Abstract
Head and neck squamous cell carcinomas (HNSCC) are common human malignancies with poor clinical outcomes. The 5-year survival rates for patients with advanced stage HNSCC have not changed appreciably in the past few decades, underscoring a dire need for improved therapeutic options. Recent studies have elucidated a key signaling axis, the EGFR-STAT3-Bcl-XL signaling axis, that is aberrantly activated in a majority of HNSCC and contributes to the proliferation and survival of malignant cells. Considerable effort is being placed on developing highly specific inhibitors of different components of this pathway. This review highlights the progress that is being made towards achieving potent inhibition of the EGFR-STAT3-Bcl-XL signaling axis in HNSCC and the promising therapeutic strategies that are currently under development for this disease.
Keywords: EGFR, cetuximab, STAT3, STAT3 decoy, Bcl-XL, ABT-737
The term “head and neck cancer” refers to malignancies that originate on the mucosal surfaces of the oral cavity, pharynx, and larynx. Worldwide, head and neck cancer represents the sixth most common cancer and is associated with considerable morbidity and mortality[1]. Histological examination has revealed that more than 90% of head and neck cancers exhibit squamous cell characteristics. Thus, head and neck cancers are commonly labeled as head and neck squamous cell carcinomas (HNSCC; also SCCHN).
Three key risk factors for HNSCC have been identified: tobacco consumption, alcohol consumption, and human papilloma virus (HPV) infection. Roughly three quarters of all HNSCC cases can be attributed to tobacco or alcohol use[2]–[4]. Although this represents a particularly high percentage of HNSCC cases, it is noteworthy that approximately 25% of cases do not coincide with a history of tobacco or alcohol usage. HPV infection is most closely associated with HNSCCs arising in the oropharynx[4]–[7]. Although HPV infection rates may vary by country or region, the prevalence of HPV infection in oropharyngeal HNSCC may exceed 50% in some areas[8]. Furthermore, while the overall prevalence of HNSCC is modestly declining in some countries, the incidence of HPV-positive HNSCC is increasing in certain countries, including the United States[9],[10]. The increase in HPV-positive HNSCC is particularly prevalent among young patients[10]. Intriguingly, HPV-positive HNSCC patients typically have better prognosis than HPV-negative HNSCC patients[11]–[13]. This has led to the conclusion that HPV-positive HNSCC represents a distinct disease entity[14] and has raised hope that it may be possible to target unique features of HPV-positive disease to achieve even further improvements in outcomes. In this regard, epidemiologic evaluation of the impact of HPV vaccines on oropharyngeal HNSCC may become available in the not-too-distant future.
The major treatment options for HNSCC include surgery, radiation, and chemotherapy. The specific treatment options are largely dependent on the stage and anatomical site of the tumor. Roughly one-third of newly diagnosed HNSCC patients present with stage I or II disease that is locally contained. Tumors in these patients are typically removed by surgery or treated with radiation, and a majority of the patients are cured[15]. A little over half of newly diagnosed HNSCC patients present with locally advanced stage III or IV disease. Depending on the anatomic site of the tumor, primary treatment may involve surgery or a combination of chemotherapy and radiation. For high risk patients, surgery may be followed by intensive chemoradiation, and vice versa. Unfortunately, the aggressive treatment strategies necessitated for advanced stage HNSCC are associated with considerable cytotoxicities and may result in deficits in speaking and swallowing, as well as cosmetic disfigurement. Moreover, approximately 50% of patients who are treated for locally advanced HNSCC will suffer recurrence within 2 years[16]. The most serious forms of HNSCC are metastatic or recurrent. The prognoses for these patients are poor (median survival of 6–10 months), and their therapies are largely aimed at prolonging survival and reducing symptoms[17]. Treatment options for metastatic or recurrent HNSCC again include surgery, radiation, and/or chemotherapy, but expectation of cure is very limited.
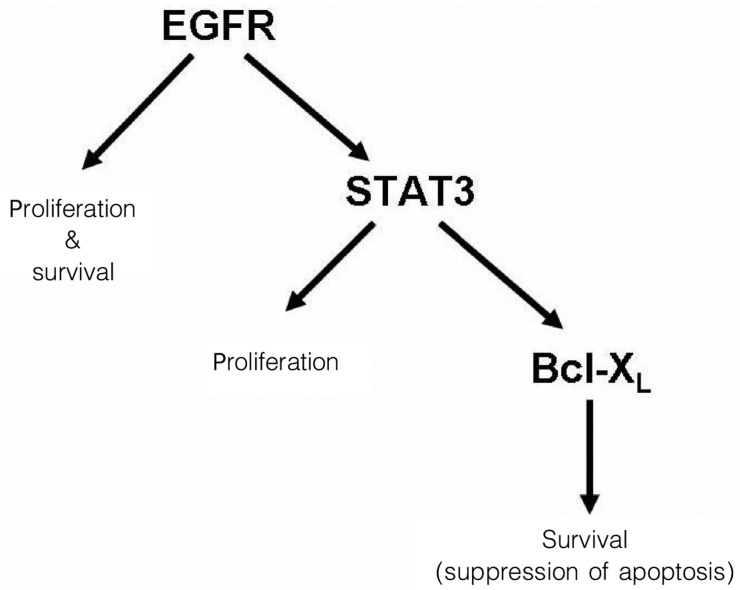
Over the past several decades, substantial improvements have been made in surgical techniques for HNSCC, as well as techniques for the administration of radiation and chemotherapy. These improvements have led to minimization of cosmetic disfigurement and preservation of normal tissue architecture and function. However, despite these advances, it is striking that the 5-year survival rates for patients with advanced stage HNSCC have not changed substantially for the past 4 decades, remaining around 50%[4],[17]. This dramatic and grim fact underscores the dire need to develop new therapeutic approaches and reagents that can be used to achieve selective and potent elimination of HNSCC tumors. This review describes the impact of dysregulated signaling via the EGFR-STAT3-Bcl-XL signaling axis (Figure 1) on HNSCC tumor growth and presents promising new approaches that are being developed and evaluated for targeting this critical pathway.
