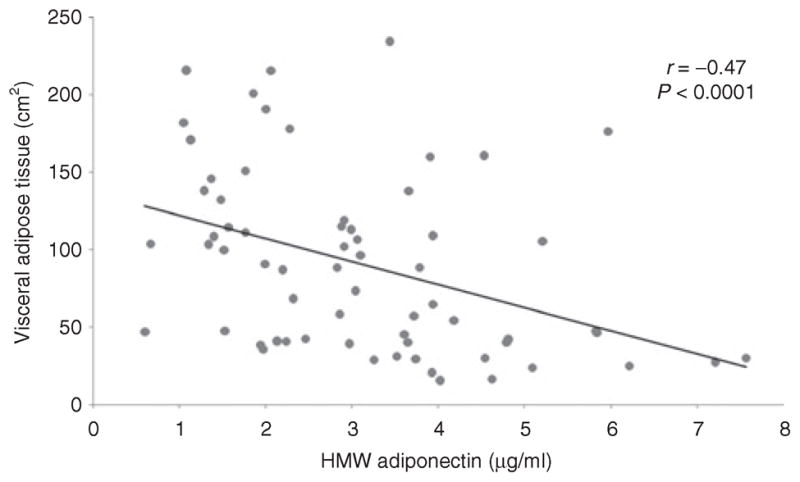
Regression analysis of high-molecular weight (HMW) adiponectin on visceral adipose tissue (VAT). There is an inverse association between HMW adiponectin and VAT.
HMW adiponectin was inversely associated with serum triglycerides (r = −0.46, P < 0.0001) (Figure 4), which remained significant after controlling for VAT and total body fat (P = 0.01 and P = 0.0009, respectively), whereas there was no association with serum cholesterol (P = 0.07 to 0.2).
Figure 4.
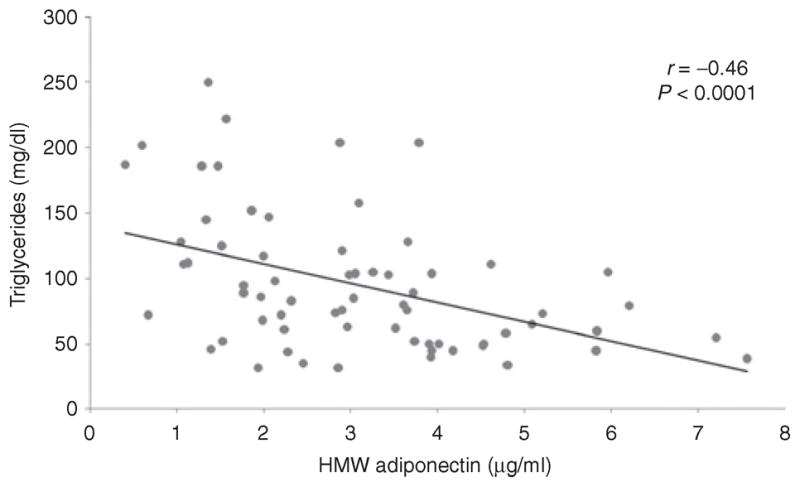
Regression analysis of high-molecular weight (HMW) adiponectin on serum triglycerides. There is an inverse association between HMW adiponectin and serum triglycerides.
There was an inverse association between HMW adiponectin and weight (r = −0.30, P = 0.02) and BMI (r = −0.31, P = 0.01). There was no significant association with adiponectin and age (P = 0.43).
In order to determine which fat depot is the strongest predictor of adiponectin levels, we performed forward stepwise regression modeling. When HMW adiponectin was entered as a dependent variable and IMCL-TA, IHL, VAT, and serum triglycerides, as independent variables in a forward stepwise regression model, IHL explained 25% of the variability of adiponectin (r2 = 0.25, P = 0.0005) and serum triglycerides explained 9% of the variability of adiponectin (r2 = 0.9, P = 0.02).
When IHL was entered as a dependent variable and HMW adiponectin, VAT, IMCL-TA, and serum triglycerides as independent variables in a forward stepwise regression model, HMW adiponectin explained 24% of the variability (r2 = 0.24, P = 0.0005) and IMCL-TA explained 10% of the variability (r2 = 0.1, P = 0.02) of IHL.
