We discuss in this review recent developments in our understanding of the role of the autonomic nervous system in creating atrial fibrillation (AF) substrate and on how these findings relate to rapidly evolving therapeutic strategies (e.g. ablation, surgery) to disrupt autonomic signaling in atrial fibrillation.
AF is the most common sustained arrhythmia disturbance and is associated with significant morbidity and mortality. The morbidity and mortality associated with AF are especially increased in the setting of congestive heart failure, with up to half of all patients with heart failure having concomitant AF1.
Several mechanisms contribute to the electrophysiological and structural substrate for AF, including fibrosis, stretch, oxidative stress and altered calcium (Ca2+) handling characteristics2. In addition, neurohumoral factors have been invoked for their possible contribution to the creation of AF substrate. An important neurohumoral factor that has been studied fairly extensively for its involvement in AF is the autonomic nervous system3. Both the sympathetic and parasympathetic nervous system have been shown to play a role in the genesis of AF4, 5.
Over the last few years, the pulmonary veins (PVs) and posterior left atrium (PLA) have been shown to play a significant role in the genesis of AF. These regions have been shown to possess unique structural, molecular and electrophysiological characteristics, all of which appear to contribute to AF substrate. The autonomic characteristics of this region of the atrium have also been explored6, 7,8. Since the development of new ablative and surgical techniques over the last few years to treat AF, several investigators have also attempted to target the neural innervation of the atria and PVs at the time of ablation and/or surgery. These attempts have including attempts at generalized denervation of the atria as well as more targeted atrial denervation by using atrial electrograms - specifically complex fractionated atrial electrograms (CFAEs) that are frequently noted in the fibrillating atrium - to identify regions of high autonomic activity. An increased understanding of the role of the autonomic nervous system in AF has also been accompanied by attempts to better image the neural innervation of the atria, in order to better guide ablative strategies for AF.
In this review, we examine the contribution of both clinical and animal studies to our understanding of the role of the autonomic nervous system in AF. We specifically review the studies in the recent literature (i.e. over the last decade) that have: a) assessed the relative role of the vagal and sympathetic nervous system in the genesis and maintenance of AF, b) assessed the autonomic profile of focal AF i.e. AF arising from the PVs and PLA, c) explored the role of autonomic triggers in the creation of AF substrate in the setting of structural heart disease, specifically heart failure, d) assessed the contribution of the autonomic nervous system to the characteristics of AF electrograms e.g. CFAEs, e) assessed the feasibility of achieving autonomic denervation of the atria by means of ablation or surgery, e) examined new ways to image the autonomic innervation of the atria, especially in light of recently developed ablative strategies targeted at the neural innervation of the atria and f) explored new and novel gene-based therapies directed at the autonomic nervous system in AF.
Potential role of the Autonomic Nervous System in the Creation of Substrate for Atrial Fibrillation
Earlier studies suggested that exercise-induced AF may be sympathetically driven; in contrast, the parasympathetic nervous system may be contributing to AF in young patients with no structural heart disease9, 10. Sympathetic activation of the heart is thought to be pro-arrhythmic by increasing calcium (Ca2+) entry and the spontaneous release of Ca2+ from the sarcoplasmic reticulum.11, 12 Animal studies show that vagal stimulation contributes to the genesis of AF by non-uniform shortening of atrial effective refractory periods, thereby setting up substrate for reentry. Vagal stimulation can also lead to the emergence of focal triggers in the atrium13. More recently, both the parasympathetic and the sympathetic nervous system have been shown to play a role in AF. Amar et al showed that onset of AF was preceded by a primary increase in sympathetic drive followed by marked modulation toward vagal predominance14. Other studies also indicate that the onset of AF is associated an imbalance between these two arms of the autonomic nervous system.15–19 Studies in animal models using direct nerve recordings from the stellate ganglia, vagal nerve, as well as the intrinsic cardiac autonomic ganglia also demonstrate an interaction between the sympathetic and parasympathetic nervous system in creating paroxysmal atrial tachyarrhythmias, including AF20–23. These studies using direct nerve recordings reveal characteristic patterns sympatho-vagal discharge prior to the initiation of atrial tachyarrhythmias, both in dogs that underwent chronic rapid atrial pacing and in dogs subjected to heart failure by rapid ventricular pacing. Data from the same laboratory suggests that sympatho-vagal interactions may also be contributing to the development of sustained AF24. Sharifov et al14 showed that acetylcholine-induced AF was facilitated by isoproterenol, which decreased the concentration of acetylcholine required for AF induction and maintenance. The physiology studies by Patterson et al3 further indicate that sympathetic stimulation plays an important modulatory role in the emergence of focal drivers in the presence of an increased vagal tone. In their proposed model, Patterson et al, suggest that Ca2+ transient triggering can generate rapid discharges under conditions in which atrial repolarization is abbreviated by IKAch activation (e.g. by vagal stimulation) and the Ca2+ transient is augmented by β-adrenergic stimulation.
The above suggests that the autonomic nervous system is involved in the genesis of both AF triggers (i.e. ectopic foci that result from interaction between vagal and sympathetic stimulation) as well as the creation of a more established AF substrate that is needed for the maintenance of AF (and is enhanced in the setting of structural heart disease – see section below on Role of autonomic signaling in creating AF substrate in the setting of structural heart disease).
Autonomic profile of the PVs and PLA and its relationship to the genesis of AF
The discovery of the PVs as being an important contributor to AF has led to a renewed interest in understanding the detailed anatomy and physiology of the cardiovascular nervous system. PV ectopic foci appear to be at least partially modulated by autonomic signaling, with sympathetic stimulation with isoproterenol being frequently utilized to “bring out” these triggers in patients undergoing ablation for AF. Clinical studies have demonstrated a change in heart rate variability after PV ablation25. Several investigators have also noted Bezold-Jarisch-like or ‘vagal’ reflexes during radiofrequency ablation of the PVs. Indeed, Pappone et al3 have suggested that elimination of vagal reflexes during ablation may improve efficacy of AF ablation procedures. Vagal responsiveness also appears to decrease following ablation in the left atrium26. In fact, in some series27, adding ganglionated plexi (GP) ablation to PV isolation appears to increase ablation success for AF.
Anatomic studies of the autonomic innervation of the atria also indicate that the PVs and PLA have a unique autonomic profile. Armour and Randall several years ago demonstrated the presence of an intricate pattern of autonomic innervation in the heart with the atria being innervated by at least 5 major atrial fad pads3. More recently, Hou et al28, 29 have suggested the presence of an intricate, interconnecting neural network in the left atrium that may contribute to substrate for focal AF. In a recent human study, Chevalier et al described heterogeneity of nerve distribution in the region of the PVs and surrounding left atrium, demonstrating the presence of several gradients of innervation at discrete sites14.
In light of these prior studies, Arora et al compared the distribution and physiology of sympathetic and parasympathetic nerves among the PVs, the PLA and left atrial appendage in canine hearts.7 The PLA was the most richly innervated, with nerve bundles containing both parasympathetic and sympathetic fibers. Parasympathetic fibres predominated over sympathetic fibers within bundles. M2 receptor distribution was also most pronounced in the PLA. In a related study, Ulphani et al discovered a particularly high concentration of parasympathetic fibers in the ligament of Marshall30. The ligament of Marshall could be traced back to a major branch of the left cervical vagus nerve. Ablation of the ligament of Marshall led to an attenuation of vagal-induced ERP shortening in the left sided PVs and the PLA. The course of the ligament of Marshall along the posterior wall of the left atrium further highlights the potential importance of this region in the creation of autonomic substrate for AF. These canine studies are in agreement with human studies where Tan et al demonstrated co-localization of sympathetic and parasympathetic nerve fibers in the human left atrium3, 31. Another human study by Deneke et al32 not only demonstrates co-localization of sympathetic and parasympathetic nerves, but shows that patients with persistent AF had a shift toward a lower density of cholinergic nerves and a higher density of nerves containing adrenergic components.
A related functional study in a canine model suggests a differential electrophysiological response of the PVs and adjoining PLA from that in the rest of the left atrium in response to autonomic maneuvers6. In that physiologic study, there was a greater decrease in refractory periods in the PVs and PLA as compared to the rest of the left atrium in response to vagal stimulation. The heterogeneity of vagal responses in the left atrium in this study was found to correlate with the pattern of distribution of IKAch.