Figure 1. EGFR-STAT3-Bcl-XL, a key proliferation and survival signaling axis in head and neck squamous cell carcinomas.
EGFR Biochemistry and Involvement in HNSCC
The epidermal growth factor receptor (EGFR) has been studied intensively since the early 1980s and has been found to play an important role in a variety of human malignancies. EGFR is a 170 kDa protein that spans the plasma membrane once. The extracellular N-terminal region of EGFR contains the ligand-binding domain and is also glycosylated. The intracellular C-terminal region contains the tyrosine kinase domain. EGFR (also called ErbB1 or HER1) is a member of the HER family of receptor tyrosine kinases, which consists of four receptors, HER1-4[18],[19]. Several different ligands have been shown to bind to EGFR, including epidermal growth factor (EGF), transforming growth factor-α (TGF-α), and amphiregulin. Ligand binding induces homodimerization or heterodimerization of the receptor with other HER family members, which in turn leads to activation of the EGFR tyrosine kinase activity, followed by autophosphorylation of key tyrosine residues in the cytoplasmic region of the receptor. These phosphorylated residues then serve as docking sites for intracellular signaling molecules, leading to the activation of EGFR-mediated signaling pathways. Prominent among the signaling pathways that become activated are the RAS/RA F/MEK/ERK, PI3K/AKT, phospholipase C gamma (PLCγ), and STAT3 signaling pathways. Once activated, these pathways play important roles in mediating EGFR effects on cellular proliferation and survival.
Dysregulation of EGFR expression and signaling has been reported in a wide variety of solid malignancies. In HNSCC, EGFR is overexpressed in a majority of patient primary specimens[20]. Transcriptional mechanisms and gene amplification have been shown to lead to EGFR overexpression in HNSCC[21],[22]. Additionally, autocrine secretion of TGF-α by HNSCC tumors leads to constitutive activation of EGFR. Overexpression of TGF-α or EGFR correlates with short survival in patients with HNSCC[20],[23], with EGFR overexpression also correlating with resistance to radiation and chemotherapy in preclinical models[4]. Activating mutations of EGFR, such as those found in non-small cell lung cancer, are rare in HNSCC.
Expression of variant form of EGFR, called EGFRvIII, has been reported in 42% of primary HNSCC specimens[24], as well as in malignancies of the brain, lung, breast, prostate, and ovary[18]. EGFRvIII is a 145 kDa protein that lacks sequences encoded by exons 2–7 (amino acids 6–273), yet retains the N-terminal signal peptide and is inserted normally into the plasma membrane. The deleted sequences in EGFRvIII lie entirely within the extracellular ligand-binding region and severely curtail the binding of antibodies that recognize the extracellular region of wild-type (wt) EGFR. Importantly, the tyrosine kinase activity of EGFRvIII is constitutively activated in tumors. Thus, expression of EGFRvIII confers ligand-independent activation of receptor signaling.
Exogenous overexpression of either wt-EGFR or EGFRvIII results in cellular transformation. In view of the overexpression of these proteins in a large percentage of HNSCC tumors, numerous studies have sought to determine how endogenously overexpressed or hyperactivated EGFR or EGFRvIII contribute to HNSCC oncogenesis. These studies have used preclinical models to assess the impact of inhibiting either the expression or the function of the proteins and have relied on reagents including antisense oligonucleotides, dominant-negative mutants, and small-molecule tyrosine kinase inhibitors (TKIs). Results from these preclinical studies have determined that signaling via EGFR or EGFRvIII contributes to HNSCC cellular proliferation and patient survival, as well as tumor invasion, motility, adhesion, and angiogenesis[18],[25]. These findings, coupled with correlative studies linking EGFR with poor patient prognosis, have stimulated clinical and translational studies aimed at therapeutic targeting of EGFR in patients with HNSCC.
Therapeutic Targeting of EGFR in HNSCC
Two primary groups of agents are currently being used, or tested, for therapeutic targeting of EGFR: anti-EGFR monoclonal antibodies and small molecule tyrosine kinase inhibitors (TKIs). Four monoclonal antibodies are actively being investigated, namely cetuximab, panitumumab, zalutumumab, and nimotuzumab. Each of these antibodies recognizes and binds the extracellular region of EGFR. TKIs that have been tested in HNSCC, or are undergoing evaluation, include gefitinib, erlotinib, and lapatinib.