In order to determine the strongest predictors of insulin resistance we entered HOMAIR as a measure of insulin resistance as a dependent variable and HMW adiponectin, IHL, IMCL-TA, VAT, and triglycerides as independent variables in a forward stepwise regression model. IMCL-TA was a significant predictor of HOMAIR and explained 19% of the variability of HOMAIR (r2 = 0.19, P = 0.004), and VAT explained 9% of the variability of HOMAIR (r2 = 0.09, P = 0.03).
When using total adiponectin instead of HMW adiponectin, there was no difference in predictors of VAT, IHL, and HOMAIR and IHL was the strongest predictor of total adiponectin.
DISCUSSION
We demonstrate inverse associations of HMW and total adiponectin with IMCL and IHL independent of VAT and total body fat in premenopausal women over a wide range of BMIs. In addition, we show that HMW and total adiponectin are inverse predictors of insulin resistance, and IHL and serum triglycerides are predictors of adiponectin levels. In nondiabetic healthy women of reproductive age, adiponectin may exert its insulin-sensitizing effects through effects on intrahepatic and intramyocellular lipid content.
Adiponectin is an adipokine that is abundantly expressed in adipose tissue and is known to modulate insulin effects. Paradoxically, its concentration is reduced in subjects with obesity and the metabolic syndrome. Adiponectin is inversely associated with measures of insulin resistance and low levels of adiponectin are associated with visceral adiposity, hyperlipidemia, nonalcoholic steatohepatitis, and insulin resistance (1,23). Treatment of diabetic mice with adiponectin increases insulin sensitivity by reducing triglyceride content of muscle and liver (8). In skeletal muscle, adiponectin improves insulin sensitivity by increasing free fatty acid transport and lipid oxidation, which in turn leads to decreased muscle triglyceride content (8). In the liver, adiponectin reduces hepatic glucogenesis and free fatty acid influx into the liver, thereby decreasing hepatic triglyceride content (8,24). Consistent with these findings, we found an inverse association between both IHL and IMCL-TA and adiponectin levels; independent of VAT and total body fat mass, with IHL as the strongest predictor of adiponectin levels explaining 24% of the variability of adiponectin. This is in concordance with studies demonstrating an inverse association between adiponectin and IHL in obese Pima-Indians (10) and in subjects with nonalcoholic steatohepatitis and type 2 diabetes (1,13,25,26). However, in a study of nondiabetic healthy subjects no significant correlation between adiponectin and IHL was observed (15). The lack of significant association between adiponectin and IHL in that study may have been due to the small number and narrower range of BMI and the inclusion of both men and women in the same study.
In our study, IMCL-TA was inversely associated with adiponectin levels; independent of VAT and total body fat mass. IMCL-SOL was inversely associated with adiponectin but the association lost significance after adjusting for VAT. Prior studies in men ranging from normal-weight to obese (12) and Japanese men and women with type 2 diabetes found no significant correlations between adiponectin and IMCL-SOL (13), while there was an inverse association between adiponectin and IHL in subjects with type 2 diabetes (13). IMCL-TA was not evaluated in either study. In a prior study in obese premenopausal women we have shown stronger correlations between the IMCL of TA muscle, which is composed predominately of nonoxidative type IIb fibers, and IHL and triglycerides compared to IMCL of SOL muscle, which is composed predominately of oxidative type I fibers. IMCL-SOL correlated better with measures of insulin resistance and abdominal fat depots (18). This is concordant with a study by Koska et al. (10) that demonstrated in inverse association between adiponectin and IMCL of type II fibers but not IMCL of type I fibers in obese Pima Indians using muscle biopsies of vastus lateralis. In a group of healthy volunteers with a mean BMI of 24 kg/m2, Thamer et al. found an inverse association between IMCL-SOL and adiponectin, but there was no association with IMCL-TA. However, following a dietary lipid challenge, there was a significant inverse association between IMCL-TA and adiponectin, whereas the association with IMCL-SOL lost significance (27). These data suggest that IMCL-TA and IMCL-SOL may play different roles in the pathogenesis of adiponectin-mediated skeletal muscle insulin sensitivity. Obesity may cause lipid saturation of type I fibers, which have high lipid transport capacity, whereas type II fibers are dependent on activation of the AMP-kinase pathway regulated by adiponectin (10).