Taken together, the above studies indicate that the PVs and the adjoining PLA have a unique autonomic profile that differs from the rest of the atria, and likely contributes to the genesis of both focal triggers as well as sustained micro-reentry in this region. Indeed, even though it has been demonstrated that the normal PVs have marked heterogeneity of conduction and repolarization at baseline – with resulting substrate for reentry33- it has also been shown that microreentry within the PVs could be sustained only in the presence of isoproterenol or acetylcholine, indicating that sympathomimetic or cholinergic stimulation appear to be necessary to promote development of sustained focal activity in the PVs33.
Role of autonomic signaling in creating AF substrate in the setting of structural heart disease
Studies performed in the last few years suggest that the autonomic nervous system may also be playing a role in the genesis of AF in diseased hearts, which are known to have increased predisposition to persistent AF. Jayachandran et al3, 34 demonstrated a heterogeneous increase in sympathetic innervation in the atria in dogs subjected to rapid atrial pacing for prolonged periods of time. There is also evidence of sympathetic hyperinnervation in patients with persistent AF35. More recently, Ogawa et al,8 using direct nerve recordings from the stellate ganglia and vagal nerves, have shown increased sympathetic and vagal nerve discharge prior to the onset of atrial arrhythmias in dogs with pacing-induced HF. Indeed, the atrial tachyarrhythmias in this model were prevented by prophylactic ablation of the stellate ganglion and the T2–4 thoracic sympathetic ganglia22. In the same model of pacing induced HF, Ng et al recently demonstrated increased sympathetic as well as parasympathetic nerve growth in the left atrium1; nerve growth was most pronounced in the PVs and PLA (see figure 1). In this model, increase in sympathetic innervation was accompanied by an increase in β1-adrenergic innervation in the PVs and by an increase in sympathetic responsiveness in the PVs and PLA; this increase in sympathetic innervation is consistent with that previously noted in human AF35. The increase in parasympathetic innervation noted in the HF model of AF was paradoxically accompanied by a) no change in M2 binding, and b) a significant decrease in vagal induced ERP shortening in the left atrium. This decrease in vagal responsiveness was accounted for by an increase in acetylcholinesterase activity, with inhibition of acetylcholinesterase by physostigmine completely restoring vagal responsiveness in the left atrium. More importantly, despite this decrease in vagal responsiveness, parasympathetic tone was still an important contributor to the maintenance of AF; administration of atropine resulted in a significant decrease in the duration of induced AF, indicating the importance of parasympathetic remodeling in the creation of AF substrate. While double autonomic blockade did not result in a further decrease in AF duration, it did decrease AF dominant frequency, thus indicating the additional influences of sympathetic activity on AF characteristics.The sensitivity of activation patterns in the PVs and PLA to parasympathetic manipulation noted in this study suggests that vagal effects on conduction may be playing a role in creating substrate for AF in HF. Figure 2 shows a proposed model of how sympathetic and parasympathetic remodeling contribute to the creation of AF substrate in the setting of heart failure.
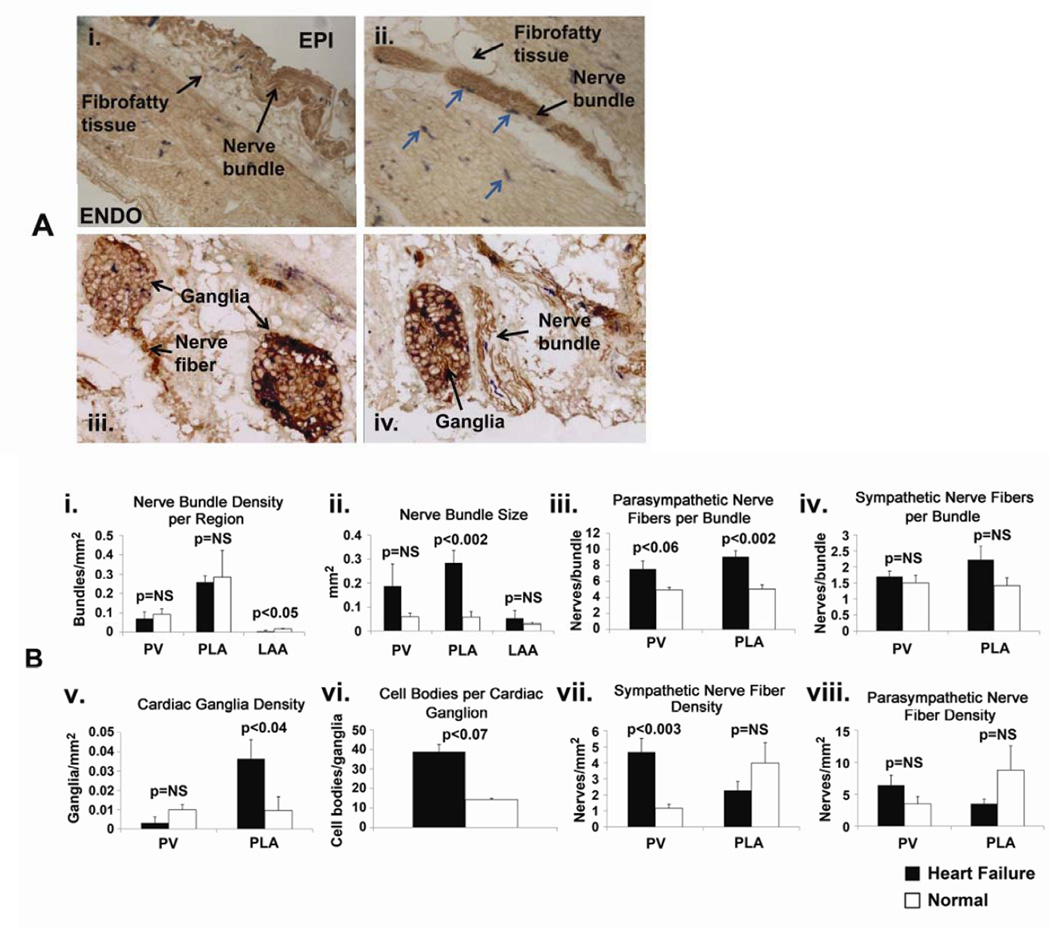
Figure 1.
Comparison of nerve density and distribution in HF vs normal atria. Panel A: Examples of sympathetic and parasympathetic nerve staining in HF atria. Sympathetic fibers were stained by dopamine beta-hydroxylase, while parasympathetic fibers were stained by acetylcholine esterase. A.i. – example of a nerve bundle located in the fibrofatty tissue overlying the epicardium (EPI) (10X). ENDO – endocardium. A.ii. – example of nerve bundles located in fibrofatty tissue on the epicardial aspect of PV (4X). Sympathetic fibers are in blue (arrows). A.iii. – examples of cardiac ganglia, with parasympathetic fibers arising from cardiac ganglion on the left side (20X). A.iv. - example of cardiac ganglia on the left and nerve bundle on the right; nerve fibers showing co-localized sympathetic (blue) and parasympathetic fibers (brown) (20X). Panel B: Quantitative analysis of nerve staining in HF vs normal atria. B.i. – nerve bundle density, B.ii. – nerve bundle size, B.iii. – number of parasympathetic nerve fibers/bundle, B.iv. – number of sympathetic nerve fibers/bundle, B.v. – density of cardiac ganglia, B.vi. – number of cell bodies/cardiac ganglion, B.vii. - density of sympathetic fibers, B.viii. – density of parasympathetic fibers.(modified from Ng et al, Autonomic Remodeling in the Posterior Left Atrium and Pulmonary Veins in Heart Failure – Creation of a Dynamic Substrate for Atrial Fibrillation. Circulation-Arrhythmia and Electrophysiology 2011;4(3):388–96).