Cetuximab is an IgG1, chimeric human/mouse monoclonal antibody that binds with high affinity to EGFR. Cetuximab exhibits two distinct mechanisms of action. First, cetuximab competitively inhibits ligand binding to EGFR, thereby inhibiting activation of EGFR tyrosine kinase activity and subsequent signaling. Second, binding of cetuximab to EGFR-expressing cells induces antibody-dependent cellular cytotoxicity mediated by host immune cells. In 2006, results were reported from a landmark, randomized phase III trial of HNSCC patients with locally advanced disease that compared cetuximab plus radiation to radiation alone[26]. The addition of cetuximab resulted in significant improvement of locoregional control (24.4 months for the combination versus 14.9 months for radiation alone) and overall survival (49.0 months versus 29.3 months)[26]. A more recent update from this trial continues to demonstrate a benefit to cetuximab inclusion (5-year survival rate of 45.6% for the combination versus 36.4% for radiation alone)[27]. On the basis of this trial, the United States Food and Drug Administration approved the use of cetuximab in patients with locally advanced HNSCC. Thus, cetuximab is the first biological agent approved for use in HNSCC and represents the first major addition to the treatment armamentarium for this disease in several decades. In metastatic HNSCC, a recent phase III randomized trial (the EXTREME study) assessed the benefit of adding cetuximab to a chemotherapy regimen of 5-fluorouracil and either cisplatin or carboplatin[28],[29]. The addition of cetuximab enhanced median overall survival from 7.4 months for chemotherapy alone to 10.1 months, leading to FDA approval for cetuximab inclusion in this chemotherapy-based treatment. Although the use of cetuximab (as well as other EGFR monoclonal antibodies or TKIs) frequently results in development of skin rash, the overall quality of life for cetuximab-treated patients does not seem adversely affected. On the contrary, inclusion of cetuximab may actually improve larygeal function and reduce pain[30].
Although the use of cetuximab represents a significant advance in HNSCC therapy and additional applications of this therapy are likely, there are several limitations to its success. First, only a minority of patients respond to cetuximab (approximately 20% or less), and it is not possible to predict which patients will benefit. No clear correlations have been observed between EGFR expression levels and response to cetuximab (or other EGFR-targeting agents). Thus, markers are needed that will allow prediction of response. Second, in the phase III trial that led to approval of cetuximab plus radiation in locally advanced disease, the addition of cetuximab ultimately did not prevent recurrence or metastasis[26]. Third, cetuximab and other EGFR-blocking antibodies fail to prevent signaling emanating from the constitutively active EGFRvIII variant, whose expression is relatively prevalent in HNSCC.
Panitumumab is a fully humanized IgG2 antibody directed against the EGFR extracellular region. This monoclonal antibody is currently being evaluated in phase III trials of locally advanced and recurrent/metastatic HNSCC[4]. Since panitumumab is fully humanized, it may be less prone to stimulate host generation of neutralizing antibodies or hypersensitive reactions. Nimotuzumab is a chimeric human/mouse IgG1 monoclonal antibody, whereas zalutumumab is a fully humanized IgG1 monoclonal antibody; both are being evaluated in advanced clinical trials outside the United States. Nimotuzumab may offer the advantage of provoking less severe skin rashes, whereas zalutumumab, being IgG1, may be particularly potent at stimulating antibody-dependent cellular cytotoxicity[31].
Monoclonal antibodies directed against EGFR are advantageous due to their high specificity for their target. However, except for an antibody (mAb 806) currently being evaluated in a different malignancy[32],[33], the antibodies described above fail to substantially inhibit signaling derived from constitutively active EGFRvIII. This suggests that small-molecule TKIs capable of inhibiting both wt-EGFR and EGFRvIII may be of value in treating HNSCC. In addition, TKIs offer the advantage of being orally bioavailable. On the other hand, a significant downside of the TKIs is a lack of specificity. In addition to inhibiting EGFR, or HER family members, the TKIs described below (gefitinib, erlotinib, lapatinib) exhibit some level of cross-inhibition of other receptor or nonreceptor tyrosine kinases.
Gefitinib and erlotinib exhibit some specificity for EGFR and are FDA-approved for use in non-small cell lung cancer. Moreover, specific activating mutants of EGFR that are found more frequently in lung cancer than in HNSCC have been shown to exhibit heightened sensitivity to gefitinib and erlotinib, underscoring the use of these compounds in lung cancer. In HNSCC, a phase III trial failed to demonstrate a benefit of adding gefitinib to methotrexate in refractory disease[34]. A phase III trial combining gefitinib and docetaxel also yielded disappointing results[35]. The future of gefitinib as a therapeutic agent in HNSCC remains unclear. In contrast, phase I/II testing of erlotinib alone or in combination with chemotherapy in metastatic, recurrent, or refractory HNSCC has suggested a potential therapeutic benefit for this TKI[4]. Phase II studies have also indicated a benefit of adding the TKI lapatinib to chemoradiation regimens. Lapatinib is unique in that it inhibits both EGFR and HER2[4],[36]. Since HER2 is commonly overexpressed in HNSCC and forms a functional heterodimer with EGFR[37]–[40], application of this or similar TKIs may have added value in HNSCC treatment.