The adiponectin gene is located on chromosome 3q26, a region associated with susceptibility to developing metabolic syndrome and type 2 diabetes (28). It has been suggested that low adiponectin levels may be a consequence of insulin resistance with compensatory hyperinsulinemia (29). We found strong inverse associations between adiponectin and measures of insulin resistance, independent of VAT and total body fat, which is in concordance with several prior studies in adults and adolescents with obesity, subjects with nonalcoholic steatohepatitis, and type 2 diabetes (1,4,11,12). However, the negative association between adiponectin and HOMAIR lost significance after controlling for IMCL and IHL, possibly due to effects of adiponectin on IMCL and IHL. IMCL have been hypothesized to play an etiologic role in obesity-induced insulin resistance (30) and hepatic lipid content has been shown to be strongly linked to the degree of insulin resistance in obesity (31). Our data support the hypothesis that the insulin-sensitizing effects of adiponectin are mediated through its effect of IMCL and IHL content.
When evaluating the association between adiponectin and different abdominal fat depots as well as total body fat mass, we found inverse associations between all abdominal fat depots, trunk and total body fat mass, with the strongest correlation between HMW adiponectin and VAT, concordant with prior studies (32,33). In contrast, a study in normal-weight men found the strongest inverse association between adiponectin and SAT and a positive correlation between adiponectin and lower extremity fat mass as determined by DXA (34). In our study there was no significant correlation between adiponectin and lower and total extremity fat mass.
Circulating adiponectin is found in several isoforms and the HMW form has been linked most strongly to the insulin-sensitizing effects of adiponectin (5–7,35,36). We could not demonstrate statistically significant difference when comparing correlation coefficients of HMW and total adiponectin using Fisher’s z-transformation. We might have been able to demonstrate a statistically significant difference had we studied a much larger number of subjects. However, these results are in concordance with a prior study that did not find significant differences in HMW and total adiponectin as a predictor of metabolic variables and insulin sensitivity prior to and following an exercise intervention (37). Another limitation of our study is that we did not measure low and medium molecular weight adiponectin. Finally, the conclusions we can draw from this study are limited by its cross-sectional design, and our findings can not imply causality or mechanism. Further studies are needed to determine the role of HMW and total adiponectin as a mediator of insulin sensitivity.
In conclusion, in healthy premenopausal women over a wide BMI range, HMW adiponectin is inversely associated with IMCL and IHL content. This suggests that adiponectin exerts positive effects on insulin sensitivity in women of reproductive by decreasing intracellular triglyceride content in skeletal muscle and liver; it is also possible that our results reflect effects of insulin on adiponectin.
Acknowledgments
This work was supported in part by National Institutes of Health Grants RO1 HL-077674, UL1 RR025758, and K23 RR-23090.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Bugianesi E, Pagotto U, Manini R, et al. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab. 2005;90:3498–3504. doi: 10.1210/jc.2004-2240. [DOI] [PubMed] [Google Scholar]
- 2.Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 3.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 4.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 5.Heidemann C, Sun Q, van Dam RM, et al. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med. 2008;149:307–316. doi: 10.7326/0003-4819-149-5-200809020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill MJ, Kumar S, McTernan PG. Adipokines and the clinical laboratory: what to measure, when and how? J Clin Pathol. 2009;62:206–211. doi: 10.1136/jcp.2007.049171. [DOI] [PubMed] [Google Scholar]
- 7.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT. Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes. 2006;55:249–259. [PubMed] [Google Scholar]
- 8.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 9.Godoy-Matos AF, Bahia LR, Domingues RC, et al. Adiponectin is related to intramyocellular lipid content in nondiabetic adults. J Endocrinol Invest. 2010;33:382–387. doi: 10.1007/BF03346608. [DOI] [PubMed] [Google Scholar]
- 10.Koska J, Stefan N, Permana PA, et al. Increased fat accumulation in liver may link insulin resistance with subcutaneous abdominal adipocyte enlargement, visceral adiposity, and hypoadiponectinemia in obese individuals. Am J Clin Nutr. 2008;87:295–302. doi: 10.1093/ajcn/87.2.295. [DOI] [PubMed] [Google Scholar]