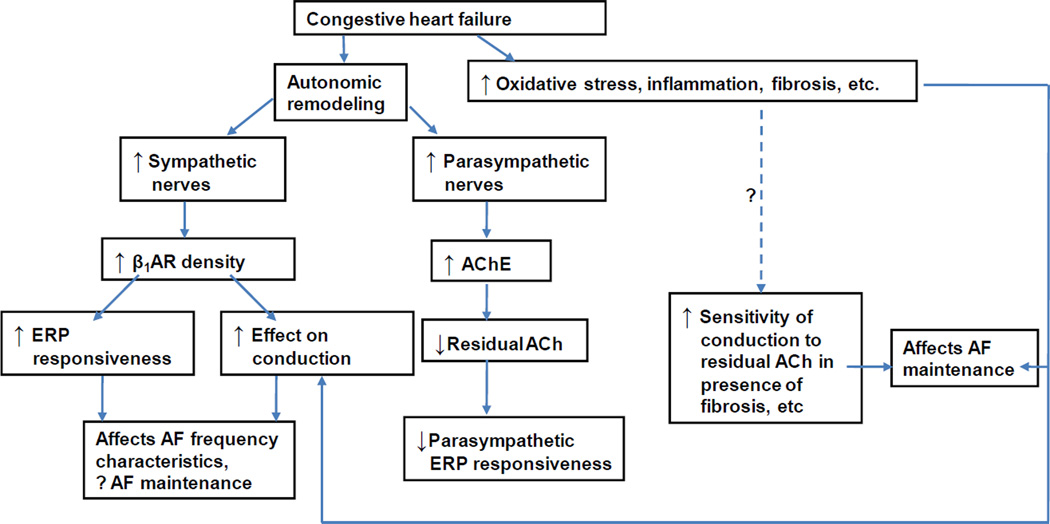
Figure 2.
Proposed model of creation of autonomic substrate for AF in CHF. The model suggests the likely presence of synergistic interactions between structural changes (fibrosis) and autonomic remodeling in the creation of AF substrate in heart failure.
ACh = acetylcholine, AChE = acetylcholinesterase, β1AR – beta-1 adrenergic receptor, ERP = effective refractory period (modified from Ng et al, Autonomic Remodeling in the Posterior Left Atrium and Pulmonary Veins in Heart Failure – Creation of a Dynamic Substrate for Atrial Fibrillation. Circulation-Arrhythmia and Electrophysiology 2011;4(3):388–96).
Figure 2 also illustrates how autonomic remodeling may be interacting with other AF mechanisms e.g. fixed structural changes in the atrium, to create the necessary substrate for the maintenance of AF in the setting of HF. In addition to alterations in the ion-channel and gap junction expression that occur in AF36, an important structural abnormality that has been studied extensively for its role in creating electrophysiological abnormalities in the atrium is fibrosis. Fibrosis promotes heterogeneity of conduction and pathways facilitating micro and macrorentry.37 The findings by Ng et al suggest that autonomic remodeling may also play a significant role in the creation of chronic AF substrate. It appears that while fibrosis may lead to conduction heterogeneity in the atrium and create a fixed substrate for reentry and consequent AF, parasympathetic and sympathetic remodeling in the PLA and PVs contribute to a more dynamic AF substrate that is dependent on the autonomic state of the left atrium.
Taken together, the above studies indicate an important role for the autonomic nervous system in the genesis of AF not only in normal hearts, but also in the setting of structural heart disease. These data underscore the potential importance of the autonomic nervous system as a suitable therapeutic target in AF in both normal and diseased hearts. The studies mentioned above also highlight the differences in autonomic remodeling in the atrium and ventricle in the setting of HF. Unlike in the ventricle, where the vagus appears to be protective against arrhythmias, the parasympathetic nervous system is clearly playing an important role in the creation of AF substrate.
Contribution of the autonomic nervous system to formation of complex atrial fractionated electrograms (CFAEs)
Over the last few years, electrophysiologically guided ablation techniques have been developed, in order to modify the arrhythmogenic substrate underlying AF. Electrogram-guided ablation procedures are the most common of these electrophysiologically-guided techniques and can be broadly divided into procedures that target atrial sites with particular electrogram characteristics in either the time domain (complex fractionated electrograms or CFAEs) or frequency components in the frequency domain (dominant frequencies). Dominant frequency (DF) is a known electrophysiologic variable by which atrial sites of periodic activity during AF can be identified. It has been suggested by several investigators that spatially organized high DF sites may play an important role in the maintenance of AF38, 39. Indeed, some studies have attempted to target high DF areas during AF ablation, with varying degrees of success40, 41. Related studies also demonstrate the heightened autonomic responsiveness of some high DF sites in patients with AF42, thereby suggesting a mechanistic role for autonomic hyperactivity in the creation of these sites.
Clinical studies performed in the last decade suggests that areas in the atrium demonstrating complex fractionated atrial electrograms (CFAE) may also represent a suitable target site for ablation; ablation at these sites appears to increase the efficacy of PV isolation procedures43, 44. One possible explanation for this improvement in ablation success is that several CFAE sites may be located in the anatomic vicinity of autonomic ganglionated plexi (GPs).45, 46 Indeed, Katritsis et al46 showed that not only did CFAEs occur over presumed GP sites in over two-thirds of patients with paroxysmal AF, but in patients that did not have CFAEs at the GP sites, CFAEs were rarely recorded elsewhere in the left atrial wall. A recent study by Pokushalov et al47 suggests that additional identification of CFAEs around the atrial regions with a positive reaction to high-frequency stimulation (HFS) might improve the accuracy of GP's boundaries location, and even enhance the success rate of AF ablation. Nonetheless, the precise relationship of CFAEs to vagal inputs is not entirely clear, especially as vagal responses are not evoked at all presumed GP sites48 or sites where CFAEs are recorded. Other data indicates that heightened vagal activity may contribute to the formation of CFAE-like EGMs45, 49. More recently, Habel et al50 showed that CFAEs organize and DF decreases in the atrium in response to autonomic blockade. Knecht et al51 also showed that CFAEs organize in response to autonomic blockade, with organization being noted in patients with paroxysmal but not persistent AF. Importantly, in the study by Knecht et al51, CFAE organization in response to double autonomic blockade was accompanied by an increase in AF cycle length, suggesting that the latter was a possible mechanism mediating autonomic responsiveness of CFAEs. Chaldoupi et al52 showed that CFAEs in the right atrial free wall and the superior/posterior wall of the left atrium are autonomically sensitive, with CFAEs in both atria organizing in the presence of double autonomic blockade. Data from our laboratory in a canine model of HF induced AF, indicates that: a) autonomic blockade significantly decreases Dominant Frequency and increases the Fractionation Interval (with a resulting decrease in CFAEs) in the PLA, and b) the autonomic responsiveness of AF electrograms (i.e. entropy of AF signals) is directly correlated with the amount and distribution of nerve-rich fatty tissue present in the myocardium53. Taken together, the findings of these studies support a role for the autonomic nervous system in contributing to AF electrograms, both in the absence and presence of structural heart disease. The contribution of autonomic nerves to time and frequency domain measures of electrogram characteristics suggests that a detailed assessment of AF electrogram content in the present of autonomic blockade may help better target autonomic ganglia during ablation.
Recent developments in imaging of the autonomic innervation of the atria – implications for AF ablation
As alluded to earlier, much of the data supporting the involvement of the autonomic nervous system in patients with AF comes from noninvasive measures of autonomic tone such as heart rate variability (HRV)25. It must be remembered, however, that HRV is a measure of autonomic modulation on the sinus node, and does not reliably quantify sympathetic and parasympathetic activity.54 As discussed earlier, more recent studies in animal models, which include data from direct nerve recordings21 as well as histological characterization of autonomic nerves7, 31, have helped shed light on the precise role of the autonomic nerves in the genesis of AF. Noninvasive methods of directly assessing neural activity in patients, e.g., with imaging-based methods, would hopefully further improve our understanding of the role of the autonomic nervous system in AF.