STAT3 Activation and Importance in HNSCC
Activation of EGFR leads to the activation of several intracellular signaling pathways, including the RAS/RAF/MEK/ERK, PI3K/AKT, PLCγ, and STAT3 pathways[18]. The STAT3 pathway plays a particularly important role in mediating cellular proliferation and apoptosis suppression in HNSCC. Activation of EGFR results in phosphorylation of several tyrosine residues in the cytoplasmic domain of the receptor protein. The STAT3 protein recognizes and binds phosphorylated tyrosines 1068 and 1086, promoting direct interaction between STAT3 and EGFR[41],[42]. The recruitment of STAT3 to the activated EGFR leads to receptor-mediated phosphorylation of STAT3 on tyrosine 705, followed by head-to-tail dimerization of the STAT3 protein. STAT3 dimerization involves recognition of phospho-Tyr705 on one STAT3 protein by the SH2 domain on the other binding partner. Once dimerized, the STAT3 proteins translocate to the nucleus where they recognize and bind STAT3 response elements in the promoters of target genes, inducing transcription[43]. In normal cells, STAT3 acts to sustain cellular survival or promote growth or differentiation[44]. However, Darnell et al.[45] have shown that a constitutively active form of STAT3 has the capacity to transform normal fibroblasts, promoting tumorigenesis. Thus, STAT3 has also been classified as an oncogene.
STAT3 was first reported to be constitutively activated in HNSCC by Grandis et al.[46],[47], who demonstrated activation downstream of autocrine TGF-α/EGFR signaling. In addition, STAT3 activation in HNSCC can occur independently of EGFR. In a subset of patients, binding of interleukin-6 (IL-6) to glycoprotein 130 (gp130) receptor leads to activation of Janus kinase 2 (JAK2), followed by JAK2-mediated phosphorylation/activation of STAT3[48]. Moreover, Src family kinases, which are frequently activated in HNSCC, can promote EGFR-independent STAT3 activation[49]. Mutation of STAT3, leading to constitutive activation, has not been reported in HNSCC.
Recent studies examining primary patient specimens have confirmed the constitutive activation and/or overexpression of STAT3 in a majority of HNSCC patients. These studies have relied extensively on immunohistochemistry of tissue sections or protein microarrays using antibodies that are specific for STAT3 that is phosphorylated on Tyr705. Importantly, cumulative findings demonstrate that high levels of activated STAT3 correlate with advanced tumor stage and poor patient prognosis[50]–[53]. STAT3 levels are highest in poorly differentiated HNSCC tumors[54]. Moreover, STAT3 is present at higher levels in normal adjacent epithelia from HNSCC patients compared with those from individuals without cancer[47]. This suggests that activation/overexpression of STAT3 represents an early event in HNSCC progression.
Molecular Targeting of STAT3
The frequent hyperactivation/overexpression of STAT3 in HNSCC has stimulated research to understand the role of STAT3 signaling in this disease. In vitro studies using HNSCC cell lines and in vivo preclinical studies involving HNSCC xenograft tumors in mice have assessed the impact of suppressing STAT3 expression or inhibiting STAT3 function. These studies have relied on a variety of reagents, including dominant-negative STAT3, antisense and small interfering RNA nucleotides, as well as duplex decoy oligonucleotides resembling STAT3 response elements. Collectively, the application of these reagents has shown that inhibition of STAT3 expression or function in HNSCC cells and tumors acts to inhibit cellular proliferation and invasion, promote apoptosis, and slow the growth of tumors in vivo[46],[47],[55]–[58]. STAT3 down-regulation or inhibition also sensitizes HNSCC to conventional chemotherapeutic drugs such as cisplatin in vitro and in vivo[55],[59].
The potential for achieving useful targeting of STAT3 in HNSCC patients depends, in part, on the cytotoxicity of inhibiting STAT3 in normal cells. Genetic deletion of STAT3 in mice results in embryonic lethality, indicating an essential, nonredundant role for STAT3 during embryogenesis[60]. In contrast, several studies have shown that loss of STAT3 does not affect the growth and survival of normal cells and adult tissues[61]–[65]. These findings raise hope that tumor-specific effects of STAT3 targeting can be achieved in patients without concurrent adverse cytotoxicities to normal tissues.
Because STAT3 is now a well-validated target in a wide variety of human malignancies, including HNSCC, there has been considerable effort to identify small-molecule STAT3 inhibitors with therapeutic potential. In early studies, phosphotyrosyl peptides and related peptidomimetics were developed that bound to the SH2 domain in STAT3 and inhibited function and biological activity[66],[67]. Subsequently, virtual and high-throughput screening methods have been used to identify several small organic molecules that disrupt STAT3 function, including S3I-201, STA-21, and Stattic[68]–[70]. In addition, several naturally occurring compounds have been identified that inhibit STAT3 phosphorylation/activation in vitro. These compounds often act by inhibiting upstream kinases, such as JAK2, and include guggulsterone[71],[72], galiellalactone[73], capsaicin[74], cucurbitacin I[75], curcumin[76], and ursolic acid[77]. Although many of these compounds are effective in inhibiting STAT3 activation in whole cells, most also hit other targets in the cell. Efforts to derive a highly specific inhibitor of STAT3 have led to the development of a STAT3 decoy oligonucleotide[55]. The STAT3 decoy is a 15-bp duplex oligonucleotide that is based on the sequence of the STAT3 regulatory element in the promoter of the c-fos gene. STAT3 decoy binds with high affinity to STAT3 protein, inhibits the expression of STAT3 target genes, promotes apoptosis, and inhibits tumor growth in vivo[55],[59]. A clinical trial involving intratumoral injection of STAT3 decoy in HNSCC patients is currently underway.