- 11.Weiss R, Dufour S, Groszmann A, et al. Low adiponectin levels in adolescent obesity: a marker of increased intramyocellular lipid accumulation. J Clin Endocrinol Metab. 2003;88:2014–2018. doi: 10.1210/jc.2002-021711. [DOI] [PubMed] [Google Scholar]
- 12.Furler SM, Gan SK, Poynten AM, et al. Relationship of adiponectin with insulin sensitivity in humans, independent of lipid availability. Obesity (Silver Spring) 2006;14:228–234. doi: 10.1038/oby.2006.29. [DOI] [PubMed] [Google Scholar]
- 13.Maeda K, Ishihara K, Miyake K, et al. Inverse correlation between serum adiponectin concentration and hepatic lipid content in Japanese with type 2 diabetes. Metabolism. 2005;54:775–780. doi: 10.1016/j.metabol.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Teranishi T, Ohara T, Maeda K, et al. Effects of pioglitazone and metformin on intracellular lipid content in liver and skeletal muscle of individuals with type 2 diabetes mellitus. Metabolism. 2007;56:1418–1424. doi: 10.1016/j.metabol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Hwang JH, Stein DT, Barzilai N, et al. Increased intrahepatic triglyceride is associated with peripheral insulin resistance: in vivo MR imaging and spectroscopy studies. Am J Physiol Endocrinol Metab. 2007;293:E1663–E1669. doi: 10.1152/ajpendo.00590.2006. [DOI] [PubMed] [Google Scholar]
- 16.Bredella MA, Ghomi RH, Thomas BJ, Miller KK, Torriani M. Comparison of 3. 0 T proton magnetic resonance spectroscopy short and long echo-time measures of intramyocellular lipids in obese and normal-weight women. J Magn Reson Imaging. 2010;32:388–393. doi: 10.1002/jmri.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bredella MA, Ghomi RH, Thomas BJ, et al. Breath-hold 1H-magnetic resonance spectroscopy for intrahepatic lipid quantification at 3 Tesla. J Comput Assist Tomogr. 2010;34:372–376. doi: 10.1097/RCT.0b013e3181cefb89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bredella MA, Torriani M, Thomas BJ, et al. Peak growth hormone-releasing hormone-arginine-stimulated growth hormone is inversely associated with intramyocellular and intrahepatic lipid content in premenopausal women with obesity. J Clin Endocrinol Metab. 2009;94:3995–4002. doi: 10.1210/jc.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 21.Borkan GA, Gerzof SG, Robbins AH, et al. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36:172–177. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 22.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 23.Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S64–S73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 25.Bajaj M, Suraamornkul S, Piper P, et al. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab. 2004;89:200–206. doi: 10.1210/jc.2003-031315. [DOI] [PubMed] [Google Scholar]
- 26.Hui JM, Hodge A, Farrell GC, et al. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46–54. doi: 10.1002/hep.20280. [DOI] [PubMed] [Google Scholar]
- 27.Thamer C, Machann J, Tschritter O, et al. Relationship between serum adiponectin concentration and intramyocellular lipid stores in humans. Horm Metab Res. 2002;34:646–649. doi: 10.1055/s-2002-38260. [DOI] [PubMed] [Google Scholar]
- 28.Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14:447–455. doi: 10.1016/s1359-6101(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 29.Cook JR, Semple RK. Hypoadiponectinemia–cause or consequence of human “insulin resistance”? J Clin Endocrinol Metab. 2010;95:1544–1554. doi: 10.1210/jc.2009-2286. [DOI] [PubMed] [Google Scholar]
- 30.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 32.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 33.Hanley AJ, Bowden D, Wagenknecht LE, et al. Associations of adiponectin with body fat distribution and insulin sensitivity in nondiabetic Hispanics and African-Americans. J Clin Endocrinol Metab. 2007;92:2665–2671. doi: 10.1210/jc.2006-2614. [DOI] [PubMed] [Google Scholar]
- 34.Frederiksen L, Nielsen TL, Wraae K, et al. Subcutaneous rather than visceral adipose tissue is associated with adiponectin levels and insulin resistance in young men. J Clin Endocrinol Metab. 2009;94:4010–4015. doi: 10.1210/jc.2009-0980. [DOI] [PubMed] [Google Scholar]
- 35.Hara K, Horikoshi M, Yamauchi T, et al. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–1362. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J. 2008;409:623–633. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]
- 37.Blüher M, Brennan AM, Kelesidis T, et al. Total and high-molecular weight adiponectin in relation to metabolic variables at baseline and in response to an exercise treatment program: comparative evaluation of three assays. Diabetes Care. 2007;30:280–285. doi: 10.2337/dc06-1362. [DOI] [PubMed] [Google Scholar]