Radionuclide-based imaging modalities that have been used to assess autonomic function of the heart include 123-I-MIBG imaging55–58 and 11C-meta-hydroxyephedrine (HED)-PET59–61. Of these, 123-I-MIBG imaging, which allows an assessment of global sympathetic function in the heart, has been the most widely studied. The role of 123-I-MIBG imaging has been evaluated in assessing the risk of worsening congestive heart failure, death from cardiac causes and the risk of developing malignant ventricular arrhythmias in patients with coronary artery disease and in the setting of idiopathic dilated cardiomyopathy12 and has been shown to have good prognostic value in assessing the risk of ventricular tachyarrhythmias. Recently, 123-I-MIBG has undergone study for its potential utility in the setting of AF. Akutsu et al62 showed in a study of 98 patients with paroxysmal AF that a reduced Heart-to-Mediastinum (H/M) ratio – a measure of 123-I-MIBG uptake derived by drawing regions of interest (ROI) over the heart and over the upper mediastinum in an anterior planar image, and taking the ratio of mean counts per pixel in the heart to the mean counts per pixel in the mediastinum55 - was a powerful independent predictor of the development of permanent AF alone and heart failure plus permanent AF. In a related study, Akutsu et al62 showed that 123-I-MIBG may be predictive of vascular events in patients with idiopathic paroxysmal AF. Lately, Arimoto et al63 have demonstrated that a high washout rate on 123-I-MIBG imaging was an independent predictor of AF recurrence in patients with paroxysmal and persistent AF that had undergone AF ablation. The authors also demonstrated a decreased H/M ratio, both in patients with paroxysmal and persistent AF. The study by Arimoto et al underscores a need for more studies examining autonomic imaging in patients undergoing AF ablation64. While 123-I-MIBG imaging is specific to the sympathetic nervous system, thus indicating the potential role of sympathetic activity in the recurrence of AF following ablation, it is possible that 123-I-MIBG imaging may also in part reflect parasympathetic activity in the atrium, especially as sympathetic and parasympathetic nerve fibers are co-localized in the majority of nerve trunks in the atrium7, 31
In a related surgical study, there was evidence of re-innervation of sympathetic nerves in patients that have undergone the MAZE procedure for AF65. These findings are consistent with animal studies that have demonstrated autonomic re-innervation, with restoration of vagal responsiveness a few weeks after epicardial, GP denervation had been performed.66 The re-innervation noted in the atrium is not unlike that noted in the ventricle after surgical denervation e.g. at the time of cardiac transplant.67 It has also been shown that ablation itself can lead to nerve growth in the atrium68, usually several weeks following ablation. Future studies are therefore needed to look at the long-term effects of ablation on 123-I-MIBG imaging.
Selective autonomic denervation of the atria—a new therapeutic target during AF ablation or surgery?
In light of the above-mentioned data supporting the role of the autonomic nervous system in the creation of AF substrate, recent years have therefore seen the development of a variety of strategies targeted at one or more GPs either surgically69, 70 or through an endocardial approach. A strategy targeting the GPs is supported by large animal studies where ablation of the autonomic ganglia at the base of the PVs was shown to contribute to the effectiveness of PV-directed ablation procedures in vagally-induce AF71, and was also found to eliminate rapid PV firing in response to high frequency stimulation72.
GP ablation – alone or together with PV isolation - has been employed in patients for both paroxysmal and persistent AF with variable success, although success rates appear to better in patients with paroxysxmal as compared to persistent AF73–75. Scanavacca et al demonstrated the feasibility of selective atrial vagal denervation - guided by evoked vagal reflexes - to treat patients with paroxysmal atrial fibrillation.76 Pokushalov et al47 have reported that regional ablation at the anatomic sites of the left atrial GP can be safely performed and enables maintenance of sinus rhythm in 71% of patients with paroxysmal AF. Calo et al77 have recently shown that that in a selected population of vagal paroxysmal AF, anatomic ablation of GPs in the right atrium is effective in about 70% of patients. Mikhaylov et al78 compared 35 subjects with paroxysmal AF that underwent anatomic GP ablation with another 35 patients that underwent circumferential PV isolation; they discovered that anatomic GP ablation yields a significantly lower success rate over the long-term follow-up period, when compared with circumferential PV isolation. However, Karitsis et al27 and others79 have demonstrated that when GP ablation is combined with PV isolation, it yields better results than PV isolation alone, with success rates approaching up to 80%27. Pokushalov et al74 reported success rates of < 40% at one year after performing isolated GP ablation for symptomatic, drug refractory persistent AF; circumferential isolation of the PVs was needed in these patients to increase the success rate of GP ablation. Recent surgical studies have also attempted to add GP ablation/excision to PV isolation, albeit with varying efficacy69, 70, 80, 81. However, it is clear while minimally invasive surgery consisting of bipolar radiofrequency pulmonary vein (PV) isolation and limited GP ablation is effective in reducing atrial fibrillation (AF) in patients with paroxysmal AF, it is less effective in those with persistent AF or long-standing persistent AF82. In the latter setting, the addition of linear lesion sets appears to increase surgical success. 82.
Despite the success rates of some of the above-mentioned studies in decreasing AF, it must be remembered that even if AF inducibility decreases in the short term following GP ablation, long-term suppression of AF is not guaranteed, in part due to the possibility of re-innervation of ablated autonomic nerves66. An added disadvantage of an anatomic ablative approach is that it inevitably causes transmural atrial tissue damage. Lastly, even though a majority of nerve trunks are located within the fat/fibrofatty tissue itself, up to a third of nerve trunks in the PLA can be located away from the fat in adjoining/underlying myocardium7. This finding suggests that anatomic ablation strategies directed at atrial fat pads may not result in complete and/or sustained denervation of the PLA.
As mentioned earlier, it appears that some CFAEs appear to be autonomically mediated, both in paroxysmal and persistent AF. It is therefore possible that ablation strategies targeted at autonomically-sensitive CFAEs may help increase efficacy of AF ablation. Future studies are needed to assess the relative efficacy of an anatomic, ‘GP-focused’ approach over a CFAE-guided approach to target the autonomic substrate underlying AF.
Novel, biological approaches targeting autonomic signaling in the atrium – role for G-protein modification
Some of the drawbacks of current ablative approaches to obtaining autonomic denervation of the atria have been discussed above, including the fact that sympathetic and parasympathetic fibers are co-localized, with the result that ablation approaches will result likely in denervation of both limbs of the autonomic nervous system. Ablation also carries the risk of damaging adjoining myocardium, as well as other surrounding structures. We and others have therefore attempted to modify autonomic influences on the atria using molecular or biological approaches. Below, we describe recent attempts by our group and others to modulate vagal signaling in the atrium by targeting Gαi proteins.
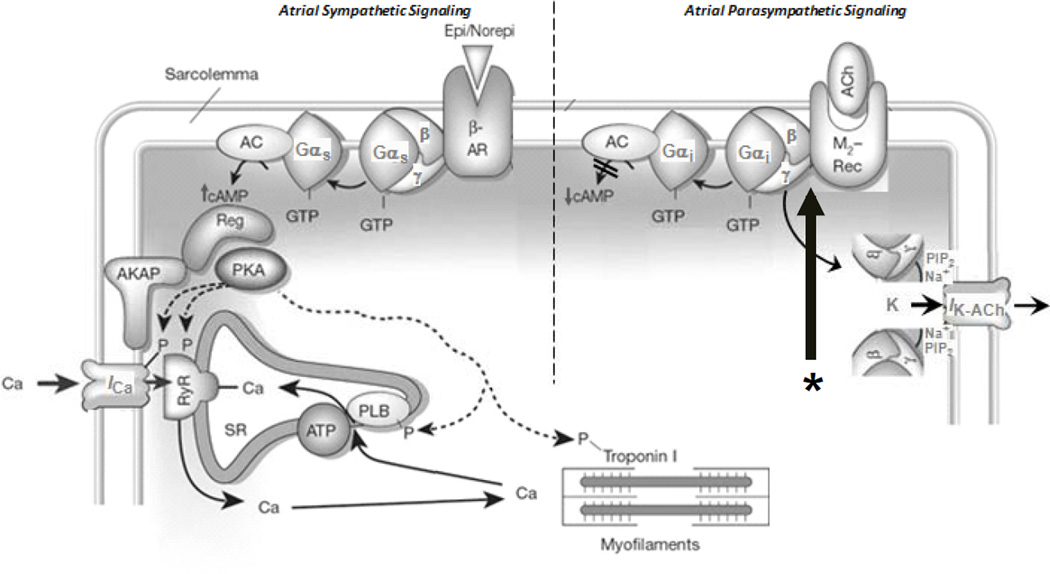
G-protein coupled receptors (GPCRs) transduce the autonomic neurohormonal signals via their respective G-proteins that either act on ion channels and Ca2+-handling proteins indirectly through second messengers (e.g., adenyl cyclase, phospholipid hydrolysis systems) or by direct protein-protein interaction (see figure 3). The inotropic and chronotropic actions of the sympathetic system on the heart occur primarily via β1 and β2-adrenergic receptors. β1-receptors comprise 70–80% of all β-receptors in the normal atrium. The stimulatory β-adrenergic (β-AR) response is initiated via Gαs leading to activation of adenyl cyclase and subsequent protein kinase A-mediated phosphorylation of L-type calcium channels, troponin I, and phospholamban, resulting in increased calcium influx and augmented contractility as well as increased calcium reuptake and enhanced relaxation. These effects of sympathetic stimulation in the atria can result in triggered atrial premature beats as well as a shortening of refractoriness. Cholinergic M2 receptors (M2R) are the primary mediators parasympathetic control of heart function, and thus M2R stimulation effects are opposite those of β-AR stimulation. M2R stimulation by acetylcholine causes inhibition of adenyl cyclase and reduces cAMP via pertussis toxin (PTx) sensitive Gαi/o proteins, which leads to a attenuated ICa-L and If. M2R-stimulated Gi also directly activates atrial GIRK (IK-ACh), which effects refractory period shortening in the atrium.