Targeting Bcl-XL in HNSCC
The oncogenic properties of STAT3 in HNSCC are undoubtedly mediated by the products of STAT3 target genes. Activated STAT3 induces the expression of a broad array of proteins that regulate cell cycle progression, suppression of apoptosis, metastasis, and angiogenesis. Additionally, STAT3 induces expression of suppressor of cytokine signaling-3 (SOCS-3), a negative regulator of STAT3 activation[65],[78]. A key characteristic of advanced stage HNSCC is chemotherapy resistance, which results, in part, from aberrant overexpression of Bcl-XL, the product of a STAT3 target gene[79]. Bcl-XL is an anti-apoptotic member of the Bcl-2 protein family and acts to inhibit activation of the intrinsic apoptosis pathway. Endogenous or exogenous overexpression of Bcl-XL typically confers resistance to chemotherapy-induced apoptosis. A majority of HNSCC primary patient specimens exhibit marked overexpression of Bcl-XL[80],[81]. Importantly, overexpression of Bcl-XL in HNSCC patients has been shown to correlate with resistance to chemotherapy and radiotherapy and with poor clinical prognosis[81]–[83].
To determine the role of Bcl-XL in HNSCC, several in vitro studies have assessed the impact of inhibiting Bcl-XL expression or function. Down-regulation of Bcl-XL protein levels using antisense oligonucleotides has been shown to sensitize HNSCC cells to chemotherapy[84]. In addition, short peptides that bind to Bcl-XL and Bcl-2 and inhibit the function of these proteins also promote apoptosis signaling and cell death in HNSCC cell lines[85],[86]. Moreover, the naturally occurring compound (-)-gossypol, which binds and inhibits Bcl-XL and Bcl-2, promotes apoptosis and sensitizes HNSCC cells to chemotherapy in vitro and inhibits the growth of HNSCC xenograft tumors in vivo[87]–[89]. Lastly, the highly selective Bcl-XL/Bcl-2 inhibitor ABT-737 was recently shown to potently synergize with conventional chemotherapeutic drugs in killing HNSCC cells[90],[91]. ABT-737 and the orally bioavailable derivative ABT-263[92] are currently undergoing testing in early clinical trials and may represent promising therapeutics in combination with chemotherapy for HNSCC.
Conclusions
There is an urgent need to develop effective therapeutic reagents and strategies that can be used to treat HNSCC, a malignancy with worldwide prevalence. Laboratory studies continue to elucidate the key signaling pathways that contribute to the transformed properties of HNSCC cells. Aberrant activation of the EGFR-STAT3-Bcl-XL signaling axis has been shown to play an important role in the progression of HNSCC. Molecular targeting of this pathway has demonstrated efficacy against HNSCC in preclinical models. Moreover, validation of EGFR as an important molecular target has been demonstrated in HNSCC patients using cetuximab antibody. The development and application of highly specific agents targeting STAT3 and Bcl-XL will likely lead to even further improvement in the outcomes of HNSCC patients in the future.
Acknowledgments
This work was supported by National Institutes of Health grants R01 CA137260 and P50 CA097190.
References
- 1.Jemal A, Siegel R, Ward E, et al. et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Hashibe M, Brennan P, Benhamou S, et al. et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 3.Hashibe M, Brennan P, Chuang SC, et al. et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:541–550. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fung C, Grandis JR. Emerging drugs to treat squamous cell carcinomas of the head and neck. Expert Opin Emerg Drugs. 2010;15:355–373. doi: 10.1517/14728214.2010.497754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mork J, Lie AK, Glattre E, et al. et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–1131. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 6.Herrero R, Castellsagué X, Pawlita M, et al. et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 7.Gillison ML, Koch WM, Capone RB, et al. et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 8.Hammarstedt L, Lindquist D, Dahlstrand H, et al. et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–2623. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi AK, Engels EA, Anderson WF, et al. et al. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 10.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103:1843–1849. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 11.Gillison ML, D'Souza G, Westra W, et al. et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 12.Fakhry C, Westra WH, Li S, et al. et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 13.Licitra L, Perrone F, Bossi P, et al. et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24:5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 14.Gillison ML. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin Oncol. 2004;31:744–754. doi: 10.1053/j.seminoncol.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–1154. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 16.Argiris A, Karamouzis MV, Raben D, et al. et al. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson MK, Forastiere AA. Reassessment of the role of induction chemotherapy for head and neck cancer. Lancet Oncol. 2006;7:565–574. doi: 10.1016/S1470-2045(06)70757-4. [DOI] [PubMed] [Google Scholar]
- 18.Grandis JR, Sok JC. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol Ther. 2004;102:37–46. doi: 10.1016/j.pharmthera.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77:400–410. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- 20.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579–3584. [PubMed] [Google Scholar]
- 21.Rubin Grandis J, Zeng Q, Tweardy DJ. Retinoic acid normalizes the increased gene transcription rate of TGF-alpha and EGFR in head and neck cancer cell lines. Nat Med. 1996;2:237–240. doi: 10.1038/nm0296-237. [DOI] [PubMed] [Google Scholar]
- 22.Sheu JJ, Hua CH, Wan L, et al. et al. Functional genomic analysis identified epidermal growth factor receptor activation as the most common genetic event in oral squamous cell carcinoma. Cancer Res. 2009;69:2568–2576. doi: 10.1158/0008-5472.CAN-08-3199. [DOI] [PubMed] [Google Scholar]
- 23.Rubin Grandis J, Melhem MF, Gooding WE, et al. et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 24.Sok JC, Coppelli FM, Thomas SM, et al. et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. 2006;12:5064–5073. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 25.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 26.Bonner JA, Harari PM, Giralt J, et al. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 27.Bonner JA, Harari PM, Giralt J, et al. et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 28.Vermorken JB, Mesia R, Rivera F, et al. et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 29.Rivera F, Garcia-Castano A, Vega N, et al. et al. Cetuximab in metastatic or recurrent head and neck cancer: the EXTREME trial. Expert Rev Anticancer Ther. 2009;9:1421–1428. doi: 10.1586/era.09.113. [DOI] [PubMed] [Google Scholar]
- 30.Mesia R, Rivera F, Kawecki A, et al. et al. Quality of life of patients receiving platinum-based chemotherapy plus cetuximab first line for recurrent and/or metastatic squamous cell carcinoma of the head and neck. Ann Oncol. 2010;21:1967–1973. doi: 10.1093/annonc/mdq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivera F, Vega-Villegas ME, Lopez-Brea MF, et al. et al. Current situation of Panitumumab, Matuzumab, Nimotuzumab and Zalutumumab. Acta Oncol. 2008;47:9–19. doi: 10.1080/02841860701704724. [DOI] [PubMed] [Google Scholar]
- 32.Jungbluth AA, Stockert E, Huang HJ, et al. et al. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc Natl Acad Sci U S A. 2003;100:639–644. doi: 10.1073/pnas.232686499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott AM, Lee FT, Tebbutt N, et al. et al. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc Natl Acad Sci U S A. 2007;104:4071–4076. doi: 10.1073/pnas.0611693104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart JS, Cohen EE, Licitra L, et al. et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1864–1871. doi: 10.1200/JCO.2008.17.0530. [DOI] [PubMed] [Google Scholar]
- 35.Argiris A, Ghebremichael M, Gilbert J, et al. et al. A phase III randomized, placebo-controlled trial of docetaxel (D) with or without gefitinib (G) in recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): a trial of the Eastern Cooperative Oncology Group (ECOG) J Clin Oncol. 2009;27:5s. abstract 6011. doi: 10.1200/JCO.2012.45.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hegymegi-Barakonyi B, Eros D, Szántai-Kis C, et al. et al. Tyrosine kinase inhibitors—small molecular weight compounds inhibiting EGFR. Curr Opin Mol Ther. 2009;11:308–321. [PubMed] [Google Scholar]
- 37.Xia W, Lau YK, Zhang HZ, et al. et al. Strong correlation between c-erbB-2 overexpression and overall survival of patients with oral squamous cell carcinoma. Clin Cancer Res. 1997;3:3–9. [PubMed] [Google Scholar]
- 38.Xia W, Lau YK, Zhang HZ, et al. et al. Combination of EGFR, HER-2/neu, and HER-3 is a stronger predictor for the outcome of oral squamous cell carcinoma than any individual family members. Clin Cancer Res. 1999;5:4164–4174. [PubMed] [Google Scholar]
- 39.Ekberg T, Nestor M, Engstrom M, et al. et al. Expression of EGFR, HER2, HER3, and HER4 in metastatic squamous cell carcinomas of the oral cavity and base of tongue. Int J Oncol. 2005;26:1177–1185. doi: 10.3892/ijo.26.5.1177. [DOI] [PubMed] [Google Scholar]
- 40.Cavalot A, Martone T, Roggero N, et al. et al. Prognostic impact of HER-2/neu expression on squamous head and neck carcinomas. Head Neck. 2007;29:655–664. doi: 10.1002/hed.20574. [DOI] [PubMed] [Google Scholar]
- 41.Shao H, Cheng HY, Cook RG, et al. et al. Identification and characterization of signal transducer and activator of transcription 3 recruitment sites within the epidermal growth factor receptor. Cancer Res. 2003;63:3923–3930. [PubMed] [Google Scholar]