Figure 3.
The figure shows the signaling cascade for sympathetic and parasympathetic signaling. The large arrow with the asterix (*) below is pointing at the M2/Gαi/o interface, to which the Gα-C-terminal peptides (see text) are directed.
The central role of G-protein signaling in autonomic function in the heart has been successfully exploited by some groups to modify electrophysiological properties of the heart. In an innovative approach, Donahue et al genetically modified the signal transduction effectors of cardiac autonomic innervation using an adenoviral vector overexpressing the Gαi protein83, 84. Infection of Gαi in the AV node suppressed baseline AV conduction and slowed heart rate during AF.
Since the parasympathetic hyperactivity has been shown to create AF substrate, we have attempted to disrupt parasympathetic signaling in the atrium by using G-protein inhibitory peptides targeting the C-terminus of the Gαi/o subunits85. A variety of studies have implicated the C-terminus of G-protein α subunits in mediating receptor/G-protein interaction and selectivity86. Since vagal signaling is known to be pro-arrhythmic in the atrium, Aistrup et al, in a proof-of-concept study85, demonstrated that atrial-selective attenuation of vagal signaling can be acutely achieved by a Gαi C-terminal peptide (Gαi2ctp or Gαi3ctp) delivered to the PLA in a targeted manner—direct myocardial injection plus electroporation. This Gαictp putatively acts by selectively disrupting M2R-Gαi coupling (see figure 3), thus impeding Gαi-mediated signal transduction. In an effort to obtain sustained inhibition of vagal signaling in the atria, Aistrup et al84, in a subsequent study, attempted constitutive administration of Gαi2ctp and Gαo1ctp (to inhibit Gαo, another G-protein known to contribute to vagal signaling in the atria87), by incorporating their cDNA into plasmid expression vectors (minigenes), delivering them into canine PLA and assessing their effects on cholinergic responsiveness. 3 days after gene delivery, they noted a significant decrease in parasympathetic responsiveness not just in the PLA, but also in the rest of the left atrium. This decrease in vagal responsiveness was accompanied by a significant decrease in vagal induced AF.
The early stage studies described above provide proof-of-concept for a gene-based approach to selectively target sympathetic and/or parasympathetic signaling in the atrium. More rigorous pre-clinical studies need to be performed, demonstrating a) longer term expression of genes targeting the autonomic nervous system and b) the safety of such an approach, especially since the G-proteins being targeted may also affect other signaling pathways in the atrium. Nonetheless, gene therapy approaches do appear to hold some promise for the treatment of AF; lately, other investigators have demonstrated the feasibility of a gene-therapy approach in successfully targeting other mechanisms in AF e.g. modification of potassium channels and atrial gap junctions88–91.
Summary
The studies presented above indicate that autonomic influences contribute to the creation of AF substrate not only in normal hearts but also in the setting of structural heart disease. Current ablative and surgical methods are therefore attempting to anatomically target autonomic GPs in patients with AF, in order to achieve autonomic denervation of the atria. Recent studies suggest that characteristic of AF electrograms (e.g. CFAEs) may also define autonomic inputs in the fibrillating atria, and may therefore be a suitable target for ablation. However, significant further investigation is necessary to optimize current ablation approaches to the atrial autonomic nervous system. Other new developments in our understanding of the role of the autonomic signaling in AF include radionuclide imaging studies in patients with AF; these studies indicate that 123-I-MIBG imaging may have prognostic value in patients with AF, including in the setting of AF ablation. Lastly, due the varying efficacy of current ablation approaches targeting the autonomic innervation of the atria, we also describe recent biological (gene-therapy) attempts to selectively disrupt parasympathetic signaling in the atria using novel G-protein inhibitory peptides; further studies are needed to fully investigate the potential of these new biological approaches to AF.
Acknowledgments
RA receives research grants from National Institutes of Health (NHLBI) Grants: 1R01HL093490, 3R01HL093490-01S1, and R21 HL088304, Everett/O’Connor Trust, Dixon Translational Research Council, Zoe Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: He is has ownership interest in Rhythm Solutions, LLC as founder and Rythmos Therapeutics as co-founder.
References
- 1.Ng J, Villuendas R, Cokic I, Schliamser JE, Gordon D, Koduri H, Benefield B, Simon J, Murthy SN, Lomasney JW, Wasserstrom JA, Goldberger JJ, Aistrup GL, Arora R. Autonomic remodeling in the left atrium and pulmonary veins in heart failure: Creation of a dynamic substrate for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:388–396. doi: 10.1161/CIRCEP.110.959650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: Implications for management. Circulation. 2011;124:2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 3.Shen MJ, Choi EK, Tan AY, Lin SF, Fishbein MC, Chen LS, Chen PS. Neural mechanisms of atrial arrhythmias. Nat Rev Cardiol. 2011;9:30–39. doi: 10.1038/nrcardio.2011.139. [DOI] [PubMed] [Google Scholar]
- 4.Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J Am Coll Cardiol. 2003;42:1262–1268. doi: 10.1016/s0735-1097(03)00955-0. [DOI] [PubMed] [Google Scholar]
- 5.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation.[see comment] Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Arora R, Ng J, Ulphani J, Mylonas I, Subacius H, Shade G, Gordon D, Morris A, He X, Lu Y, Belin R, Goldberger JJ, Kadish AH. Unique autonomic profile of the pulmonary veins and posterior left atrium. J Am Coll Cardiol. 2007;49:1340–1348. doi: 10.1016/j.jacc.2006.10.075. [DOI] [PubMed] [Google Scholar]
- 7.Arora R, Ulphani JS, Villuendas R, Ng J, Harvey L, Thordson S, Inderyas F, Lu Y, Gordon D, Denes P, Greene R, Crawford S, Decker R, Morris A, Goldberger J, Kadish AH. Neural substrate for atrial fibrillation: Implications for targeted parasympathetic blockade in the posterior left atrium. Am J Physiol Heart Circ Physiol. 2008;294:H134–H144. doi: 10.1152/ajpheart.00732.2007. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa M, Zhou S, Tan AY, Song J, Gholmieh G, Fishbein MC, Luo H, Siegel RJ, Karagueuzian HS, Chen LS, Lin SF, Chen PS. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007;50:335–343. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 9.Chen PS, Tan AY. Autonomic nerve activity and atrial fibrillation. Heart Rhythm. 2007;4:S61–S64. doi: 10.1016/j.hrthm.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coumel P. Paroxysmal atrial fibrillation: A disorder of autonomic tone? Eur Heart J. 1994;15(Suppl A):9–16. doi: 10.1093/eurheartj/15.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 11.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 12.Ter Keurs HE, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev. 2007;87:457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemirovsky D, Hutter R, Gomes JA. The electrical substrate of vagal atrial fibrillation as assessed by the signal-averaged electrocardiogram of the p wave. Pacing Clin Electrophysiol. 2008;31:308–313. doi: 10.1111/j.1540-8159.2008.00990.x. [DOI] [PubMed] [Google Scholar]