- 42.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- 43.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 44.Bromberg J, Darnell JE., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- 45.Bromberg JF, Wrzeszczynska MH, Devgan G, et al. et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 46.Grandis JR, Drenning SD, Chakraborty A, et al. et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor-mediated cell growth in vitro. J Clin Invest. 1998;102:1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grandis JR, Drenning SD, Zeng Q, et al. et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci U S A. 2000;97:4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sriuranpong V, Park JI, Amornphimoltham P, et al. et al. Epidermal growth factor receptor-independent constitutive activation of STAT3 in head and neck squamous cell carcinoma is mediated by the autocrine/paracrine stimulation of the interleukin 6/gp130 cytokine system. Cancer Res. 2003;63:2948–2956. [PubMed] [Google Scholar]
- 49.Xi S, Zhang Q, Dyer KF, et al. et al. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J Biol Chem. 2003;278:31574–31583. doi: 10.1074/jbc.M303499200. [DOI] [PubMed] [Google Scholar]
- 50.Masuda M, Suzui M, Yasumatu R, et al. et al. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–3355. [PubMed] [Google Scholar]
- 51.Nagpal JK, Mishra R, Das BR. Activation of Stat-3 as one of the early events in tobacco chewing-mediated oral carcinogenesis. Cancer. 2002;94:2393–2400. doi: 10.1002/cncr.10499. [DOI] [PubMed] [Google Scholar]
- 52.Shah NG, Trivedi TI, Tankshali RA, et al. et al. Stat3 expression in oral squamous cell carcinoma: association with clinicopatho-logical parameters and survival. Int J Biol Markers. 2006;21:175–183. doi: 10.1177/172460080602100307. [DOI] [PubMed] [Google Scholar]
- 53.Weber A, Hengge UR, Stricker I, et al. et al. Protein microarrays for the detection of biomarkers in head and neck squamous cell carcinomas. Hum Pathol. 2007;38:228–238. doi: 10.1016/j.humpath.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 54.Arany I, Chen SH, Megyesi JK, et al. et al. Differentiation-dependent expression of signal transducers and activators of transcription (STATs) might modify responses to growth factors in the cancers of the head and neck. Cancer Lett. 2003;199:83–89. doi: 10.1016/s0304-3835(03)00345-8. [DOI] [PubMed] [Google Scholar]
- 55.Leong PL, Andrews GA, Johnson DE, et al. et al. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc Natl Acad Sci U S A. 2003;100:4138–4143. doi: 10.1073/pnas.0534764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao LF, Xu DQ, Wen LJ, et al. et al. Inhibition of STAT3 expression by siRNA suppresses growth and induces apoptosis in laryngeal cancer cells. Acta Pharmacol Sin. 2005;26:377–383. doi: 10.1111/j.1745-7254.2005.00053.x. [DOI] [PubMed] [Google Scholar]
- 57.Gao LF, Wen LJ, Yu H, et al. et al. Knockdown of Stat3 expression using RNAi inhibits growth of laryngeal tumors in vivo. Acta Pharmacol Sin. 2006;27:347–352. doi: 10.1111/j.1745-7254.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 58.Masuda M, Wakasaki T, Suzui M, et al. et al. Stat3 orchestrates tumor development and progression: the Achilles' heel of head and neck cancers? Curr Cancer Drug Targets. 2010;10:117–126. doi: 10.2174/156800910790980197. [DOI] [PubMed] [Google Scholar]
- 59.Xi S, Gooding WE, Grandis JR. In vivo antitumor efficacy of STAT3 blockade using a transcription factor decoy approach: implications for cancer therapy. Oncogene. 2005;24:970–979. doi: 10.1038/sj.onc.1208316. [DOI] [PubMed] [Google Scholar]
- 60.Takeda K, Noguchi K, Shi W, et al. et al. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci U S A. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niu G, Heller R, Catlett-Falcone R, et al. et al. Gene therapy with dominant-negative Stat3 suppresses growth of the murine melanoma B16 tumor in vivo. Cancer Res. 1999;59:5059–5063. [PubMed] [Google Scholar]
- 62.Sano S, Itami S, Takeda K, et al. et al. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J. 1999;18:4657–4668. doi: 10.1093/emboj/18.17.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takeda K, Akira S. Multi-functional roles of Stat3 revealed by conditional gene targeting. Arch Immunol Ther Exp (Warsz) 2001;49:279–83. [PubMed] [Google Scholar]
- 64.Schlessinger K, Levy DE. Malignant transformation but not normal cell growth depends on signal transducer and activator of transcription 3. Cancer Res. 2005;65:5828–5834. doi: 10.1158/0008-5472.CAN-05-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frank DA. STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett. 2007;251:199–210. doi: 10.1016/j.canlet.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 66.Turkson J, Ryan D, Kim JS, et al. et al. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J Biol Chem. 2001;276:45443–45455. doi: 10.1074/jbc.M107527200. [DOI] [PubMed] [Google Scholar]
- 67.Turkson J, Kim JS, Zhang S, et al. et al. Novel peptidomimetic inhibitors of signal transducer and activator of transcription 3 dimerization and biological activity. Mol Cancer Ther. 2004;3:261–269. [PubMed] [Google Scholar]