- 14.Chou CC, Chen PS. New concepts in atrial fibrillation: Neural mechanisms and calcium dynamics. Cardiol Clin. 2009;27:35–43. doi: 10.1016/j.ccl.2008.09.003. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fioranelli M, Piccoli M, Mileto GM, Sgreccia F, Azzolini P, Risa MP, Francardelli RL, Venturini E, Puglisi A. Analysis of heart rate variability five minutes before the onset of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 1999;22:743–749. doi: 10.1111/j.1540-8159.1999.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 16.Tomita T, Takei M, Saikawa Y, Hanaoka T, Uchikawa S, Tsutsui H, Aruga M, Miyashita T, Yazaki Y, Imamura H, Kinoshita O, Owa M, Kubo K. Role of autonomic tone in the initiation and termination of paroxysmal atrial fibrillation in patients without structural heart disease. J Cardiovasc Electrophysiol. 2003;14:559–564. doi: 10.1046/j.1540-8167.2003.02462.x. [DOI] [PubMed] [Google Scholar]
- 17.Dimmer C, Tavernier R, Gjorgov N, Van Nooten G, Clement DL, Jordaens L. Variations of autonomic tone preceding onset of atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 1998;82:22–25. doi: 10.1016/s0002-9149(98)00231-8. [DOI] [PubMed] [Google Scholar]
- 18.Herweg B, Dalal P, Nagy B, Schweitzer P. Power spectral analysis of heart period variability of preceding sinus rhythm before initiation of paroxysmal atrial fibrillation. Am J Cardiol. 1998;82:869–874. doi: 10.1016/s0002-9149(98)00494-9. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann M, Kalusche D. Fluctuation in autonomic tone is a major determinant of sustained atrial arrhythmias in patients with focal ectopy originating from the pulmonary veins. J Cardiovasc Electrophysiol. 2001;12:285–291. doi: 10.1046/j.1540-8167.2001.00285.x. [DOI] [PubMed] [Google Scholar]
- 20.Tan AY, Zhou S, Ogawa M, Song J, Chu M, Li H, Fishbein MC, Lin SF, Chen LS, Chen PS. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi EK, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, Piccirillo G, Frick K, Fishbein MC, Hwang C, Lin SF, Chen PS. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–2623. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa M, Tan AY, Song J, Kobayashi K, Fishbein MC, Lin SF, Chen LS, Chen PS. Cryoablation of stellate ganglia and atrial arrhythmia in ambulatory dogs with pacing-induced heart failure. Heart Rhythm. 2009;6:1772–1779. doi: 10.1016/j.hrthm.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa M, Zhou S, Tan AY, Song J, Gholmieh G, Fishbein MC, Luo H, Siegel RJ, Karagueuzian HS, Chen LS, Lin SF, Chen PS. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007;50:335–343. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 24.Shen MJ, Choi EK, Tan AY, Han S, Shinohara T, Maruyama M, Chen LS, Shen C, Hwang C, Lin SF, Chen PS. Patterns of baseline autonomic nerve activity and the development of pacing-induced sustained atrial fibrillation. Heart Rhythm. 2011;8:583–589. doi: 10.1016/j.hrthm.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi Y, Kumagai K, Nakashima H, Saku K. Long-term effects of box isolation on sympathovagal balance in atrial fibrillation. Circ J. 2010;74:1096–1103. doi: 10.1253/circj.cj-09-0899. [DOI] [PubMed] [Google Scholar]
- 26.Verma A, Saliba WI, Lakkireddy D, Burkhardt JD, Cummings JE, Wazni OM, Belden WA, Thal S, Schweikert RA, Martin DO, Tchou PJ, Natale A. Vagal responses induced by endocardial left atrial autonomic ganglion stimulation before and after pulmonary vein antrum isolation for atrial fibrillation. Heart Rhythm. 2007;4:1177–1182. doi: 10.1016/j.hrthm.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 27.Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modification: A randomized study. Heart Rhythm. 2011;8:672–678. doi: 10.1016/j.hrthm.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 28.Hou Y, Scherlag BJ, Lin J, Zhang Y, Lu Z, Truong K, Patterson E, Lazzara R, Jackman WM, Po SS. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: Effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol. 2007;50:61–68. doi: 10.1016/j.jacc.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 29.Hou Y, Scherlag BJ, Lin J, Zhou J, Song J, Zhang Y, Patterson E, Lazzara R, Jackman WM, Po SS. Interactive atrial neural network: Determining the connections between ganglionated plexi. Heart Rhythm. 2007;4:56–63. doi: 10.1016/j.hrthm.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Ulphani JS, Arora R, Cain JH, Villuendas R, Shen S, Gordon D, Inderyas F, Harvey LA, Morris A, Goldberger JJ, Kadish AH. The ligament of marshall as a parasympathetic conduit. Am J Physiol Heart Circ Physiol. 2007;293:H1629–H1635. doi: 10.1152/ajpheart.00139.2007. [DOI] [PubMed] [Google Scholar]
- 31.Tan AY, Li H, Wachsmann-Hogiu S, Chen LS, Chen PS, Fishbein MC. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: Implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006;48:132–143. doi: 10.1016/j.jacc.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 32.Deneke T, Chaar H, de Groot JR, Wilde AA, Lawo T, Mundig J, Bosche L, Mugge A, Grewe PH. Shift in the pattern of autonomic atrial innervation in subjects with persistent atrial fibrillation. Heart Rhythm. 2011;8:1357–1363. doi: 10.1016/j.hrthm.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Arora R, Verheule S, Scott L, Navarrete A, Katari V, Wilson E, Vaz D, Olgin JE. Arrhythmogenic substrate of the pulmonary veins assessed by high-resolution optical mapping. Circulation. 2003;107:1816–1821. doi: 10.1161/01.CIR.0000058461.86339.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jayachandran JV, Sih HJ, Winkle W, Zipes DP, Hutchins GD, Olgin JE. Atrial fibrillation produced by prolonged rapid atrial pacing is associated with heterogeneous changes in atrial sympathetic innervation. Circulation. 2000;101:1185–1191. doi: 10.1161/01.cir.101.10.1185. [DOI] [PubMed] [Google Scholar]
- 35.Gould PA, Yii M, McLean C, Finch S, Marshall T, Lambert GW, Kaye DM. Evidence for increased atrial sympathetic innervation in persistent human atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:821–829. doi: 10.1111/j.1540-8159.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 36.Gollob MH. Cardiac connexins as candidate genes for idiopathic atrial fibrillation. Curr Opin Cardiol. 2006;21:155–158. doi: 10.1097/01.hco.0000221574.95383.6f. [DOI] [PubMed] [Google Scholar]
- 37.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: Atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 38.Kalifa J, Tanaka K, Zaitsev AV, Warren M, Vaidyanathan R, Auerbach D, Pandit S, Vikstrom KL, Ploutz-Snyder R, Talkachou A, Atienza F, Guiraudon G, Jalife J, Berenfeld O. Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation. 2006;113:626–633. doi: 10.1161/CIRCULATIONAHA.105.575340. [DOI] [PubMed] [Google Scholar]
- 39.Okumura Y, Watanabe I, Kofune M, Nagashima K, Sonoda K, Mano H, Ohkubo K, Nakai T, Hirayama A. Characteristics and distribution of complex fractionated atrial electrograms and the dominant frequency during atrial fibrillation: Relationship to the response and outcome of circumferential pulmonary vein isolation. J Interv Card Electrophysiol. 2011 Dec 17; doi: 10.1007/s10840-011-9637-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Atienza F, Almendral J, Jalife J, Zlochiver S, Ploutz-Snyder R, Torrecilla EG, Arenal A, Kalifa J, Fernandez-Aviles F, Berenfeld O. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm. 2009;6:33–40. doi: 10.1016/j.hrthm.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin YJ, Tsao HM, Chang SL, Lo LW, Hu YF, Chang CJ, Tsai WC, Suenari K, Huang SY, Chang HY, Wu TJ, Chen SA. Role of high dominant frequency sites in nonparoxysmal atrial fibrillation patients: Insights from high-density frequency and fractionation mapping. Heart Rhythm. 2010;7:1255–1262. doi: 10.1016/j.hrthm.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 42.Habel N, Muller JG, Znojkiewicz P, Thompson N, Calame J, Calame S, Noori A, Gallo A, Lustgarten DL, Sobel BE, Spector PS. The impact of pharmacologic sympathetic and parasympathetic blockade on atrial electrogram characteristics in patients with atrial fibrillation. Pacing Clin Electrophysiol. 2011;34:1460–1467. doi: 10.1111/j.1540-8159.2011.03212.x. [DOI] [PubMed] [Google Scholar]