- 68.Siddiquee K, Zhang S, Guida WC, et al. et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci U S A. 2007;104:7391–7396. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song H, Wang R, Wang S, et al. et al. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci U S A. 2005;102:4700–4705. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schust J, Sperl B, Hollis A, et al. et al. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13:1235–1242. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 71.Kim ES, Hong SY, Lee HK, et al. et al. Guggulsterone inhibits angiogenesis by blocking STAT3 and VEGF expression in colon cancer cells. Oncol Rep. 2008;20:1321–1327. [PubMed] [Google Scholar]
- 72.Ahn KS, Sethi G, Sung B, et al. et al. Guggulsterone, a farnesoid X receptor antagonist, inhibits constitutive and inducible STAT3 activation through induction of a protein tyrosine phosphatase SHP-1. Cancer Res. 2008;68:4406–4415. doi: 10.1158/0008-5472.CAN-07-6696. [DOI] [PubMed] [Google Scholar]
- 73.Hellsten R, Johansson M, Dahlman A, et al. et al. Galiellalactone is a novel therapeutic candidate against hormone-refractory prostate cancer expressing activated Stat3. Prostate. 2008;68:269–280. doi: 10.1002/pros.20699. [DOI] [PubMed] [Google Scholar]
- 74.Bhutani M, Pathak AK, Nair AS, et al. et al. Capsaicin is a novel blocker of constitutive and interleukin-6-inducible STAT3 activation. Clin Cancer Res. 2007;13:3024–3032. doi: 10.1158/1078-0432.CCR-06-2575. [DOI] [PubMed] [Google Scholar]
- 75.Blaskovich MA, Sun J, Cantor A, et al. et al. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- 76.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloyl-methane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 77.Pathak AK, Bhutani M, Nair AS, et al. et al. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol Cancer Res. 2007;5:943–955. doi: 10.1158/1541-7786.MCR-06-0348. [DOI] [PubMed] [Google Scholar]
- 78.Leeman RJ, Lui VW, Grandis JR. STAT3 as a therapeutic target in head and neck cancer. Expert Opin Biol Ther. 2006;6:231–241. doi: 10.1517/14712598.6.3.231. [DOI] [PubMed] [Google Scholar]
- 79.Catlett-Falcone R, Landowski TH, Oshiro MM, et al. et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 80.Drenning SD, Marcovitch AJ, Johnson DE, et al. et al. Bcl-2 but not Bax expression is associated with apoptosis in normal and transformed squamous epithelium. Clin Cancer Res. 1998;4:2913–2921. [PubMed] [Google Scholar]
- 81.Trask DK, Wolf GT, Bradford CR, et al. et al. Expression of Bcl-2 family proteins in advanced laryngeal squamous cell carcinoma: correlation with response to chemotherapy and organ preservation. Laryngoscope. 2002;112:638–644. doi: 10.1097/00005537-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 82.Pena JC, Thompson CB, Recant W, et al. et al. Bcl-xL and Bcl-2 expression in squamous cell carcinoma of the head and neck. Cancer. 1999;85:164–170. doi: 10.1002/(sici)1097-0142(19990101)85:1<164::aid-cncr23>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 83.Homma A, Furuta Y, Oridate N, et al. et al. Prognostic significance of clinical parameters and biological markers in patients with squamous cell carcinoma of the head and neck treated with concurrent chemoradiotherapy. Clin Cancer Res. 1995;5:801–806. [PubMed] [Google Scholar]
- 84.Sharma H, Sen S, Lo Muzio L, et al. et al. Antisense-mediated downregulation of anti-apoptotic proteins induces apoptosis and sensitizes head and neck squamous cell carcinoma cells to chemotherapy. Cancer Biol Ther. 2005;4:720–727. doi: 10.4161/cbt.4.7.1783. [DOI] [PubMed] [Google Scholar]
- 85.Shangary S, Johnson DE. Peptides derived from BH3 domains of Bcl-2 family members: a comparative analysis of inhibition of Bcl-2, Bcl-x(L) and Bax oligomerization, induction of cytochrome c release, and activation of cell death. Biochemistry. 2002;41:9485–9495. doi: 10.1021/bi025605h. [DOI] [PubMed] [Google Scholar]
- 86.Li R, Boehm AL, Miranda MB, et al. et al. Targeting antiapoptotic Bcl-2 family members with cell-permeable BH3 peptides induces apoptosis signaling and death in head and neck squamous cell carcinoma cells. Neoplasia. 2007;9:801–811. doi: 10.1593/neo.07394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bauer JA, Trask DK, Kumar B, et al. et al. Reversal of cisplatin resistance with a BH3 mimetic, (-)-gossypol, in head and neck cancer cells: role of wild-type p53 and Bcl-xL. Mol Cancer Ther. 2005;4:1096–1104. doi: 10.1158/1535-7163.MCT-05-0081. [DOI] [PubMed] [Google Scholar]
- 88.Oliver CL, Bauer JA, Wolter KG, et al. et al. In vitro effects of the BH3 mimetic, (-)-gossypol, on head and neck squamous cell carcinoma cells. Clin Cancer Res. 2004;10:7757–7763. doi: 10.1158/1078-0432.CCR-04-0551. [DOI] [PubMed] [Google Scholar]
- 89.Wolter KG, Wang SJ, Henson BS, et al. et al. (-)-gossypol inhibits growth and promotes apoptosis of human head and neck squamous cell carcinoma in vivo. Neoplasia. 2006;8:163–172. doi: 10.1593/neo.05691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oltersdorf T, Elmore SW, Shoemaker AR, et al. et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 91.Li R, Zang Y, Li C, et al. et al. ABT-737 synergizes with chemotherapy to kill head and neck squamous cell carcinoma cells via a Noxa-mediated pathway. Mol Pharmacol. 2009;75:1231–1239. doi: 10.1124/mol.108.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tse C, Shoemaker AR, Adickes J, et al. et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]