- 43.Arruda M, Natale A. Ablation of permanent AF: adjunctive strategies to pulmonary veins isolation: targeting AF NEST in sinus rhythm and CFAE in AF. J Interv Card Electrophysiol. 2008;23:51–57. doi: 10.1007/s10840-008-9252-z. [DOI] [PubMed] [Google Scholar]
- 44.Porter M, Spear W, Akar JG, Helms R, Brysiewicz N, Santucci P, Wilber DJ. Prospective study of atrial fibrillation termination during ablation guided by automated detection of fractionated electrograms. J Cardiovasc Electrophysiol. 2008;19:613–620. doi: 10.1111/j.1540-8167.2008.01189.x. [DOI] [PubMed] [Google Scholar]
- 45.Lin J, Scherlag BJ, Zhou J, Lu Z, Patterson E, Jackman WM, Lazzara R, Po SS. Autonomic mechanism to explain complex fractionated atrial electrograms (cfae) J Cardiovasc Electrophysiol. 2007;18:1197–1205. doi: 10.1111/j.1540-8167.2007.00976.x. [DOI] [PubMed] [Google Scholar]
- 46.Katritsis D, Giazitzoglou E, Sougiannis D, Voridis E, Po SS. Complex fractionated atrial electrograms at anatomic sites of ganglionated plexi in atrial fibrillation. Europace. 2009;11:308–315. doi: 10.1093/europace/eup036. [DOI] [PubMed] [Google Scholar]
- 47.Pokushalov E, Romanov A, Artyomenko S, Shirokova N, Turov A, Karaskov A, Katritsis DG, Po SS. Ganglionated plexi ablation directed by high-frequency stimulation and complex fractionated atrial electrograms for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2012 Apr 5; doi: 10.1111/j.1540-8159.2012.03392.x. doi: 10.1111/j.1540-8159.2012.03392.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Katritsis D, Sougiannis D, Batsikas K, Giazitzoglou E, Mersinias J, Katritsis G, Po SS. Autonomic modulation of complex fractionated atrial electrograms in patients with paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2011;31:217–223. doi: 10.1007/s10840-011-9558-0. [DOI] [PubMed] [Google Scholar]
- 49.Oh S, Kong HJ, Choi EK, Kim HC, Choi YS. Complex fractionated electrograms and af nests in vagally mediated atrial fibrillation. Pacing Clin Electrophysiol. 2010;33:1497–1503. doi: 10.1111/j.1540-8159.2010.02834.x. [DOI] [PubMed] [Google Scholar]
- 50.Habel N, Muller JG, Znojkiewicz P, Thompson N, Calame J, Calame S, Noori A, Gallo A, Lustgarten DL, Sobel BE, Spector PS. The impact of pharmacologic sympathetic and parasympathetic blockade on atrial electrogram characteristics in patients with atrial fibrillation. Pacing Clin Electrophysiol. 2011;34:1460–1467. doi: 10.1111/j.1540-8159.2011.03212.x. [DOI] [PubMed] [Google Scholar]
- 51.Knecht S, Wright M, Matsuo S, Nault I, Lellouche N, Sacher F, Kim SJ, Morgan D, Afonso V, Shinzuke M, Hocini M, Clementy J, Narayan SM, Ritter P, Jais P, Haissaguerre M. Impact of pharmacological autonomic blockade on complex fractionated atrial electrograms. J Cardiovasc Electrophysiol. 2010;21:766–772. doi: 10.1111/j.1540-8167.2009.01712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaldoupi SM, Linnenbank AC, Wittkampf FH, Boldt LH, H VANW, VJ VAND, Doevendans PA, Hauer RN, JM DEB, Loh P. Complex fractionated electrograms in the right atrial free wall and the superior/posterior wall of the left atrium are affected by activity of the autonomic nervous system. J Cardiovasc Electrophysiol. 2012;23:26–33. doi: 10.1111/j.1540-8167.2011.02145.x. [DOI] [PubMed] [Google Scholar]
- 53.Koduri HNJ, Cokic I, Aistrup G, Gordon D, Wasserstrom J, Kadish A, Lee R, Passman R, Knight B, Goldberger J, Arora R. Contribution of fibrosis and the autonomic nervous system to atrial fibrillation electrograms in heart failure. Circ Arrhythm Electrophysiol. doi: 10.1161/CIRCEP.111.970095. (accepted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piccirillo G, Ogawa M, Song J, Chong VJ, Joung B, Han S, Magrì D, Chen LS, Lin S-F, Chen P-S. Power spectral analysis of heart rate variability and autonomic nervous system activity measured directly in healthy dogs and dogs with tachycardia-induced heart failure. Heart Rhythm. 2009;6:546–552. doi: 10.1016/j.hrthm.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chirumamilla A, Travin MI. Cardiac applications of 123i-mibg imaging. Semin Nucl Med. 2011;41:374–387. doi: 10.1053/j.semnuclmed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Ji SY, Travin MI. Radionuclide imaging of cardiac autonomic innervation. J Nucl Cardiol. 2010;17:655–666. doi: 10.1007/s12350-010-9239-x. [DOI] [PubMed] [Google Scholar]
- 57.Travin MI. Cardiac neuronal imaging at the edge of clinical application. Cardiol Clin. 2009;27:311–327. doi: 10.1016/j.ccl.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 58.Arora R, Ferrick KJ, Nakata T, Kaplan RC, Rozengarten M, Latif F, Ng K, Marcano V, Heller S, Fisher JD, Travin MI. I-123 mibg imaging and heart rate variability analysis to predict the need for an implantable cardioverter defibrillator. J Nucl Cardiol. 2003;10:121–131. doi: 10.1067/mnc.2003.2. [DOI] [PubMed] [Google Scholar]
- 59.Fallavollita JA, Banas MD, Suzuki G, deKemp RA, Sajjad M, Canty JM., Jr 11c-meta-hydroxyephedrine defects persist despite functional improvement in hibernating myocardium. J Nucl Cardiol. 2010;17:85–96. doi: 10.1007/s12350-009-9164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luisi AJ, Jr, Suzuki G, Dekemp R, Haka MS, Toorongian SA, Canty JM, Jr, Fallavollita JA. Regional 11c-hydroxyephedrine retention in hibernating myocardium: Chronic inhomogeneity of sympathetic innervation in the absence of infarction. J Nucl Med. 2005;46:1368–1374. [PubMed] [Google Scholar]
- 61.Mazzadi AN, Andre-Fouet X, Duisit J, Gebuhrer V, Costes N, Chevalier P, Rodriguez C, Schott JJ, Le Marec H, Guicheney P, Le Bars D, Janier M. Cardiac retention of [11c]hed in genotyped long qt patients: A potential amplifier role for severity of the disease. Am J Physiol Heart Circ Physiol. 2003;285:H1286–H1293. doi: 10.1152/ajpheart.00276.2003. [DOI] [PubMed] [Google Scholar]
- 62.Akutsu Y, Kaneko K, Kodama Y, Li HL, Suyama J, Shinozuka A, Gokan T, Hamazaki Y, Tanno K, Kobayashi Y. Iodine-123 mibg imaging for predicting the development of atrial fibrillation. JACC Cardiovasc Imaging. 2011;4:78–86. doi: 10.1016/j.jcmg.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Arimoto TTH, Igarashi M, Sekiguchi Y, Sato A, Koyama T, Yamasaki H, Machino T, Kuroki K, Aonuma K. High washout rate of iodine-123-metaiodobenzylguanidine imaging predicts the outcome of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22:1297–1304. doi: 10.1111/j.1540-8167.2011.02123.x. [DOI] [PubMed] [Google Scholar]
- 64.Arora R. Sympathetic imaging with 123-i-mibg--a new way to predict recurrences after af ablation. J Cardiovasc Electrophysiol. 2011;22:1305–1308. doi: 10.1111/j.1540-8167.2011.02176.x. [DOI] [PubMed] [Google Scholar]
- 65.Mabuchi M, Imamura M, Kubo N, Morita K, Noriyasu K, Tsukamoto T, Yasuda K, Tamaki N. Sympathetic denervation and reinnervation after the maze procedure. J Nucl Med. 2005;46:1089–1094. [PubMed] [Google Scholar]
- 66.Sakamoto S, Schuessler RB, Lee AM, Aziz A, Lall SC, Damiano RJ., Jr Vagal denervation and reinnervation after ablation of ganglionated plexi. J Thorac Cardiovasc Surg. 2010;139:444–452. doi: 10.1016/j.jtcvs.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buendia-Fuentes F, Almenar L, Ruiz C, Vercher JL, Sanchez-Lazaro I, Martinez-Dolz L, Navarro J, Bello P, Salvador A. Sympathetic reinnervation 1 year after heart transplantation, assessed using iodine-123 metaiodobenzylguanidine imaging. Transplant Proc. 2011;43:2247–2248. doi: 10.1016/j.transproceed.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 68.Okuyama Y, Pak HN, Miyauchi Y, Liu YB, Chou CC, Hayashi H, Fu KJ, Kerwin WF, Kar S, Hata C, Karagueuzian HS, Fishbein MC, Chen PS, Chen LS. Nerve sprouting induced by radiofrequency catheter ablation in dogs. Heart Rhythm. 2004;1:712–717. doi: 10.1016/j.hrthm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 69.Edgerton JR, Brinkman WT, Weaver T, Prince SL, Culica D, Herbert MA, Mack MJ. Pulmonary vein isolation and autonomic denervation for the management of paroxysmal atrial fibrillation by a minimally invasive surgical approach. J Thorac Cardiovasc Surg. 2010;140:823–828. doi: 10.1016/j.jtcvs.2009.11.065. [DOI] [PubMed] [Google Scholar]
- 70.Edgerton JR, Edgerton ZJ, Weaver T, Reed K, Prince S, Herbert MA, Mack MJ. Minimally invasive pulmonary vein isolation and partial autonomic denervation for surgical treatment of atrial fibrillation. Ann Thorac Surg. 2008;86:35–38. doi: 10.1016/j.athoracsur.2008.03.071. discussion 39. [DOI] [PubMed] [Google Scholar]
- 71.Lemola K, Chartier D, Yeh YH, Dubuc M, Cartier R, Armour A, Ting M, Sakabe M, Shiroshita-Takeshita A, Comtois P, Nattel S. Pulmonary vein region ablation in experimental vagal atrial fibrillation: Role of pulmonary veins versus autonomic ganglia. Circulation. 2008;117:470–477. doi: 10.1161/CIRCULATIONAHA.107.737023. [DOI] [PubMed] [Google Scholar]
- 72.Lu Z, Scherlag BJ, Lin J, Yu L, Guo JH, Niu G, Jackman WM, Lazzara R, Jiang H, Po SS. Autonomic mechanism for initiation of rapid firing from atria and pulmonary veins: Evidence by ablation of ganglionated plexi. Cardiovasc Res. 2009;84:245–252. doi: 10.1093/cvr/cvp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pokushalov E, Romanov A, Artyomenko S, Turov A, Shirokova N, Katritsis DG. Left atrial ablation at the anatomic areas of ganglionated plexi for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2010;33:1231–1238. doi: 10.1111/j.1540-8159.2010.02800.x. [DOI] [PubMed] [Google Scholar]
- 74.Pokushalov E, Romanov A, Artyomenko S, Turov A, Shugayev P, Shirokova N, Katritsis DG. Ganglionated plexi ablation for longstanding persistent atrial fibrillation. Europace. 2010;12:342–346. doi: 10.1093/europace/euq014. [DOI] [PubMed] [Google Scholar]
- 75.Pokushalov E, Romanov A, Shugayev P, Artyomenko S, Shirokova N, Turov A, Katritsis DG. Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2009;6:1257–1264. doi: 10.1016/j.hrthm.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 76.Scanavacca M, Pisani CF, Hachul D, Lara S, Hardy C, Darrieux F, Trombetta I, Negrao CE, Sosa E. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006;114:876–885. doi: 10.1161/CIRCULATIONAHA.106.633560. [DOI] [PubMed] [Google Scholar]
- 77.Calo L, Rebecchi M, Sciarra L, De Luca L, Fagagnini A, Zuccaro LM, Pitrone P, Dottori S, Porfirio M, de Ruvo E, Lioy E. Catheter ablation of right atrial ganglionated plexi in patients with vagal paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:22–31. doi: 10.1161/CIRCEP.111.964262. [DOI] [PubMed] [Google Scholar]
- 78.Mikhaylov E, Kanidieva A, Sviridova N, Abramov M, Gureev S, Szili-Torok T, Lebedev D. Outcome of anatomic ganglionated plexi ablation to treat paroxysmal atrial fibrillation: A 3-year follow-up study. Europace. 2011;13:362–370. doi: 10.1093/europace/euq416. [DOI] [PubMed] [Google Scholar]
- 79.Zhou Q, Hou Y, Yang S. A meta-analysis of the comparative efficacy of ablation for atrial fibrillation with and without ablation of the ganglionated plexi. Pacing Clin Electrophysiol. 2011;34:1687–1694. doi: 10.1111/j.1540-8159.2011.03220.x. [DOI] [PubMed] [Google Scholar]
- 80.Nitta T, Ishii Y, Sakamoto S. Surgery for atrial fibrillation: Recent progress and future perspective. Gen Thorac Cardiovasc Surg. 2012;60:13–20. doi: 10.1007/s11748-011-0849-2. [DOI] [PubMed] [Google Scholar]
- 81.Bagge L, Blomstrom P, Nilsson L, Einarsson GM, Jideus L, Blomstrom-Lundqvist C. Epicardial off-pump pulmonary vein isolation and vagal denervation improve long-term outcome and quality of life in patients with atrial fibrillation. J Thorac Cardiovasc Surg. 2009;137:1265–1271. doi: 10.1016/j.jtcvs.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 82.Edgerton JR, Jackman WM, Mahoney C, Mack MJ. Totally thorascopic surgical ablation of persistent af and long-standing persistent atrial fibrillation using the "dallas" lesion set. Heart Rhythm. 2009;6:S64–S70. doi: 10.1016/j.hrthm.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 83.Donahue JK, Heldman AW, Fraser H, McDonald AD, Miller JM, Rade JJ, Eschenhagen T, Marban E. Focal modification of electrical conduction in the heart by viral gene transfer. Nat Med. 2000;6:1395–1398. doi: 10.1038/82214. [DOI] [PubMed] [Google Scholar]
- 84.Aistrup GL, Cokic I, Ng J, Gordon D, Koduri H, Browne S, Arapi D, Segon Y, Goldstein J, Angulo A, Wasserstrom JA, Goldberger JJ, Kadish AH, Arora R. Targeted non-viral gene-based inhibition of galphai/o-mediated vagal signaling in the posterior left atrium decreases vagal induced af. Heart Rhythm. 2011;8:1722–1729. doi: 10.1016/j.hrthm.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aistrup GL, Villuendas R, Ng J, Gilchrist A, Lynch TW, Gordon D, Cokic I, Mottl S, Zhou R, Dean DA, Wasserstrom JA, Goldberger JJ, Kadish AH, Arora R. Targeted g-protein inhibition as a novel approach to decrease vagal atrial fibrillation by selective parasympathetic attenuation. Cardiovasc Res. 2009;83:481–492. doi: 10.1093/cvr/cvp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oldham WM, Hamm HE. Heterotrimeric g protein activation by g-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- 87.Boknik P, Grote-Wessels S, Barteska G, Jiang M, Müller FU, Schmitz W, Neumann J, Birnbaumer L. Genetic disruption of g proteins, gi2α or goα, does not abolish inotropic and chronotropic effects of stimulating muscarinic cholinoceptors in atrium. Br J Pharmacol. 2009;158:1557–1564. doi: 10.1111/j.1476-5381.2009.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Soucek R, Thomas D, Kelemen K, Bikou O, Seyler C, Voss F, Becker R, Koenen M, Katus HA, Bauer A. Genetic suppression of atrial fibrillation using a dominant-negative ether-a-go-go-related gene mutant. Heart Rhythm. 2012;9:265–272. doi: 10.1016/j.hrthm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 89.Bikou O, Thomas D, Trappe K, Lugenbiel P, Kelemen K, Koch M, Soucek R, Voss F, Becker R, Katus HA, Bauer A. Connexin 43 gene therapy prevents persistent atrial fibrillation in a porcine model. Cardiovasc Res. 2011;92:218–225. doi: 10.1093/cvr/cvr209. [DOI] [PubMed] [Google Scholar]
- 90.Trappe K, Thomas D, Bikou O, Kelemen K, Lugenbiel P, Voss F, Becker R, Katus HA, Bauer A. Suppression of persistent atrial fibrillation by genetic knockdown of caspase 3: A pre-clinical pilot study. Eur Heart J. 2011 Jul 23; doi: 10.1093/eurheartj/ehr269. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 91.Igarashi T, Finet JE, Takeuchi A, Fujino Y, Strom M, Greener ID, Rosenbaum DS, Donahue JK. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation. 2012;125:216–225. doi: 10.1161/CIRCULATIONAHA.111.053272. [DOI] [PMC free article] [PubMed] [Google Scholar]